Abstract
The goal of this study was to create nomograms of echocardiographic two-dimensional valve dimensions based on a large group of children without heart disease. Children aged 0–18 years underwent standard echocardiographic evaluation. Referring diagnoses were chest pain, heart murmur, or syncope. Only patients with a structurally normal heart and normal systolic and diastolic function were included. All four valves were measured at their maximal dimensions. A total of 748 children (314 girls and 434 boys) met the inclusion criteria. Mean values and standard deviations were calculated, and z value nomograms based on body surface area were developed. Surprisingly, the boys had larger valve dimensions at all ages. These valve dimension differences were statistically significant for three of four valves even after adjustment for the differences in body sizes. The difference may be due to higher circulating blood volume in boys compared to that in girls. Because the differences are subtle, they reach statistical significance only when evaluated in a large group of subjects. Presented normal value data will be helpful in following cardiology patients and evaluating intervention strategy in patients with valve hypoplasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Knowledge of cardiac valve dimensions is important in managing patients with congenital and acquired heart diseases. Currently, published pediatric two-dimensional (2-D) normative data are based on a relatively small (48 –196) number of subjects [3, 10, 16]. The aim of this study was to create z value nomograms of echocardiographic valve dimensions based on a larger group of children without heart disease.
Materials and Methods
The subjects were children aged 0–18 years referred to the Cincinnati Children's Hospital echocardiography laboratory. Referring diagnoses were chest pain, heart murmur, or syncope evaluation. Only patients with structurally normal heart and normal systolic and diastolic function were included. Exclusion criteria were arterial hypertension, chronic renal failure, the presence of oncologic disease, or any other conditions that could affect cardiac function or geometry. The weight, height, and race of each patient were recorded at the time of the echocardiography evaluation.
The studies were performed using a Philips Sonos 5500 (Andover, MA, USA) or GE Vivid 5 (Milwaukee, WI, USA) echocardiography system. Measurements were performed off-line using Cardiology Analysis System software (Digisonics, Houston, TX, USA). The valves were measured at their maximal dimensions using the distance between “hinge points” at the level of the annulus. Aortic valves were measured in the parasternal long-axis view in systole, pulmonary valves in the parasternal short-axis view in systole, and mitral and tricuspid valves in the apical four-chamber view in diastole.
Results
A total of 748 children met inclusion criteria. There were 314 girls and 434 boys. Their ages ranged from 0 to 18 years. Racial distribution was as follows: Caucasian, 629; African American, 80; Asian, 9; Hispanic, 2; biracial, 11; unknown, 17.
Data were plotted vs body surface area (BSA) and height. Mean values and standard deviations were calculated. Analysis of covariance (ANCOVA) modeling was performed, initially to determine whether to use height, BSA (calculated by Dubois formula), or a combination as the indexing variable, and then to compare the regression lines (slopes and intercepts) for the boys and girls for each valve.
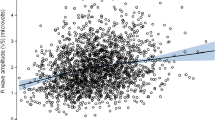
Visual inspection of smoothed regression curves indicated that the relationships between each valve and either BSA or height were very similar for boys and girls, with the curves being slightly higher for boys in each case. It was determined that a log transformation was appropriate to make each of the valve distributions suitably near normal. Furthermore, log transformation of BSA and height yielded the strongest correlations with all of the valves. Correlations were stronger with BSA than with height for all four valves. Correlations with BSA were 0.81, 0.91, 0.89, and 0.84 for tricuspid, aortic, pulmonary, and mitral valves, respectively, for boys and 0.76, 0.91, 0.89, and 0.80, respectively, for girls. Corresponding correlations with height were 0.72, 0.83, 0.81, and 0.75 for boys and 0.70, 0.86, 0.87, and 0.73 for girls. The relationships between the valve measurements and BSA were significantly different between boys and girls for three of the four valves (p < 0.05) (Table 1). We found that although the slopes were the same for girls and boys, the intercepts were different. Thus, the boys had higher means after adjusting for differences in BSA. This gender difference was also observed when valve measurements were adjusted for both BSA and height simultaneously in the ANCOVA models. Furthermore, there was no independent effect due to height for any valve when it was included in the model with BSA. Therefore, the nomograms presented in Figs. 1, 2, 3, 4 were developed separately for boys and girls using results of regression analyses taking the form y = α + β(ln(BSA)), where y is the natural logarithm of the mean normal value for the valve (cm), ln is natural logarithm, BSA is body surface area (m2), α is the intercept, and β is the regression coefficient. These nomograms are based on the following relationship: z = [ ln(valve) - ln(mean normal value)]/root mean square error.
Discussion
This is the largest study of pediatric normal valves that provides standardized 2-D measurements in pediatric patients. It reflects valve sizes in children from the greater Cincinnati metropolitan area.
