Abstract
Ventricular dysfunction in patients after Fontan-like operations (FLOs) is a serious complication that might contribute to poor long-term results. Ischemic heart disease will have debilitating consequences on a Fontan heart. Ten patients (15.8 ± 5.01 years) after FLO had transesophageal echocardiography and cardiac catheterization 9.3 ± 4.2 years after surgery. Myocardial perfusion was assessed by NH3-positron emission tomography (rest/adenosine) and compared with that of 10 healthy adults (26.1 ± 6.3 years). Ventricular function was normal in 4 and reduced in 6 patients; end systolic and end diastolic meridional wall stress was significantly elevated in the FLO group. Coronary angiography revealed no stenosis of the coronaries. Compared to normals, myocardial blood flow (MBF) at rest was higher in the FLO group (0.99 ± 0.25 vs 0.77 ± 0.17 ml/g/min, p < 0.05), whereas MBF after vasodilatation (2.12 ± 0.78 vs 3.10 ± 0.85 ml/g/min, p < 0.05) and coronary flow reserve (CFR) was reduced (2.5 ± 0.88 vs 4.1 ± 1.01, p < 0.05), especially in those with impaired ventricular function. Coronary vascular resistance after vasodilatation was elevated in the FLO group (38.2 ± 17.4 vs 24.5 ± 8.3 mmHg/ml/g/min, p < 0.05). Altered MBF, increased meridional wall stress, and impaired CFR are common findings in FLO. Attenuated CFR and reduced ventricular function are significantly correlated and may be risk factors for the long-term outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since the first report of a total atriopulmonary connection by Fontan and Baudet [10], advances in operative techniques and postoperative management have been accompanied by improvements in early survival [2], despite application of the Fontan approach to patients with complex forms of single ventricle and patients with less favorable hemodynamics [19].
There is concern about the long-term outcome of circulation driven by a single ventricular chamber [11]. The long-term sequelae—namely thromboembolic complications, suboptimal flow dynamics, ventricular dysfunction, arrhythmias [9, 35], protein-losing enteropathy [14], and reduced exercise capacity [7]—are without doubt a complex and multifactorial problem, resulting from subtle and continuous changes in the various areas of circulation. The systemic ventricle, the only power source of the Fontan circulation, is bound to play a vital role in the long-term outcome of these patients. Decreased ventricular function, both systolic and diastolic, is known to occur in many “Fontan” patients. This feature has been associated with increased ventricular mass, the morphological type of the ventricle, and impaired coronary artery flow [17]. One can expect that ischemic heart disease will have much more debilitating consequences on a Fontan heart than on biventricular circulation, and this might be a major cause of problems in the future.
We therefore studied, as a part of the routine follow-up, the coronary pattern and myocardial perfusion in patients after Fontan-like operations (FLOs) and compared the results with hemodynamic parameters obtained by cardiac catheterization and echocardiography.
Methods
Patients
Six female and 4 male patients (15.8 ± 5.01 years) who had a FLO at a mean age of 5.9 ± 3.7 years were investigated 9.3 ± 4.2 years after surgery. The systemic ventricle had the morphology of a right one in 6 patients and that of a left one in 4 of the patients. Eight patients had different modifications of the Fontan procedure (atriopulmonary connection in 6 patients, and incorporation of the residual right ventricular chamber and pulmonary valve in 2 patients); 2 patients had a total cavopulmonary anastomosis (TCPC) with Kaye–Damus–Stansel operation due to a restrictive ventricular septal defect. The patients were in good clinical condition without signs of protein-losing enteropathy; 8 patients were in sinus rhythm, and 2 had atrioventricular dissociation in form of a junctional escape rhythm.
Ten healthy young adults (25.6 ± 5.4 years) without cardiovascular disease on the basis of the absence of symptoms or risk factors, normal resting electrocardiogram, and normal exercise test were used as a control group for positron emission tomography (PET) [22].
Study Protocol
All patients had a routine checkup including clinical examination, electrocardiogram (ECG), transthoracic and transesophageal ultrasound (TEE), cardiac catheterization, and PET imaging with N-13 ammonia at rest and after vasodilatation with adenosine.
TEE and cardiac catheterization with selective coronary angiography were performed under general anesthesia using the femoral artery approach. We used an electronic 7.5-MHz probe for transesophageal imaging (Vingmed System 5) to assess ventricular function, atrioventricular valve insufficiency, aortic valve insufficiency, and the presence of obstruction of the atriopulmonary or cavopulmonary anastomosis.
