Abstract
To report our experience of coarctation stent therapy in small children weighing less than 30 kg, with the low profile dilatable Valeo stent and review the literature on coarctation stent therapy in this patient population. Coarctation stent implantation was undertaken in 14 consecutive children using the Bard Valeo Stent. Demographic, angiographic, echocardiographic and clinical data were reviewed retrospectively. The median age at the time of procedure was 5.1 (2.6–7.5) years and median weight was 20.8 (14.7–27) kg. There was improvement in median coarctation diameter from 4 (1.3–5.2) to 9.5 (5.8–12.7) mm, p < 0.001; and a reduction in the median peak pressure gradient across the coarctation from 35 (20–49) to 9 (0–15) mmHg, p < 0.001. Median stent recoil was 7.9 (0–20)%. There was one case of access related complication that resolved without sequelae. Follow-up was a median of 15 (3.0–57.2) months. CT angiogram performed at a median time of 3.3 (2.6–10.2) months post procedure showed no aortic wall injury and preserved stent integrity in all cases. Two children underwent re-intervention for stent dilation and further stent implantation due to in-stent stenosis and somatic growth after 3 years. Six of fourteen children remained on a single antihypertensive agent post-intervention at last follow-up. Implantation of the dilatable Valeo stent is a feasible treatment strategy in native or recurrent coarctation in small children, accepting that additional stent implantation may be necessary with somatic growth. Further study is required to determine longer-term stent efficacy and clinical outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decade, intravascular stenting has become a well-accepted alternative to surgical repair for older children and adults with coarctation of aorta [1, 2]. Stent implantation is minimally invasive and has consistently proven to provide superior gradient reduction with less disruption of the aortic wall compared to balloon angioplasty alone [3,4,5,6]. These advantages are technically difficult to apply to small children, as most stents with a potential for further expansion to accommodate growth have a relatively large profile necessitating high calibre delivery sheaths, with increased risk of vascular access complications. Indeed, published evidence on coarctation stenting in small children is limited. Its application in this patient population is often based on evolving clinical experience, extrapolation of efficacy from adult studies and a rational strategy for future management.
When stent therapy is chosen for management of aortic coarctation in children, staged re-intervention is inevitable due to somatic growth. In selected high-risk and younger patients, several procedures for balloon dilation or further stent implantation are considered to be part of the trade-off in avoidance of surgery. The Valeo stent was first used in our centre for a 15 kg child who presented with acute cerebral haemorrhage and severe hypertension related to coarctation of the aorta [7]. Since then, this stent has been used consecutively in small children considered appropriate for stenting of native or recurrent coarctation. Here, we report our early experience of coarctation stenting in children weighing less than 30 kg using the Valeo stent and review the literature in this patient population.
Methods
All patients with a diagnosis of coarctation of the aorta who underwent implantation of the Bard Valeo stent at a single tertiary cardiology unit were identified from the departmental cardiology database (HeartSuite™). Patient demographic, echocardiographic and clinical data were collected from medical notes and HeartSuite™. Angiographic and haemodynamic data including the diameter of the coarctation and pressure gradients before and after stent implantation were collated from catheterisation reports and angiograms. The degree of stent recoil was calculated from the difference between the diameters of the fully inflated balloon during implantation and that of the expanded stent after balloon deflation. Stent integrity and aortic wall complications were evaluated with a contrast-enhanced computed tomography (CT) routinely performed at 3 months post procedure. An ultrasound study of the femoral and iliac arteries was also performed to assess for any evidence of peripheral vessel complications due to vascular access with the delivery sheath. Data are expressed as medians, ranges and percentages as appropriate. Comparisons before and after stent implantation were performed using the paired Student’s t test and a p value <0.05 was considered significant. All statistical analyses were performed using GraphPad InStat version 3.01 and GraphPad Prism version 6.05 (GraphPad Soft- ware, San Diego, CA).
