Abstract
Beeswax, honey, and live in-hive worker bees were collected in this study from eight districts in Khyber Pakhtunkhwa, Pakistan. The concentration of seven essential elements (copper, calcium, zinc, iron, nickel, chromium and manganese) and two non-essentials (lead and cadmium) were determined. All of the samples were found to have a random distribution of metal concentrations. The plentiful metals with high concentrations in the gathered samples were discovered to be calcium, iron, and zinc. The health concerns related with metal intake in honey were assessed using the Average Daily Dose (ADD), Hazard Quotients (HQs) and Hazard Index (HI) models. Children were found to have higher estimated health risk values for the components assessed in all samples than adults. All of the computed ADD values were lower than the matching reference (RfD) values. The matching HI values of metals in various honeys were found to be less than one, implying that honey consumption in the studied area has no non carcinogenic risk. Cancer risks (CR) was also calculated for intake of Pb, Cr, Ni and Cd in honey in the selected districts. The CR values for Cr and Cd exceeded 1E-4 in various districts such as Karak, Kohat, Nowshera, Bajur, Dir Upper, and Mohmand Agency, signifying that there was a small danger involved. In the case of Karak, the Ni CR value was greater than the allowed limits. As a result, it is important to keep an eye on the concentration of these metals in honey because anthropogenic input could raise their concentration in the future, posing a health danger.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Honey is a natural substance made by honeybees (Apismellifera and Apis dorsta) from floral nectar. Honey has been consumed by humans since almost 5500 years ago (Samarghandian et al. 2017). Because of its nutritional and physiological advantages, honey is a significant part of the human diet (Alvarez-Suarez et al. 2010). Honey's composition is determined on the type of flower from which it is derived (El Sohaimy et al. 2015). A 100 g of honey generates 300 kcal of energy which is prompt source of energy for the human body due to the presence of simple, inverted sugars like glucose and fructose (Saxena et al. 2010). It also contains oligosaccharides such as anderose and panose (trisaccharide), as well as enzymes such as amylase, peroxidase, catalase, and acid phosphorylase. Amino acids, Vitamin B6, trace vitamin B, niacin, Vitamin C, folic acid, iron, minerals, zinc, and antioxidants are also found in honey (Fatimah et al. 2013; Meo et al. 2017). Honey's antioxidant capabilities enable it to remove free radicals from the human body. Honey has benefitted people for a long time because of its medicinal and therapeutic properties, i.e., it is ingested alone or in combination with other substances for a variety of therapies (Khalil et al. 2010; Molan 2002). Honey can be used to treat a variety of wounds due to its antibacterial qualities, diabetes, pressure, leg ulcers, burns, and cuts can all be treated with it (Yaqub et al. 2020). Throughout the world honey is utilized as a source of food and for therapeutic purposes, hence it is critical that it remains pure (Wieczorek et al. 2014). Aside from its nutritional and medicinal properties, honey is employed as a barometer to assess environmental quality in areas contaminated by heavy metals (Porrini et al. 2003), radiation, and pesticides (Čelechovská and Vorlova 2001; Leita et al. 1996). The elemental makeup of flowers, as well as their botanical and geographical origin, influence metal concentrations in different honey kinds (Pisani et al. 2008; Rashed and Soltan 2004). External sources of these metals include pollution from industrial smelters, industrial unit emissions, and faulty honey processing and maintenance practices (Wang et al. 2010). Bees come into contact with plants, air, water, and soil in addition to hunting for nectar, honeydew, pollen, and plant exudates across such a vast territory.Where there is pollution in the environment, they become contaminated as well and transfer toxins into the hive or in the raw materials obtained (Dobrinas et al. 2006; Bogdanov 2006). Because hazardous metal accumulation in the human body causes side effects, for human nutrition and safety honey quality particularly elemental content must be assessed (Tuzen and Soylak 2005). Honey's trace element concentration varies, but it is usually less than 1.0 μgg−1 (Dourado et al. 2019). Essential elements that play a basic role in the maintenance of biochemical and physiological functions in living organisms include Cr, Ca, Cu, Co, Fe, Mg, K, Mo, Mn, Na, P, Ni, Se and Zn. Different