Ventricular dimensions and myocardial thickness were calculated by transesophageal imaging of the systemic ventricle (SV) in a 60° to 90° craniocaudal long-axis projection. The anterior and posterior ventricular endocardial borders and the epicardial border of the ventricular posterior wall were manually digitized. The ventricular dimensions were measured at end systole and end diastole; ventricular dimensions at end diastole were measured at the onset of the QRS complex; and the dimensions at end systole were measured at the smallest vertical dimension between the anterior and posterior wall endocardium. Systolic and end diastolic pressures were obtained simultaneously by cardiac catheterization.
From SV short-axis diameter (D), SV posterior wall thickness (H) and systolic and end diastolic pressures (P), end systolic and end diastolic meridional wall stress (ESSm and ESDm) were calculated as follows according to Grossman et al. [12].

Normal values for wall stress were obtained in 10 patients (23.3 ± 6.2 years) with persistent foramen ovale (PFO) and structural normal hearts, which had interventional PFO closure because of paradoxical embolism.
For calculation of the hemodynamic parameters, oxygen consumption was measured during cardiac catheterization (Deltatrac II Metabolic monitor, Hoyer Bremen). Selective coronary angiography was performed by manual injection of contrast medium using 4- or 5-Fr coronary catheters. The angiograms were interpreted by two experienced pediatric cardiologists blinded to patient data and myocardial flow parameters. Ventricular function, shape and course of the coronary arteries, and the site of the drainage of the coronary sinus were analyzed visually.
Myocardial blood flow (MBF) was quantified noninvasively at rest and during adenosine-induced vasodilatation by dynamic PET with N-13 ammonia. The regions of interest for MBF quantification were defined in the systemic ventricular free wall. After positioning the patient, a transmission scan was acquired for correction of photon attenuation. Subsequently, N-13 ammonia (approximately 0.3 mCi/kg) was injected intravenously at rest, and a dynamic sequence of 21 frames was acquired over 20 minutes. After 50 minutes to allow for decay of N-13 ammonia, adenosine (0.14 mg/g/min) was continuously infused for 5 minutes. Two minutes after the onset of adenosine infusion, a second dose of N-13 ammonia was administered, and a dynamic imaging sequence similar to the rest study was started. Heart rate, blood pressure, and a 12-lead ECG were monitored continuously during the procedure. MBF at rest and during hyperemia were quantified using a volumetric sampling approach and a validated three compartment model [16].
Because of the relation of MBF at rest with the rate pressure product (RPP) as an index of cardiac work [4], resting flow was normalized to the corresponding RPP. In addition to quantification of global MBF, regional myocardial perfusion was analyzed visually. Summed images of tracer distribution in the last three frames of the dynamic sequence were examined for the presence of reversible or persistent defects in six myocardial segments (apex, distal and proximal anterior, lateral, and inferior and septal wall).
To obtain an index of coronary vascular resistance (CVR), the difference between mean aortic blood pressure and coronary sinus pressure (measured invasively in FLO and estimated in healthy controls) as a measure of coronary perfusion pressure was divided by blood flow values at rest and during adenosine infusion.
Statistics
Significance was defined as p < 0.05. Comparisons between values of coronary flow dynamics of patients and the control group were made using the Mann–Whitney test. Correlation between coronary flow dynamics and hemodynamic data obtained by cardiac catheterization and echocardiography was performed using Spearman’s correlation coefficient.
Results
Echocardiography
Ventricular function was normal in four [ejection faction (EF) > 0.50, fractional shortening (FS) > 0.30]) and reduced in six patients (EF > 0.45; FS > 0.25). Atrioventricular valve insufficiency was apparent in all patients; in nine it was graded as trivial, and in one it was graded as moderate. The systemic ventricle had the morphology of a right ventricle in six and the morphology of a left ventricle in four patients.
Compared to normals, meridional end systolic and end diastolic wall stress was significantly elevated in the FLO group (ESSm: 81.2 ± 18.1 vs 54.2 ± 2.4 g/cm2, p < 0.05; ESDm: 15.4 ± 5.8 vs 6.6 ± 2.1 g/cm2, p < 0.05) (Table 1). Within the FLO group, no significant correlation between wall stress and myocardial flow parameters was found.