Stent Description
The Valeo stent has a triple helix design laser cut from a 316-L stainless steel tube. The stent is pre-mounted on a low profile nylon balloon accepting a 0.035-inch guide wire and accommodating inflation pressures up to 14 atm. The stent is available in diameters between 6 and 10 mm and lengths between 18 and 56 mm. The required sheath size is 6 F for stents mounted on 6–8 mm balloons and 7 F for the larger stents mounted on 9–10 mm balloons. The open cell architecture of the stent allows it to be post dilated with minimal shortening [8, 9]. The 6–8 mm Valeo stents can be maximally dilated to 13 mm before fracturing. In our case series, only the 9- and 10- mm Valeo stents are used to take advantage of its maximal expandability to 20 mm [9].
Implantation Technique
All studies were performed under general anaesthesia. The femoral artery was accessed with ultrasound guidance and a femoral artery angiogram performed through a 5 F sheath to evaluate the adequacy of vessel diameter to accommodate the required delivery sheath. The coarctation was crossed with a J-tip Terumo glide wire (Terumo Medical Corporation, New Jersey, USA) and a suitable end-hole catheter. The catheter was withdrawn across the narrowing and the pressure gradient across the coarctation recorded. The coarctation was then re-crossed and angiography performed with a 5 F pigtail catheter using a right anterior oblique and lateral projection. The stent size was selected based on the diameter of the distal transverse arch and descending aorta above the diaphragm at maximum systolic expansion. The length of the chosen stent was based on the distance from the left subclavian artery to approximately 15 mm beyond the site of coarctation. The pre-mounted Valeo stent was prepared and advanced to the coarctation site via a 7 F destination sheath (Terumo Medical Corporation, New Jersey, USA) over a super stiff Amplatzer wire (St Jude Medical, Minnesota, USA) positioned in the right subclavian or axillary artery. Optimal stent positioning was assessed with hand injections of contrast through the side arm of the destination sheath. The stent was then dilated gradually to nominal pressure or until adequate de-waisting was seen on fluoroscopy. Once the stent was deployed, the balloon was deflated and removed carefully into the sheath. Repeat angiography and pullback gradient were performed with a multitrack catheter over the existing guide wire. The guide wire and catheter were then retrieved carefully to avoid disruption of the stent. Haemostasis was achieved by direct pressure to the entry point.
Results
Fourteen consecutive patients underwent implantation of the Valeo stent for the treatment of aortic coarctation between July 2012 and January 2017. The median age at the time of the procedure was 5.1 (2.6–7.5) years and median weight was 20.8 (14.7–27) kg. Eleven patients had isolated coarctation of the aorta; one patient had William’s syndrome with fracture and re-stenosis of previous coarctation stent (Genesis PG3110, Cordis Corp, Miami, FL, USA); two had other cardiac anomalies (Table 1).
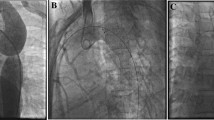
The median diameter of the common femoral artery on initial angiogram was 4.85 (2.8–6) mm and the 10 mm × 26 mm Valeo stent was delivered via a 7 F sheath in all cases. One patient required implantation of two (9 and 10 mm × 26 mm) stents to cover the coarcted segment and hypoplastic transverse arch. The stents were readily visible with a fluoroscopy rate of 15 frames per second and tracked easily to the target site. There was significant improvement in median coarctation diameter from 4 (1.3–5.2) to 9.5 (5.8–12.7) mm and a significant reduction in the median peak systolic pressure gradient from 35 (20–49) mmHg to 9 (0–15), p < 0.001. Median percent stent recoil in the middle of the stent was 7.9 (0–20)%. The median procedure time was 52 (36–73) min, median screening time was 10 (7–22) min and median radiation exposure was 238 (114–1163) cGy/cm2. Table 1 documents the angiographic and haemodynamic results for each stent implant. Figure 1 shows the representative angiograms of two patients in our series before and after stent implantation.