elements play different role for example, Cu and Fe are involved in physiological balance (i.e., homeostasis), Ca is involved in vascular, endocrine and neuromuscular function, Mg in endocrine function (i.e., blood glucose control), Mn in immune function and in physiological balance (i.e., homeostasis, coagulation) and K is involved in vascular function (i.e., blood pressure regulation), and biochemical reactions. Phosphorous plays role in multiple physiological functions, Na is involved in vascular and neuromuscular function (i.e., transmission of nerve impulses), while Zn is involved in many biochemical and enzymatic reactions. However, the bioaccumulation of these essential elements above certain thresholds results in toxicity (Marquès et al. 2021). On the other hand, non-essential elements play no role in the human body and they can be toxic even at low concentrations. These include metals and metalloids such as aluminum (Al), cadmium (Cd), arsenic (As), lead (Pb), mercury (Hg), tin (Sn) and antimony (Sb). These toxic elements can damage vital organs such as the brain, kidney or liver, among others. Health effects that are physical such as chronic pain, blood pressure alteration, blood composition change, as well as psychological, such as anxiety, passivity disorders, as well as neurodegenerative diseases and cancer may be caused by long-term exposureto non-essential elements (Yang and Massey 2019). Thus, the determination of metal concentrations in the tissues of honeybees (Apis mellifera) could be a valuable tool for biomonitoring of metal pollution and estimating human health risks (Tomczyk et al. 2020).
Honey consumption is a popular practice among Pakistanis at breakfast, family gatherings, and social gatherings. As a result, the current study was conducted to determine and compare heavy metal levels in honey, beeswax and bees taken from apiaries in different areas of Khyber Pakhtunkhwa, Pakistan. One of the study's goals was to estimate the risk level for those living in places where honey is consumed.
Experimental
Chemicals
In the present study, analytical grade reagents, super-pure nitric acid (HNO3) and hydrogen peroxide (H2O2) were used. Throughout the study, high purity water (18.2 Ωcm−1 resistivity) was used. Glassware was cleaned by soaking it overnight in diluted HNO3 (10% v/v) and then rinsing it with high quality water before use. Dilution of the stock solution with 1 percent (v/v) HNO3 yielded the standard solutions used in the calibration processes.
Study Area and Sampling
Beeswax (20 g), honey (100 g), and live in-hive worker bees (n = 30) were collected in June and July of 2019–2020 from different districts in Khyber Pakhtunkhwa Pakistan (Kohat, Karak, Bajur, Dir Upper, Nowshehra, Mohmand Agency, Dir Lower, and Khyber Agency) that covered a wide range of landscapes, from intensive farming areas to grasslands, holm oak woodlands, mountainous (Fig. 1). Four apiaries and five hives from each apiary were chosen for sampling in each district. During the collection period, the hives had no veterinary treatment, and the bee colonies appeared to be in good health. Cutting a comb part from each of the selected hives at a given location and pooling them together to generate a single sample representative of each apiary was used to get honey and beeswax samples. A pool of bees from five hives was used to collect worker bee samples from lateral combs. To avoid additional emerging individuals, no bees were captured near the brood nest. The samples were transported to the laboratory in an insulated cooler and kept at 20 °C until they were analyzed.
Sample Preparation
Honey samples were cooked in a water bath at 65 °C to dissolve any fine crystals, as per conventional protocols. These were then filtered to remove any coarse particles that could interfere with the analysis. After that the samples were chilled and weighed for further analysis (Belouali et al. 2008).
Acid Digestion of Honey Samples
Exactly 1 g of pre-mixed and homogenized honey was put into conical flask. 4 mL hydrogen peroxide and 8 mL pure nitric acid were also added to the flask. The mixture was then boiled on a water bath for 4 h till dryness. After cooling to room temperature, 2% nitric acid (in de-ionized water) was added to dissolve the dry bulk, and the contents were filtered into a 10-ml volumetric flask using Whatman no. 42 filter paper. De-ionized water was used to bring the solution up to the required volume. In order to correct the effect of the blank in parallel, a reagent blank was also digested with the same digestion conditions. The digested samples were kept in the fridge until they were analyzed (Tuzen et al. 2007).