Positron Emission Tomography
PET imaging was performed at a mean of 6.6 ± 5.9 days after cardiac catheterization. During hyperemia, both groups demonstrated a significant increase in heart rate and RPP whereas no statistically significant change was observed in systolic and diastolic blood pressure (Table 2).
MBF at rest, normalized to the corresponding RPP, was significantly higher in the FLO group of patients compared to the MBF of healthy young adults (0.99 ± 0.25 vs 0.77 ± 0.17 ml/g/min, p < 0.05). Adenosine-induced vasodilatation resulted in significantly increased MBF in both groups, but MBF was significantly attenuated in the patient group (2.12 ± 0.78 vs 3.10 ± 0.85 ml/g/min, p < 0.05).
As a result of increased MBF at rest and reduced MBF after vasodilatation, coronary flow reserve (CFR) was markedly attenuated in the FLO group of patients compared to the healthy adolescents (2.50 ± 0.88 vs 4.09 ± 1.01, p < 0.05).
CVR at rest tended to be lower in the FLO group without statistical significance (98.3 ± 41.3 vs 112.4 ± 24.2 mmHg/ml/g/min), whereas CVR after vasodilatation by adenosine was significantly elevated in patients after FLO (38.2 ± 17.4 vs 24.5 ± 8.3 mmHg/ml/g/min, p < 0.05).
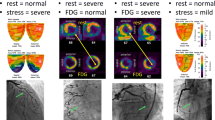
Visual analysis of the PET images revealed no persistent or adenosine-induced perfusion defects of the systemic ventricle.
Cardiac Catheterization
No patient had an obstruction within the atriopulmonary or cavopulmonary anastomosis. Seven patients had a dominant right coronary artery, two patients had left coronary dominance, and one patient had an intermediate type. Two patients had coronary venous drainage by an unroofed coronary sinus into the pulmonary venous atrium, and in six patients the coronary sinus drained into the systemic venous atrium. Two patients had a mixed coronary venous drainage into the pulmonary and systemic venous atrium. Six patients had a tortuous course of the distal coronary arteries (five in the right coronary artery and one in the left coronary artery) and visible intratrabecular communications to the systemic ventricle. The cardiac index was significantly reduced in all patients (2.34 L/min/m2 ± 0.57); end diastolic (6.3 ± 2.24 mmHg) and mean pulmonary artery pressure (8.6 ± 2.01 mmHg) were within the normal range in all patients, as was pulmonary artery resistance (2.02 ± l.2 Units x m 2). Systemic vascular resistance was slightly elevated (26.8 ± 8.2 Em2). The mean systemic venous pressure (10.3 ± 1.85 mmHg) was significantly higher than the mean pressure within the pulmonary venous atrium (5.6 ± 2.8 mmHg), resulting in a mean transthoracic gradient of 3.5 ± 1.1 mmHg (Table 3).
Comparison of MBF dynamics obtained by PET and catheter findings as cardiac index, end diastolic, pulmonary artery, systemic, and pulmonary venous pressure did not show any trend or statistical correlation. The site of venous drainage of the coronary sinus, age at operation, and preoperative time interval of cyanosis had no influence on MBF and CFR.
Patients with a tortuous course of the coronary arteries tended to have a higher CVR after vasodilatation than those without this anomaly; however, there was no statistical difference (49.4 ± 18.5 vs 31.3 ± 10.4 mmHg/ml/g/min). All patients with impaired ventricular function belonged to the subgroup of patients with a tortuous course of the coronary arteries; five patients had right and one had a left ventricular morphology of the systemic ventricle.
Hyperemic myocardial blood flow (MBFstress) and CFR were more attenuated in patients with reduced systolic ventricular function (EF < 0.45) than in those with normal function (EF > 0.50) (MBFstress: 1.7 ± 0.5 vs 2.9 ± 0.3 ml/g/min, p < 0.05; CFR: 2.2 ± 0.25 vs 2.7 ± 0.3, p < 0.05). Additionally, CFR was reduced more in patients with right ventricular morphology of the systemic ventricle than in those with left morphology (2.2 ± 0.87 vs 3.1 ± 0.55 ml/g/min, p < 0.05).