a Lateral retrograde aortogram of patient 14 demonstrating discrete juxtaductal coarctation of the aorta with significant collateralisation and b the expanded Valeo stent in position with vessel calibre matching. c Angiogram of patient 5 showing a long segment coarctation within a previous Genesis stent and d improved calibre of the distal arch post implantation of the Valeo stent
There were no intra-procedural complications. Acute loss of peripheral arterial pulse was encountered in one patient approximately 6 h post procedure, which recovered completely with low dose systemic tissue plasminogen activator regimen. Vascular ultrasound performed prior to discharge and at 3 weeks post procedure showed normal flow in the femoral and iliac arteries with no evidence of thrombosis or vessel wall injury. Another patient reported transient left sided weakness and slurred speech 11 days post procedure. CT head showed no acute cerebral infarction or haemorrhage and the patient had no neurological deficit at the last follow-up.
Follow-up duration was a median of 15 months (range 3 months to 4.8 years) (Table 1). All patients underwent a contrast-enhanced CT angiogram of the aorta at a median time of 3.3 (2.6–10.2) months post procedure, which showed no stent fracture or aortic wall complications. Adequate radiographic relief of stenosis on CT angiogram, defined as final percentage minimal stent diameter of greater than 75% of the descending aorta at diaphragmatic level was achieved in thirteen cases. In one patient, accurate CT measurement of stent was not possible due to motion-induced artefacts, however there was good vessel calibre matching and minimal stent narrowing.
Vascular ultrasound of the common femoral and iliac arteries was also performed in 11 of the 14 children at a median time of 4.5 (2.6–43) months post procedure to assess for access related vascular injury. All studies showed normal flow in the abdominal aorta to the bifurcation of both iliac arteries and the proximal femoral arteries with no evidence of obstruction, vessel wall damage or collateral formation. One child was unable to attend the ultrasound appointment but latest clinic follow-up confirmed good volume femoral pulses. Two patients had normal angiograms of the femoral artery during subsequent cardiac catheterization.
Two patients (case 1 and 2) underwent re-intervention due to a combination of mid stent re-stenosis and somatic growth. Patient 1 (previously published as a case report) had a gradual increase in velocity of the descending aorta up to 3.5 m/s, which led to repeat catheterisation at 13.1 months post procedure. This revealed a gradient of 15 mmHg across the slightly slender transverse arch but there was no angiographic evidence of stent stenosis or gradient across the stent (Fig. 2a). Subsequent evaluation 4.2 years later revealed a 5 mmHg gradient across the transverse arch and mild mid stent stenosis with a gradient of 10 mmHg. The stent was re-dilated with a 12 × 30 mm Cristal Balloon, resulting in complete resolution of gradient across the stent and aortic arch (Fig. 2). In case 2, repeat cardiac catheterisation after 3.3 years demonstrated mid stent re-stenosis with a gradient of 30 mmHg and growth of the rest of the aorta. There was a residual gradient of 14 mmHg post dilation with a 12 mm × 30 mm Cristal Balloon, hence further implantation of a 39-mm CP stent on a 14-mm Balloon-in-Balloon (BIB) catheter via an 11 F Mullins sheath was performed with complete abolishment of the gradient in the then 39 kg patient (Fig. 3). All patients were normotensive at the most recent clinic review. Eleven of fourteen patients were on a single antihypertensive agent pre-intervention; and post-intervention, six remained on treatment.