Mineral Analysis
The concentration of lead, chromium, copper, nickel, calcium, cadmium, zinc, iron, and manganese in honey, beeswax and bees samples was measured using Atomic Absorption Spectrophotometer (Analytik Jena ContrAA 700 Germany) at the National Physical and Standard Laboratory Islamabad. Repeated analyses of the samples against verified reference metal standard materials 1000 ± 4 mg/L, in 2% HNO3 (Sigma-Aldrich) were undertaken to ensure precession and accuracy.
Instrumental Conditions and Limits of Detection
The method detection limit (MDL) is the lowest concentration of an analyte that can be detected and reported with 99% certainty. It can be stated as the amount of analyte that provides a signal equal to T-test times the standard deviation of the blank (Miller and Miller 2005). The detection limits were calculated by multiplying the reagent blank's standard deviation by three. In our study reagent blank solutions were digested and analyzed for metal concentrations of Pb, Cr, Cu, Ni, Ca, Cd, Zn, Fe and Mn using Flame Atomic absorption spectrophotometer (FAAS). The instrumental conditions along with detection limits for the selected metals are given in Table 1.
Precision and Accuracy of the Method
Repeatability and recovery experiments of matrix spike were used to assess the precision and accuracy of the analytical method. In the recovery investigation, three replicate honey samples were spiked with a known concentration of metal standard solution. After that, the tainted samples were digested in the same way that real samples were. The relative standard deviation was computed using Eq. (1),
and the relative standard deviation (RSD) of the three replicate results was expressed as precision.
Accuracy of the method was calculated using the following relation (Eq. 2) (Iqbal et al. 2010).
The data regarding validation of analytical method are shown in supplementary Table S1.
Health Risk Estimation
Estimated Daily Intake (EDI)
In May 2020, a questionnaire-based survey on daily honey intake was done in selected districts using the dietary recall method and food frequency approach, which are commonly used in food consumption surveys. A total of 400 adult men and women were randomly selected from the selected districts in Khyber Pakhtunkhwa. They were asked to recollect how much honey they ate and how often they ate it. The participants were also requested to exhibit the spoon they usually used in order to improve accuracy.
The average content of each heavy metal in honey and the ingestion rate (IR) were used to calculate the estimated daily intake (ADD) of heavy metals (Pb, Cr, Cu, Ni, Ca, Cd, Zn, Fe and Mn). The following equation-3 was used to calculate the ADD for each metal.
where ADD stands for average daily consumption (µg kg1 day−1). The heavy metal concentration in honey (µg kg−1) is denoted by the letter C. The honey intake rate (kg/person/day) is referred to as IR. The rate of ingestion (Kg/person/day) was calculated to be 0.003 kg/person/day for adults and 0.0009 kg/person/day for youngsters based on our questionnaire survey. Bw stands for body weight (Kg), and the average body weight for adult male was taken 70 kg, for female adults 67.5 kg and for children as 30 kg.
Hazard Quotient (HQ)
The human health risk due to consumption of honey was calculated using the USEPA Region III Risk-Based Concentration table (Fallahi and Alijani 2012). The following relationship (Eq. 4) was used to estimate the risk level (non-carcinogenic) due to pollutant exposure using the target hazard quotient (THQ).
where ADD denotes the average daily intake of metal (µg kg−1 day−1), and Rfd denotes the USEPA-recommended reference doses (µg kg−1 day−1) derived from WHO's provisional tolerable weekly intake (PTWI) (Gruszecka-Kosowska and Mazur-Kajta 2016; Ji et al. 2015).
Hazard Index (HI)
The hazard index (HI) is basically the sum of THQs of each metals and is calculated using Eq. 5 (Mao et al. 2014)
Cancer Risks
The cancer slope factor (CSF), which is the risk produced by a lifetime average dose of 1 mg/ kg/ body weight (BW)/ day and is contaminant specific, is used to calculate incremental lifetime cancer risk (ILCR) and is given by Eq. 6
where the lifetime average daily dose exposure to the chemical is represented by chronic daily intake of chemical (CDI) in mg kg−1 BW day−1. The slope factors for Pb, Cr, Ni and Cd are 0.0085, 0.38, 0.84 and 0.5(mg/kg/day)−1, respectively. CDI can be calculated using the following Eq. 7.
where estimated daily intake of metal via consumption of honey is represented by EDI, EFr stands for exposure frequency (365 days year−1), ED stands for exposure duration (55 years), and the average exposure time for a non-carcinogen in days (Efr × ED) is represented by ATn (Orisakwe et al. 2012).