Despite having a restrictive ventricular septal defect and a Kaye–Damus–Stansel anastomosis, CFR was less attenuated in the two patients with TCPC and left ventricular morphology compared to those after Fontan modification (3.5 ± 0.03 vs 2.2 ± 0.76).
Discussion
Quantitative assessment of coronary microcirculation was compared with hemodynamic parameters in patients after FLO. MBF is regulated by hydrostatic forces, anatomic factors, metabolic control, and autoregulation [24]. It correlates well with myocardial oxygen consumption [1], which is, in turn, mostly determined by myocardial tension development, external work, heart rate, and contractility [17]. A decrease in MBF in the normal metabolic state results in reduced ventricular performance [23, 30, 34].
Pulmonary vascular impedance and ventricular systolic performance have been widely accepted as important physiologic variables in determining outcome after Fontan operation, providing the impetus to perform the operation at an increasingly younger age. However, recently attention has been focused on ventricular diastolic performance. The observation of Norwood [3], that in some patients the ventricular wall thickness/diameter ratio markedly increases after Fontan operation in temporal correlation with tachycardia and low cardiac output state, suggested the following hypothesis: Acute removal of a ventricular volume overload in the presence of a relatively unchanging ventricular muscle mass results in geometric alterations that can impair diastolic performance after FLO [3, 25]. Wall stress is an important determinant of myocardial oxygen consumption, myocardial contractile state, and diastolic function [29]. The increase in systolic wall stress, the measure of afterload most closely related to systolic function, results in a decline in ventricular performance, as seen in our FLO patients.
High wall stress has been associated with less favorable prognosis because of electrical instability and myocardial hypoperfusion [29]. Thus, not only is the oxygen supply reduced (decreased coronary flow reserve) but also at the same time oxygen demand is enhanced (increased wall stress). One can hypothesize that this mismatch between supply and demand might lead to the occurrence of potential subendocardial ischemia. Patients with a single systemic ventricle experience a significant age- and load-related deterioration in myocardial mechanics, characterized by progressive development of a more spherical shape [28]. Wall stress that exceeds the passive and tensile forces that normally maintain the prolate ellipsoidal shape of the ventricle may play a role in the transition to a more spherical configuration, which provides a mechanical disadvantage for the systemic function of the ventricle.
Coronary flow has been difficult to study in patients after correction or palliation of congenital heart defects because of the lack of a reliable and noninvasive means of measuring myocardial perfusion. However, PET has become an experimentally validated and clinically accepted method of measuring coronary flows in adults as well as children after repair or palliation of congenital heart defects [5, 13, 27]. Our findings, in agreement with the findings of Donnelly et al. [5], indicate that PET provides technically acceptable imaging of myocardial perfusion in patients with single ventricular morphology. Our study demonstrates a significant attenuation of hyperemic MBF and CFR in comparison to healthy volunteers. Is it possible to compare myocardial blood flow parameters of a systemic ventricle of right ventricular morphology with those of a normal morphologic left systemic ventricle? Murray and coworkers [20] demonstrated that chronic pressure overload of the morphologic right ventricle by pulmonary artery banding in conscious dogs is associated with significant hypertrophy of the right ventricle. Right ventricle hypertrophy is characterized by a substantial increase in blood flow per gram of the right ventricular myocardium comparable with myocardial flow dynamics of a normal left ventricle. Several animal model studies have demonstrated that hyperemic response and minimal coronary vascular resistance are similar for the morphologic left ventricle and hypertrophied systemic right ventricle [21]. Similar results have been obtained in human studies; therefore, it should be feasible to compare the myocardial flow dynamics of a morphologic right systemic ventricle with the flow pattern of a normal left ventricle [6].
Coronary blood flow is an important feature in ventricular performance, and we demonstrated a significant correlation between MBF, CFR, and ventricular function in patients after FLO. In the preoperative volume-overloaded, functionally univentricular heart, in which coronary perfusion may be altered by increased ventricular wall stress resulting in impaired ventricular function, abnormal coronary angiogenesis [31] and a pathologic expression of contractile proteins [26] may occur as the first negative effects, resulting in a poor outcome in the postoperative period. The unloading operation may counteract this process and improve the long-term results in these patients when it is performed early.
The importance of coronary venous pressure as a determinant of the pressure gradient across the coronary vascular bed, and its influence on coronary blood flow, is well-known [17].