As valve dimensions undergo changes through infancy and childhood, their measurements need to be correlated to a given size of the body. Previously published studies used BSA [1, 3, 8, 9, 13, 14], height [5, 10, 16], or, for neonate assessment, weight [7, 12, 18, 19]. Sheil et al. [16] showed that in correlating with aortic root diameter, BSA was not superior to height. In order to find a normalizing factor that would address this issue more closely, we evaluated valve dimensions indexing to BSA and height, separately and combined. In our group of patients, BSA was clearly a better independent predictor, and height gave no added explanatory value in the models.
There is no universally accepted method of measuring cardiac valve dimensions. Roman et al. [14] applied the recommendations of the American Society of Echocardiography regarding M-mode measurements [15] to 2-D measurements. Sheil et al. [16] showed that systolic aortic valve dimensions were consistently greater than the diastolic ones, and they used systolic dimensions for analysis. As have many other investigators [3, 6, 9], we measured valves at their maximal dimensions (i.e., aortic and pulmonary valves in the early systole and mitral and tricuspid valves in late diastole).
An interesting finding of this study is the small but statistically significant gender differences in valve dimensions after adjustment for differences in body sizes. Valve dimension gender difference has been shown in adult studies. Assessing 135 adults, Roman et al. [14] showed gender differences in the absolute values of aortic root dimensions, including the aortic annulus, although the values were similar when indexed to BSA. The authors concluded that sex-specific differences in aortic root dimensions were due to the differences in body sizes. Previously performed pediatric studies based on much smaller groups of subjects show no gender difference [3, 6, 9, 14, 16, 17]. However, prepubertal gender difference in left ventricular mass has been shown by de Simone et al. [4]. These authors studied 424 children from Cincinnati Children's Hospital. Although the gender difference in left ventricular mass, before puberty was not statistically significant, in each age group boys had 5%–8% higher left ventricular mass than girls. The authors observed that the gender difference in left ventricular mass was parallel to differences in body height. A small sex difference in left ventricular mass was found in children 7–11 years old in the Bogalusa Heart Study [2]. The authors concluded that sex differences play a considerable role in the determination of heart size. Additional evidence of prepubertal gender differences in heart measurements was presented by O'Leary et al. [11]. When evaluating diastolic function in 223 children, the authors found that boys had a slightly larger mitral E wave and pulmonary vein diastolic time velocity integrals as well as lower ratio of pulmonary vein systolic-to-diastolic time velocity integrals than were observed in girls. The differences reached statistical significance. The data showing that prepubertal boys have higher left ventricular mass [2, 4] and higher transmitral flow [11] are in agreement with our findings of larger valve dimensions in boys. We speculate that these collective data may reflect higher circulating blood volume in prepubertal boys compared to that in prepubertal girls. Because the differences are subtle, they reach statistical significance only when evaluated in a large group of subjects.
Study Limitations
Our study has some limitations. First, there may be a possible decrease in the precision of the measurements of mitral, tricuspid, and pulmonary valve diameters, because the measurements were performed in the direction of lateral, rather than axial, resolution of the equipment. We chose these views because they are used routinely by the majority of echocardiography laboratories. Second, for the smallest values for each valve, a small difference in BSA has a great effect on the value of z scores, making these nomograms of possible lesser use in the smallest patients.
References
T Akiba M Yoshikawa S Otaki et al. (1986) ArticleTitleEchocardiographic measurements of left ventricle in normal infants and children Tohoki J Exp Med 149 31–37 Occurrence Handle1:STN:280:DyaL283ptVertA%3D%3D
GL Burke RA Arcilla WS Culpepper et al. (1987) ArticleTitleBlood pressure and echocardiographic measures in children: the Bogalusa Heart Study Circulation 75 106–114 Occurrence Handle2947739 Occurrence Handle1:STN:280:DyaL2s%2FnvVOntg%3D%3D