Results and Discussion
Metal Concentration of Honey, Beeswax and Bees
Table 2 depicts metals concentration (µg g−1) of honey, beeswax and bees from apiaries in selected districts in Khyber Pakhtunkhwa. These are discussed one by one as follows;
Lead (Pb)
Lead (Pb) is non-essential element and extremely hazardous to living things. This substance is regarded as one of the most significant sources of pollution in the environment. Pb levels below 1.0 mg/kg are considered acceptable (Harmanescu et al. 2007). Lead has no function in the human body and its presence can cause hazardous effects. Organs such as the liver, kidneys, heart, and male gonads can be severely damaged by lead and cadmium exposure (Nega et al. 2020). The highest mean values of Pb in our samples were 0.413 μgg−1, 0.431 μgg−1 and 0.592 μgg−1 for honey, beeswax and bees from Nowshera, respectively. Vehicular emission on the nearby roadway are the most important explanation for the high Pb concentration here. Vehicles, fossil fuels, the metal industry, and refineries are said to be the most significant contributors of Pb pollution (Markert et al. 2003). A study conducted in Sindh province, Pakistan has shown Pb concentration in honey samples in the range of 0.01–0.14 μgg−1 (Lanjwani and Channa 2019), while in Morocco and Saudi Arabia, the concentration of Pb in honey was 1.88 μgg−1 and 0.37 μgg−1, respectively (Belouali et al. 2008; Osman et al. 2007).
Chromium (Cr)
According to our findings, honey, honeybees, and bee wax samples from Nowshera had highest mean concentration of chromium that was 0.278 μgg−1, 0.200 μgg−1, and 0.188 μgg−1, respectively. Excessive soluble Cr is hepatotoxic and nephrotoxic. According to the Expert Group on Vitamins and Minerals (EVM), a total daily intake of roughly 0.15 mg chromium (III)/kg body weight/day which is equal to (10 mg/person/day) is expected to be safe, however the WHO recommended that chromium supplementation should not exceed 250 μg day−1 (Vettorazzi et al. 2020). This high concentration of Cr in honey samples could be attributed to the fact that a number of tanneries are operating in Peshawar and their effluents are passing through Charsadda and the nearby Nowshehra. The direct use of the effluents by bees as well as irrigation of vegetables, plants with polluted water can lead to an increase level of Cr in these samples and subsequent in honey. The Environmental Protection Agency lists chromium (Cr) as one of the 129 priority pollutants and one of the 14 most toxic heavy metals with the maximum permissible limit (MPL) as 1.5 μgg−1(You et al. 2018). The most prevalent way for the general public to be exposed to chromium is by ingestion of chromium-containing soil, food, and water (Aghamirlou et al. 2015). The mean Cr concentration in our study was lower than that found in honey samples collected in Chile, that was 1.92 μgg−1 (Fredes and Montenegro 2006), and Nigeria, 0.32 μgg−1 (Achudume and Nwafor 2010), while higher than honey samples from Slovenia, 0.11 μgg−1 (Golob et al. 2005).
Copper (Cu)
The highest mean concentration of copper in honey, beeswax, and bees samples from Dir (U) was 1.902 μgg−1, 0.142 μgg−1, and 0.433 μgg−1, respectively. Copper is essential for the health of all living things, including humans. A surplus of Cu, on the other hand, has been linked to cellular and tissue destruction (Wilson disease), as well as a number of negative impacts and human disorders. As a result, daily copper consumption from various sources, such as food, must be considered (Aghamirlou et al. 2015). Animal fertilizers, pesticides, sewage, ashes, metal industries including iron and steel industry are the most significant sources of Cu pollution. According to the Codex Alimentarius Commission's on Standards, the maximum Cu value that must be found in sweet foods like sugar and honey is 5 μgg−1 (Leblebici and Aksoy 2008). The concentrations of Cu in this study do not exceeded these limitations. Copper concentration of 0.25–1.30 μgg−1 (Tuzen 2002) in honey samples from the Black Sea region of Turkey and 1.8μgg−1 (Yılmaz and Yavuz 1999; Conti 2000), from Turkey's south-eastern Anatola has been reported in the literature.