Elevated pressures, secondary to systemic venous hypertension after FLO, impair coronary perfusion and thereby depress myocardial function. When coronary sinus pressure is increased, there should be no change in coronary arterial flow until venous pressure becomes elevated to a threshold level; beyond this threshold, increasing the coronary sinus venous pressure will cause a redistribution of venous blood flow to noncoronary sinus channels, a change in the perfusion pressure flow relationship, and a resultant decrease in coronary blood flow [18].
Experimental data indicate that mean coronary sinus pressure exceeding 15 mmHg significantly decreases coronary arterial flow, coronary sinus outflow, and concomitantly left ventricular ejection fraction [33]. In our patient group, mean right atrium and left atrium pressure did not exceed 15 mmHg. This could be the reason why no correlation could be demonstrated between MBF dynamics and the site of coronary venous drainage into either the systemic venous or pulmonary venous atrium. Nevertheless, on the basis of well-documented experiments, every attempt should be made intraoperatively to achieve the lowest right atrial and coronary sinus pressure after FLO.
Angiographically, a tortuous course of the distal coronary arteries and ventriculoarterial communications most likely created by dilatated thebesian veins was demonstrated, especially in those patients with right coronary dominance and systemic right ventricular morphology. These patients also had a markedly attenuated ventricular function estimated by echocardiography and a significantly decreased MBF assessed by PET imaging.
In the normal heart the majority of coronary blood flow to the systemic left ventricle occurs during diastole [1, 8, 32]. There is also a small amount of antegrade flow that occurs throughout systole, with a short period of flow reversal at the beginning and at the end of systole [24]. However, in the normal morphologic right ventricle, flow is continuous throughout the cardiac cycle, with systolic flow somewhat greater than diastolic flow [24]. This was demonstrated to change to a more left ventricular profile when the right ventricular pressure increases to systemic pressure [15, 24], as would be the case in patients with a Fontan circulation. In the case of double-outlet right ventricle and mitral atresia, the morphologic right ventricle becomes the systemic ventricle and the coronary system should mimic the flow pattern of a left ventricle. Could the tortuous course of the coronary arteries and the demonstrated intratrabecular communications to the systemic ventricle be a reaction to the high systemic pressure acting on the morphologically right ventricular coronaries, especially because this coronary pattern is seen in adults with systemic arterial hypertension? Could it be explained as a compensatory mechanism to achieve adequate myocardial perfusion of a morphologic right ventricle under the condition of systemic pressures? To answer these questions, more detailed studies in patients with a morphologic right systemic ventricle after Fontan operation must be performed.
Conclusion
Abnormal coronary arteries, altered myocardial flow dynamics, and impaired CFR are common findings in patients after FLO; attenuated MBF and reduced ventricular function, especially in right ventricular morphology, are significantly correlated and seem to progress with time. Because ischemic heart disease will have negative effects on the Fontan heart, these are important risk factors. No significant correlation between the site of the coronary venous drainage and the myocardial perfusion could be established.
Study Limitations
Limitations of this study are mainly derived from the small number and the variety of underlying morphologies and from ethical constraints concerning radiation exposure in children. From an ethical standpoint, recruitment of age-matched control groups for any radionuclide study is difficult in childhood. No published blood flow data in normal children at any age are available, and young adult volunteers > l8 years of age were used in the current study as a normal control group. Therefore, age-related influences on the results cannot be ruled out completely.