Serial angiograms of patient 1 (initial angiograms of coarctation and stenting previously published7). a Lateral aortogram 13 months post Valeo stent implantation showed no stent re-stenosis. b, c Lateral and frontal angiograms 4.3 years later showing mild mid stent stenosis and a slender transverse arch. d Angiographic result post dilation with a 12 × 30 mm Cristal balloon
Serial lateral retrograde aortogram of patient 2 during the initial coarctation stenting (upper panel) at 25 kg and at re-intervention (lower panel) 3.3 years later at 39 kg. The upper panel shows a severe discrete coarctation and b good result post implantation of Valeo stent c with post dilation with a 12 × 30 mm Cristal balloon. The lower panel shows angiograms 3.3 years later with d significant in-stent re-stenosis and growth of the aorta. e Angiographic result post dilation with 12 × 30 mm Cristal balloon and f further implantation of a 39-mm CP stent on 14-mm BIB balloon within the Valeo stent
Discussion
The first reported use of stent therapy for aortic coarctation was in 1991. Since then, multiple series of coarctation stent implantation have been reported in tandem with advances in stent technology [1,2,3,4, 10, 11]. The use of endovascular stents in small children however remains debated due to challenges associated with relatively large delivery sheaths and keeping pace with somatic growth. A literature search revealed relatively few studies that included children less than 30 kgs and/or between 1 and 10 years of age treated with stent implantation for native or recurrent coarctation of the aorta [12,13,14,15,16,17,18,19,20,21]; (Table 2). The majority of the studies included adolescents and adults and often, data of the younger children treated were not analysed separately. All studies reported excellent immediate results with low complications, however longitudinal data on clinical outcome and stent performance especially when implanted in early childhood are lacking. Thanapoulos et al. reported the longest follow-up of 6 years with 85% of their cohort achieving normotension without medication and a 4% incidence of late stent fracture. Importantly, 14 of 74 patients (aged <8 years) in their cohort underwent uncomplicated stent dilation due to growth related stenosis 5–6 years after the initial procedure, although it is unlikely that the stents were dilated to maximum diameter at that point [14]. A variety of stents have been used in this weight group, including the Palmaz stents (Cordis, Warren, NewJersey); Intrastent (EV3, Plymouth, Minnesota) and Cheatham-Platinum (CP) stents (Numed, Hopkinton, New York). The most commonly used Palmaz ‘8’ and’10’ series, Genesis XD and Intrastent Mega LD have an expandable maximal diameter of 18–20 mm but stents shorten up to 50% [17, 22, 23]. In addition, flaring of the sharp edges of the stent struts (especially those with closed cell configuration) at its maximum expanded diameter has been implicated in aortic wall injury. The CP stent was specifically developed to treat aortic coarctation, and is arranged in a zig pattern with the 6 and 8 zig/row configurations dilatable up to 18 and 28 mm, respectively. The latter is a better choice for coarctation but has a larger profile, limiting its use in small children [23]. The incidence of peripheral vessel injury related to vascular access appeared to be relatively high but the majority of studies had small patient number in this weight group and the delivery sheath size of the variety of stents used was not clearly defined. Thus, the quest continues for an ideal stent with a low enough profile to allow introduction in smaller children, yet dilatable to adult sizes without compromising performance.
The Bard Valeo stent represents a significant advance in pre-mounted stent design with favourable characteristics for use in various vascular lesions associated with congenital heart disease in small children. Several studies have reported excellent short term stent efficacy primarily in the setting of pulmonary artery stenosis or hypoplasia, and less commonly for ductal stenting in hypoplastic left heart syndrome, obstructed modified Blalock Taussig shunt, restrictive foramen ovale and systemic or pulmonary vein stenosis [8,9,10, 24, 25]. The use of Valeo stent in aortic coarctation has been previously reported in seven cases to the best of our knowledge [7, 9, 10]. The principle advantages of the Valeo stent when applied to aortic coarctation in smaller children are its low profile characteristic and hybrid cell architecture, which has improved flexibility and long-term serial dilation ability. Technically, we found the 7 F hydrophilic and kink-resistant destination sheath to be a good option for stent delivery with a relatively low risk for arterial injury. Other centres have reported re-mounting of the 9- and 10- mm stents onto smaller balloons to further reduce the insertion profile, yet taking advantage of the future dilation potential of the larger Valeo stent [9]. One of the patients in our series had probable transient ischaemic attack more that a week post procedure, which could be related to wire-induced right subclavian artery injury causing late embolization. Thrombus formation and embolization from the coarctation stent appeared unlikely to be the cause, as clot propagation would be distal to the head vessels. The patient was on aspirin therapy, which was maintained for 6 months, and was neurologically intact at last follow-up.