PEF Daubeney EH Blackstone RG et al. Weintraub (1999) ArticleTitleRelationship of the dimension of cardiac structures to body size: an echocardiographic study in normal infants and children Cardiol Young 9 402–410 Occurrence Handle10476831 Occurrence Handle1:STN:280:DyaK1MvgsVCrsw%3D%3D Occurrence Handle10.1017/S1047951100005217
G Simone Particlede RB Devereux SR Daniels RA Meyer (1995) ArticleTitleGender differences in left ventricular growth Hypertension 26 979–983 Occurrence Handle7490158
A Evangelista HG Casillo Particledel T Gonsales-Alujas et al. (1996) ArticleTitleNormal values of valvular annular areas. Comparison of the results of a necroscopy and an echocardiographic series Rev Espaniola de Cardiol 49 111–116 Occurrence Handle1:STN:280:DyaK2s7gsF2ksA%3D%3D
HP Gutgesell JT Bricker EV Colvin LA Latson EP Hawkins (1984) ArticleTitleAtrioventricular valve annular diameter: two-dimensional echocardiographic–autopsy correlation Am J Cardiol 53 1652–1655 Occurrence Handle6731311 Occurrence Handle10.1016/0002-9149(84)90596-4 Occurrence Handle1:STN:280:DyaL2c3ivVantw%3D%3D
DA Halon N Amitai MS Gotsman BS Lewis (1979) ArticleTitleSerial echocardiography during the first 3 month of life in normal neonates Eur J Cardiol 9 393–404 Occurrence Handle456397 Occurrence Handle1:STN:280:DyaE1M3htlCrtw%3D%3D
WL Henry J Ware JM Gardin et al. (1978) ArticleTitleEchocardiographic measurements in normal subjects Circulation 57 278–285 Occurrence Handle618615 Occurrence Handle1:STN:280:DyaE1c%2Fmt12mtw%3D%3D
DH King EO Smith JC Huhta HP Gutgesell (1985) ArticleTitleMitral and tricuspid valve annular diameter in normal children determined by two-dimensional echocardiography Am J Cardiol 55 787–789 Occurrence Handle3976526 Occurrence Handle1:STN:280:DyaL2M7ksFGltg%3D%3D
SM Nidorf MH Picard MO Triulzi et al. (1992) ArticleTitleNew perspectives in the assessment of cardiac chamber dimensions during development and adulthood J Am Coll Cardiol 19 983–988 Occurrence Handle1552123 Occurrence Handle1:STN:280:DyaK383gt1Gqsg%3D%3D Occurrence Handle10.1016/0735-1097(92)90282-R
PW O'Leary K Durongpisitkul T Cordes et al. (1998) ArticleTitleDiastolic ventricular function in children: a Doppler echocardiographic study establishing normal values and predictors of increased ventricular end-diastolic pressure Mayo Clinic Proc 73 616–628 Occurrence Handle10.4065/73.7.616
HJA Rimoldi M Lev (1963) ArticleTitleA note on the concept of normality and abnormality in quantification of pathologic findings in congenital heart disease Pediatr Clin North Am 10 589–591
CLL Roge NH Silverman PA Hart RM Ray (1978) ArticleTitleCardiac structure growth pattern determined by echocardiography Circulation 57 285–290 Occurrence Handle618616 Occurrence Handle1:STN:280:DyaE1c%2Fmt12mtA%3D%3D
MJ Roman RB Devereux R Kramer-Fox J O'Loughlin (1989) ArticleTitleTwo-dimensional echocardiographic aortic root dimensions in normal children and adults Am J Cardiol 64 507–512 Occurrence Handle2773795 Occurrence Handle1:STN:280:DyaL1MzntFGjtg%3D%3D
DJ Sahn A DeMaria J Kisslo A Weyman (1978) ArticleTitleRecommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements Circulation 58 1072–1083 Occurrence Handle709763 Occurrence Handle1:STN:280:DyaE1M%2Fktl2msw%3D%3D
ML Sheil O Jenkins G Sholler (1995) ArticleTitleEchocardiographic assessment of aortic root dimensions in normal children based on measurements of a new ratio of aortic size independent of growth Am J Cardiol 75 711–715 Occurrence Handle7900666 Occurrence Handle10.1016/S0002-9149(99)80659-6 Occurrence Handle1:STN:280:DyaK2M3gvVKjtQ%3D%3D
AR Snider MA Enderlein DF Teitel RP Juster (1984) ArticleTitleTwo-dimensional echocardiographic determination of aortic and pulmonary artery sizes from infancy to adulthood in normal subjects Am J Cardiol 53 218–224 Occurrence Handle6691264 Occurrence Handle10.1016/0002-9149(84)90715-X Occurrence Handle1:STN:280:DyaL2c7gtFaqtw%3D%3D
R Solinger F Elbl K Minhas (1973) ArticleTitleEchocardiography in the normal neonate Circulation 47 108–118 Occurrence Handle4686588 Occurrence Handle1:STN:280:DyaE3s%2FpsFKmsw%3D%3D
TA Tacy RP Vermilion A Ludomirsky (1995) ArticleTitleRange of normal valve annulus size in neonates Am J Cardiol 75 541–543 Occurrence Handle7864012 Occurrence Handle10.1016/S0002-9149(99)80605-5 Occurrence Handle1:STN:280:DyaK2M7mvVajtQ%3D%3D
Acknowledgment
We thankWendi Long for her help in conducting the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00246-007-9164-1.
Rights and permissions
About this article
Cite this article
Zilberman, M.V., Khoury, P.R. & Kimball, R.T. Two-Dimensional Echocardiographic Valve Measurements in Healthy Children: Gender-Specific Differences . Pediatr Cardiol 26, 356–360 (2005). https://doi.org/10.1007/s00246-004-0736-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-004-0736-z