Nickel (Ni)
Nickel is a trace element that is required for the survival of various animal species, microbes and plants. Excessive Ni intake beyond threshold limit is detrimental to the people. Nickel-eczema, lung inflammation, fibrosis, emphysema, and malignancies are all serious side effects due to Ni (Zhong et al. 2016). Nickel was detected in all types of samples from Karak and Dir lower, while in Mohmand agency it was detected only in honey samples. The mean Nickel concentration in honey, beeswax, and bees samples from Karak was 0.905 μgg−1, 0.251 μgg−1, and 0.675 μgg−1, respectively, according to our findings. The concentration of nickel in samples from other districts was below the detection limit of the instrument. The mean concentration of nickel in the Karak samples was higher as compared other districts, which indicates that there are more industrial sources of nickel there. The amount of nickel consumed through food is dependent on a number of factors, including the source of Ni and the distance from the contamination source. The literature has shown that nickel concentration in honey ranged from 1.02 to 1.48 μgg−1in Pakistan (Farooq and Maqbool 2008), and 0.01 to 1.04 μgg−1 in Chile (Fredes and Montenegro 2006). Nickel is found in the air, water, and soil, and it is widely dispersed across the soil profile. The current investigation found that the content of nickel was below permitted limits that is 1 μgg−1 (Lanjwani and Channa 2019).
Calcium (Ca)
Calcium is the body's most prevalent mineral. The bones and teeth contain 99 percent of the calcium. The remaining 1% is found in the blood. Nerve conduction, muscle contraction, and blood clotting all require calcium. Calcium will be extracted from bone and injected into the blood to save blood calcium levels if blood calcium levels are abnormal. As a result, it is critical to consume adequate calcium to keep blood and bone calcium levels in check (Aghamirlou et al. 2015). Honey, beeswax, and bees samples from Dir Upper had the highest mean Ca contents of 7.393 μgg−1, 4.481 μgg−1 and 3.318 μgg−1, respectively. When compared with other studies, the samples under investigation contain large amount of Ca.
Cadmium (Cd)
The concentration of Cd in all the samples was below the limit of detection except for the samples from Nowshera and Kohat. However, the highest mean concentration was found in the samples from Nowshera which was lower than 0.01–0.38 reported for honey samples from Sindh province, Pakistan (Dhahir and Hemed 2015), and Manuka honey (0.27 μgg−1) and honeys from Turkey (0.90 μgg−1) (Tuzen et al. 2007). Cd concentrations in samples were lower than WHO/FAO allowed levels (0.25 μgg−1) (Kebebe 2019). The main Cd sources are: mining and smelting, the usage of phosphate fertilizers, and its presence in sewage sludge. Another source in honey may be through contact with surfaces contaminated with Cd rom metal plating, pigments, and plastics. Cd is a common toxin with a wide range of effects. Its negative health effects are especially severe in infants, children and pregnant women (Bilandžić et al. 2011).
Zinc (Zn)
The higher mean concentrations of zinc were found in Dir Upper honey, beeswax and bees samples, with values of 6.045 µgg−1, 5.707 µgg−1, and 2.901 µgg−1, respectively. Zn is a mineral that is found in approximately 100 enzymes and is considered an antioxidant. It is commonly second most prevalent transition metal in organisms after Fe, and it is the only transition metal found in all enzyme tribes (Broadley et al. 2007). It is possible that the amount of Zn in honey depends on the sort of flowers that bees eat. Zinc, being a heavy metal, tends to bio-accumulate in the fatty tissues of aquatic species, including fish, and is known to affect fish reproductive function. Chronic exposure to Cu and Zn has been linked to Parkinson's disease, according to some studies (Rashed and Soltan 2004). Acute Zn poisoning is caused by inhaling the metal at work, and symptoms include nausea, vomiting, diarrhea, drowsiness, and fever (World Health Organization 2000). The maximum Zn value that must be found in sweet foods such as sugar and honey, according to Codex Alimentarius Commission regulations, is 5 µgg−1 (Leblebici and Aksoy 2008). The resultant values shown in Table 2 indicate that the concentration of Zn in honey from Dir Upper exceeded the recommended value i.e. 5 µgg−1.