References
RM Berne MN Levy (1992) Cardiovascular Physiology, 6th edn. Mosby-Year Book St. Louis 219–231
F Cetta RH Feldt PW O’Leary et al. (1996) ArticleTitleImproved early morbidity and mortality after Fontan operation: the Mayo Clinic experience 1987–1992. J Am Coll Cardiol 28 480–486 Occurrence Handle10.1016/S0735-1097(96)00135-0 Occurrence Handle1:STN:280:BymA1MzjsVY%3D Occurrence Handle8800129
AJ Chin WH Franklin BA Andrews et al. (1993) ArticleTitleChanges in ventricular geometry early after Fontan operation. Ann Thorac Surg 56 1359–1365 Occurrence Handle1:STN:280:ByuD1M7os1Y%3D Occurrence Handle8267437
J Czernin P Muller S Chan et al. (1993) ArticleTitleInfluence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 88 62–69 Occurrence Handle8319357
JP Donnelly DM Raffel BL Shulkin et al. (1998) ArticleTitleResting coronary flow and coronary flow reserve in human infants after repair or palliation of congenital heart defects as measured by positron emission tomography. J Thorac Cardiovasc Surg 115 103–110 Occurrence Handle9451052
DB Doty CB Wright LF Hiratzka et al. (1984) ArticleTitleCoronary reserve in volume-induced right ventricular hypertrophy from atrial septal defect. Am J Cardio 54 1059–1063 Occurrence Handle1:STN:280:BiqD2czlsFI%3D
DJ Driscoll GK Danielson FJ Puga et al. (1986) ArticleTitleExercise tolerance and cardiorespiratory response to exercise after the Fontan operation for tricuspid atresia or functional single ventricle. J Am Coll Cardiol 7 1087–1094 Occurrence Handle1:STN:280:BimC28bjtl0%3D Occurrence Handle3958365
EO Feigl (1983) ArticleTitleCoronary physiology. Physiol Rev 63 1–205 Occurrence Handle1:STN:280:BiyC3Mfgtlc%3D Occurrence Handle6296890
SB Fishberger G Wernovsky TL Gentles et al. (1997) ArticleTitleFactors influencing the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg 113 80–86 Occurrence Handle1:STN:280:ByiC2cfosVI%3D Occurrence Handle9011705
F Fontan E Baudet (1971) ArticleTitleSurgical repair of tricuspid atresia. Thorax 26 240–248 Occurrence Handle1:STN:280:CS6B38fkvVU%3D Occurrence Handle5089489
TL Gentles JE Mayer K Gauvrean et al. (1997) ArticleTitleFontan operation in five hundred consecutive patients: factors influencing early and later outcome. J Thorac Cardiovasc Surg 114 376–391 Occurrence Handle1:STN:280:ByiH2M%2FitVY%3D Occurrence Handle9305190
W Grossman D Jones LP McLaurin (1975) ArticleTitleWall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56 56–64 Occurrence Handle1:STN:280:CSqC1cbovVc%3D Occurrence Handle124746
M Hauser FM Bengel A Kuehn et al. (2001) ArticleTitleMyocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and Ross operation. Circulation 103 1875–1880 Occurrence Handle1:STN:280:DC%2BD3M3msFymsg%3D%3D Occurrence Handle11294806
J Hess K Kruizinga CMA Bijleveld et al. (1984) ArticleTitleProtein-losing enteropathy after Fontan operation. J Thorac Cardiovasc Surg 88 606–609 Occurrence Handle1:STN:280:BiqD3M7jtVA%3D Occurrence Handle6482492
JIE Hoffman GD Buckberg (1976) ArticleTitleTransmural variations in myocardial perfusion. Prog Cardiol 5 37–89
GD Hutchins M Schwaiger KC Rosenspire et al. (1990) ArticleTitleNoninvasive quantification of regional blood flow in human heart using N-13 ammonia and dynamic PET imaging. J Am Coll Cardiol 15 1032–1042 Occurrence Handle2312957
MN Ilbawi FS Idriss AJ Muster et al. (1986) ArticleTitleEffects of elevated coronary sinus pressure on left ventricular function after the Fontan. J Thorac Cardiovasc Surg 92 231–237 Occurrence Handle1:STN:280:BimB1czisFc%3D Occurrence Handle3736081
H Laks J Milliken J Perloff et al. (1984) ArticleTitleExperience with the Fontan procedure. J Thorac Cardiovasc Surg 8 939–951
JE Mayer Jr H Helgason RA Jonas et al. (1986) ArticleTitleExtending the limits for modified Fontan procedures. J Thorac Cardiovasc Surg 92 1021–1028 Occurrence Handle1:STN:280:BiiD283mtlY%3D Occurrence Handle3784586
PA Murray H Baig MC Fishbein SF Vatner (1979) ArticleTitleEffects of experimental right ventricular hypertrophy on myocardial blood flow in conscious dogs. J Clin Invest 64 421–427 Occurrence Handle1:STN:280:CSaB3MnnvVE%3D Occurrence Handle156735