Despite early concerns, the stainless steel frame of the Valeo stent has been shown to have sufficient radial strength to overcome most vascular stenoses encountered in congenital heart lesions [8, 24, 26]. Bench testing has demonstrated superior radial strength of the Valeo stent compared to other closed or open cell stents used for treatment of aortic coarctation in small children such as the EV3 Intrastent double strut LD and Palmaz P308 stent [24, 27, 28]. We observed variable stent recoil ranging up to one-fifth of the anticipated diameter measured at the waist of the stent overlying the coarctation site. However, subsequent evaluation with CT angiogram performed at 3 months post procedure showed adequate radiographic relief of coarctation and preserved stent integrity in all cases.
The feasibility of in vivo stent re-dilation without causing significant vessel disruption has been confirmed by both animal studies and clinical data in older children and adults [29,30,31,32]. The larger Valeo stents (9 and 10 mm) can be dilated up to 20 mm diameter with minimal shortening [8, 9]. Uncertainty remains about the radial strength especially when serial dilation is performed. It appears intuitive that open cell stents will lose radial strength with increasing diameter. However, Bratincsak et al. recently reported increased radial strength and stiffness of commonly used balloon expandable stents (including open cell designs) when dilated to maximal expansion diameter compared to the nominal diameter [33]. We believe that maintenance of radial strength may not be as important in the later stages when the vessel has remodelled to the extent that it no longer requires high opposing force. Furthermore, larger adult size stents could be telescoped inside the Valeo stent for resistant lesions if required in the future when the use of a larger delivery system is no longer a limitation (Fig. 3).
In this series, there was a significant reduction in pressure gradient with improvement in the arch morphology after stent implantation. However, patients with coarctation repair are known to experience a disproportionate burden of residual hypertension despite good anatomical result by stent implantation or surgery [34]. The majority of children in our group remain on antihypertensive medication at a median follow-up of 16 months despite a significant reduction in cuff systolic blood pressure acutely post-intervention. The introduction of stent material in the aorta which is non-compliant and non-pulsatile has unknown consequences on long-term blood pressure control in a growing child especially during exercise or stress [35,36,37]. In this respect, development of bioabsorbable stents that adapt to vessel remodelling is a promising alternative and has been used in coronary revascularisation. Experience with use of these stents in congenital heart disease is presently limited [38, 39].
Based on our experience, we have generally offered coarctation stenting with the Valeo stent in children more than 12 kgs; however this is dependant on the size of the femoral artery and aortic arch anatomy. The CP stent, which requires at least a 10 F introducer sheath, is considered in children weighing more than 20 kgs. When the femoral artery is not of sufficient size to accommodate a 7 F sheath or if the child is under 12 kgs, balloon angioplasty of native coarctation is undertaken. It is our observation that the majority of patients where we have undertaken balloon angioplasty as the initial procedure have required stent implantation at a later date. Moreover, stent implantation has consistently been proven to provide superior gradient reduction with less aortic wall injury compared to balloon angioplasty in the medical literature [1,2,3,4,5,6]. Therefore, we have considered it reasonable to extrapolate our large experience with stent implantation in older children and adults to the younger age group provided the stent has the potential to be dilated to an adult size. The pros and cons of both catheter-based options, however, are fully discussed with the parents before the procedure. Surgical repair of coarctation remains the first line treatment for neonates and infants under 12 months in our institution.
Lifelong surveillance is imperative following intervention for coarctation of aorta. In these young children post coarctation stenting, we recommend an ultrasound of the femoral vessels at the initial 6-week follow-up and contrast CT of the aorta at three to 6 months to assess aortic wall and stent integrity. Repeat CT is considered at 5-year intervals, in the absence of intervening clinical or echocardiographic concerns at annual reviews [40]. Regular chest X-rays are no longer undertaken as the incidence of stent fracture is low with the current generation of stents and stent integrity can be difficult to assess in the open cell stents on plain X-ray [40].