PA Murray SF Vatner (1981) ArticleTitleAbnormal coronary vascular response to exercise in dogs with severe right ventricular hypertrophy. J Clin Invest 67 1314–1323 Occurrence Handle1:STN:280:Bi6C1M3jtFM%3D Occurrence Handle6453133
O Muzik SM Paridon TP Singh et al. (1996) ArticleTitleQuantification of myocardial blood flow and flow reserve in children with a history of Kawasaki disease and normal coronary arteries using positron emission tomography. J Am Coll Cardiol 28 757–762 Occurrence Handle10.1016/S0735-1097(96)00199-4 Occurrence Handle1:STN:280:BymA28fns1Q%3D Occurrence Handle8772768
G Osakada OM Hess KP Gallather et al. (1983) ArticleTitleEnd-systolic dimensions in wall thickness relations during myocardial ischemia in conscious dogs: a new approach for defining regional function. Am J Cardiol 51 1750–1758 Occurrence Handle1:STN:280:BiyB38josl0%3D Occurrence Handle6858885
SM Paridon DJ Fisher (1990) Regulation of myocardial blood flow and oxygen consumption. A Garson JT Bricker DG McNamara (Eds) The Science and Practice of Pediatric Cardiology Lea Febiger Philadelphia 221–231
DJ Penny ML Rigby AN Redington (1991) ArticleTitleAbnormal patterns of intraventricular flow and diastolic filling after the Fontan operation: evidence of incoordinate ventricular wall motion. Br Heart J 66 375–378 Occurrence Handle1:STN:280:By2D1c3nt10%3D Occurrence Handle1747299
K Schwartz L Carrier AM Lompre et al. (1992) ArticleTitleContractile proteins and sarcoplasmic reticulum calcium-ATPas gene expression in the hypertrophied and failing heart. Basic Res Cardiol 87 IssueID(Suppl 1) 285–290 Occurrence Handle1:CAS:528:DyaK3sXpvFenuw%3D%3D Occurrence Handle1386731
TP Singh RA Humes O Muzik et al. (2001) ArticleTitleMyocardial flow reserve in patients with a systemic right ventricle after atrial switch repair. J Am Coll Cardiol 37 2120–2125 Occurrence Handle10.1016/S0735-1097(01)01283-9 Occurrence Handle1:STN:280:DC%2BD3MzlvFaisQ%3D%3D Occurrence Handle11419897
T Sluysmans SP Sanders M van der Velde et al. (1992) ArticleTitleNatural history and patterns of recovery of contractile function in single left ventricle after Fontan operation. Circulation 86 1753–1761 Occurrence Handle1:STN:280:ByyD1c3osVM%3D Occurrence Handle1451247
Y Sugishata K Iida S Ohtsuka et al. (1994) ArticleTitleVentricular wall stress revisited: a keystone of cardiology. Jpn Heart J 35 577–587 Occurrence Handle7830323
R Tennant CJ Wiggers (1935) ArticleTitleThe effects of coronary occlusion on myocardial contractions. Am J Physiol 112 351–361
RJ Tomanek RJ Torry (1994) ArticleTitleGrowth of the coronary vasculature in hypertrophy: mechanics and model dependence. Cell Mol Biol Res 40 129–136 Occurrence Handle1:CAS:528:DyaK2cXmslOltLs%3D
J Trimble J Powney (1979) ArticleTitleContribution of myocardial contractility to myocardial perfusion. Am J Physiol 236 H121–H126 Occurrence Handle1:STN:280:CSaC28zgslM%3D Occurrence Handle434162
P Uhlig R Baer G Vlahakes et al. (1981) ArticleTitleEffect of coronary sinus pressure elevation on coronary flow [abstract]. Circulation 64 IssueID(Suppl 4) 38
SF Vatner (1980) ArticleTitleCorrelation between acute reductions in myocardial blood flow and function in the conscious dog. Circ Res 47 201–207 Occurrence Handle1:STN:280:Bi%2BB2cvksFw%3D Occurrence Handle7397952
HS Weber WE Hellenbrand CS Kleinman et al. (1989) ArticleTitlePredictors of rhythm disturbances and subsequent morbidity after the Fontan operation. Am J Cardiol 64 762–767 Occurrence Handle1:STN:280:By%2BD38zit1c%3D Occurrence Handle2801527
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hauser, M., Bengel, F., Kühn, A. et al. Myocardial Perfusion and Coronary Flow Reserve Assessed by Positron Emission Tomography in Patients after Fontan-like Operations . Pediatr Cardiol 24, 386–392 (2003). https://doi.org/10.1007/s00246-002-0355-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-002-0355-5