This study is limited by its retrospective single-centre design and small patient sample. Longer-term follow-up will be needed to assess stent efficacy and confirm the capacity for re-dilation to adult dimensions.
Conclusions
On the basis of bench testing and our limited clinical experience, we believe that the Valeo stent is a valuable addition to the armamentarium of the paediatric interventionalist for coarctation stent therapy in children between 15 and 30 kgs. Early results from our case series show that implantation of the dilatable Valeo stent is a feasible strategy in native or recurrent coarctation in small children, accepting that additional stent implantation may be necessary with somatic growth. Further study with follow-up into adulthood is needed to determine stent efficacy especially post dilation and clinical outcome in the longer term.
References
Forbes TJ, Kim DW, Du W et al (2011) Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (congenital cardiovascular interventional study consortium). J Am Coll Cardiol 58:2664–2674
Carr JA (2006) The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol 47:1101–1107
Chessa M, Carrozza M, Butera G et al (2005) Results and mid-long-term follow-up of stent implantation for native and recurrent coarctation of the aorta. Eur Heart J 26:2728–2732
Hamdan MA, Maheshwari S, Fahey JT et al (2001) Endovascular stents for coarctation of the aorta: initial results and intermediate-term follow-up. J Am Coll Cardiol 38:1518–1523
Johnston J, Kelley RI, Feigenbaum A et al (1997) Mutation characterization and genotype-phenotype correlation in Barth syndrome. Am J Hum Genet 61:1053–1058
O’Laughlin MP, Perry SB, Lock JE et al (1991) Use of endovascular stents in congenital heart disease. Circulation 83:1923–1939
Shepherd E, Connolly GM, Morgan G (2013) Using the Valeo dilatable stent in coarctation stenting for small children: expanding the inclusion criteria for coarctation stenting? BMJ Case Rep. doi:10.1136/bcr-2013-202095
Stern HJ, Baird CW (2009) A premounted stent that can be implanted in infants and re-dilated to 20 mm: introducing the Edwards Valeo lifestent. Catheter Cardiovasc Interv 74:905–912
Travelli FC, Sullivan PM, Takao C et al (2016) The Valeo stent: a pre-mounted, open-cell, large stent for use in small children with CHD. Cardiol Young 26:1187–1193
Butera G, Giugno L, Basile D et al (2015) The Edwards Valeo lifestents in the treatment and palliation of congenital heart disease in infants and small children. Catheter Cardiovasc Interv 86:432–437
Golden AB, Hellenbrand WE (2007) Coarctation of the aorta: stenting in children and adults. Catheter Cardiovasc Interv 69:289–299
Bondanza S, Calevo MG, Marasini M (2016) Early and long-term results of stent implantation for aortic coarctation in pediatric patients compared to adolescents: a single center experience. Cardiol Res Pract 2016:4818307. doi:10.1155/2016/4818307
Baykan A, Narin N, Ozyurt A et al (2014) Cheatham platinum stent implantation in children with coarctation of the aorta: single-centre short-term, intermediate-term, and long-term results from Turkey. Cardiol Young 24:675–684
Thanopoulos BD, Giannakoulas G, Giannopoulos A et al (2012) Initial and six-year results of stent implantation for aortic coarctation in children. Am J Cardiol 109:1499–1503
Cheatham JP (2001) Stenting of coarctation of the aorta. Catheter Cardiovasc Interv 54:112–125
Thanopoulos BD, Hadjinikolaou L, Konstadopoulou GN et al (2000) Stent treatment for coarctation of the aorta: intermediate term follow up and technical considerations. Heart 84:65–70
Mohan UR, Danon S, Levi D et al (2009) Stent implantation for coarctation of the aorta in children <30 kg. JACC Cardiovasc Interv 2:877–883
Schaeffler R, Kolax T, Hesse C et al (2007) Implantation of stents for treatment of recurrent and native coarctation in children weighing less than 20 kilograms. Cardiol Young 17:617–622
Forbes TJ, Garekar S, Amin Z et al (2007) Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc Interv 70(2):276–285
Magee AG, Brzezinska-Rajszys G, Qureshi SA et al (1999) Stent implantation for aortic coarctation and recoarctation. Heart 82:600–606
Suarez de Lezo J, Pan M, Romero M et al (1995) Balloon-expandable stent repair of severe coarctation of aorta. Am Heart J 129:1002–1008
Peters B, Ewert P, Berger F (2009) The role of stents in the treatment of congenital heart disease: current status and future perspectives. Ann Pediatr Cardiol 2:3–23
Ebeid M (2003) Balloon expandable stents for coarctation of the aorta: review of current status and technical considerations. Images Paediatr Cardiol 5:25–41
Kudumula V, Noonan P, Taliotis D et al (2014) Implantation and preliminary follow-up of the Bard Valeo stent in pulmonary artery stenosis. Catheter Cardiovasc Interv 84:197–203
Ovaert C, Luciano D, Gaudart J et al (2015) The VALEO(R) vascular stent for cardiovascular lesions in children. EuroIntervention 10:1326–1331
Kuecherer HF, Just A, Kirchheim H (2000) Evaluation of aortic compliance in humans. Am J Physiol Heart Circ Physiol 278:H1411–H1413
Tominaga R, Kambic HE, Emoto H et al (1992) Effects of design geometry of intravascular endoprostheses on stenosis rate in normal rabbits. Am Heart J 123:21–28
Ing F (2002) Stents: what’s available to the pediatric interventional cardiologist? Catheter Cardiovasc Interv 57:374–386
Morrow WR, Palmaz JC, Tio FO et al (1993) Re-expansion of balloon-expandable stents after growth. J Am Coll Cardiol 22:2007–2013
Duke C, Rosenthal E, Qureshi SA (2003) The efficacy and safety of stent redilatation in congenital heart disease. Heart 89:905–912
Ing FF, Grifka RG, Nihill MR et al (1995) Repeat dilation of intravascular stents in congenital heart defects. Circulation 92:893–897
Toro-Salazar OH, Steinberger J, Thomas W et al (2002) Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol 89:541–547
Bratincsak A, Moore JW, Gulker B et al (2015) Breaking the limit: mechanical characterization of overexpanded balloon expandable stents used in congenital heart disease. Congenit Heart Dis 10:51–63
Canniffe C, Ou P, Walsh K et al (2013) Hypertension after repair of aortic coarctation–a systematic review. Int J Cardiol 167:2456–2461
Eicken A, Pensl U, Sebening W et al (2006) The fate of systemic blood pressure in patients after effectively stented coarctation. Eur Heart J 27:1100–1105
Chen SS, Donald AE, Storry C et al (2008) Impact of aortic stenting on peripheral vascular function and daytime systolic blood pressure in adult coarctation. Heart 94:919–924
Bentham JR, English K, Ballard G et al (2013) Effect of interventional stent treatment of native and recurrent coarctation of aorta on blood pressure. Am J Cardiol 111:731–736
Veeram Reddy SR, Welch TR, Wang J et al (2015) A novel design biodegradable stent for use in congenital heart disease: mid-term results in rabbit descending aorta. Catheter Cardiovasc Interv 85:629–639
Alexy RD, Levi DS (2013) Materials and manufacturing technologies available for production of a pediatric bioabsorbable stent. Biomed Res Int 2013:1379–1385. doi:10.1155/2013/137985
Chakrabarti S, Kenny D, Morgan G et al (2010) Balloon expandable stent implantation for native and recurrent coarctation of the aorta- prospective computed tomography assessment of stent integrity, aneurysm formation and stenosis relief. Heart 96:1212–1216
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Kang, SL., Tometzki, A., Taliotis, D. et al. Stent Therapy for Aortic Coarctation in Children <30 kg: Use of the Low Profile Valeo Stent. Pediatr Cardiol 38, 1441–1449 (2017). https://doi.org/10.1007/s00246-017-1682-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1682-x