Iron (Fe)
The highest mean Fe concentration was found in the samples came from Mohmand agency which was 12.639 µgg−1, 14.673 µgg−1, and 11.138 µgg−1, respectively. In the general population, Fe excess as a result of dietary intake is uncommon. According to the Codex Alimentarius Commission's on Standards, the maximum Fe value that must be found in sweet foods such as sugar and honey is 15 µgg−1. Because the Fe concentrations in all of the samples examined were below the recommended limit, the Fe levels found do not pose a health risk to consumers. Iron (Fe) is a trace mineral that is needed for life and plays a unique role in the body (Alimentarius 2001). The tetramer hemoglobin protein contains Fe, which is required for the transport of oxygen and carbon dioxide via the bloodstream. As a result, a sufficient amount of it must be present in all food ingredients. The meteorological conditions of the observation site may be to blame for the varying iron contents (Djama et al. 2011).
Manganese (Mn)
In the present study, the highest mean concentration of Mn in the honey, beeswax and bees from Nowshera was found to be 0.574, 0.366 and 0.0124 μgg−1, respectively. The higher results of honeys from Nowshera could be due to their position in the city, where numerous activities that allow the release of metal from sources such as batteries, glasses, and other items are common (Oluyemi et al. 2008). The manganese levels found in this investigation are consistent with those discovered in Ethiopian honey samples (ranging from 0.008 to 0.33 μgg−1 (Nega et al. 2020). Manganese is a low-toxicity element with significant biological relevance. Industrial emissions, fossil fuel combustion, and erosion of manganese-containing soils all release manganese into the environment. Manganese can also be discharged into the environment when the gasoline additive methylcyclopentadienyl manganese tricarbonyl (MMT) is used (Oroian et al. 2016). As a result, manganese is present in all humans, and manganese is a natural component of the human body.
Principle Component and Cluster Analysis
The variables Pb, Cr, Cu, Ni, Cd, Zn, Fe, and Mn were used to perform principal component analysis (PCA). Concentrations obtained in honey samples for each metal were the observations and the cases were the various samples taken from various districts. PCA was used to extract four components/factors (Table 3). The overall variance was 42.65% due to the first component, 67.46% due to the second factor, 84.13% due to the third factor, and 96.31% due to the fourth factor. Cr, Pb, and Cd contributed the most to the first factor. Because the elemental concentration in honey is dependent on various factors such as climate, human activities, geographical location, the second factor had the greatest impact for Zn, Cu, the third for Ni and Fe, and the fourth for Cu and Cr, respectively. Loadings and scores for the first and second principal components of the PCA analysis are shown in Fig. 2. The graph scores show spatial distribution of the samples as a function of each component studied which in turn shows the relationship among the samples. These graphs of loadings, based on the variables researched, provide for the visualization of correlations between variables as well as the understanding of sample grouping. On the positive axis, PC1 clustered the majority of honey samples taken from Dir Upper, Nowshera, Dir Lower, Bajaur, Kohat, and Karak. Khyber Agency and Mohmand Agency were put on the PC1's negative axis. Non-essential elements (Cr and Cd) concentrations were substantially associated, with the greatest amounts seen in honey samples from the Nowshera, Karak, and Dir upper regions.
The high level of Pb, Cr, and Cd in the Nowshera district’s samples, Pb and Ni in Karak could be due to environmental contamination in the collection regions. Improper handling during honey harvesting and subsequent treatment also lead to the contamination of honey. Metal levels in honey are related to minerals in plants, which in turn depends upon the availability of mineral in the soil thereby reflecting honey production location. Nectar collected from plants is used by bees to produce honey, so metal levels in honey are related to minerals in plants, which in turn depends on the availability of mineral in the soil. Pollution of air, water and soil around the hives also contribute to the presence of Pb, Cd, and Cr in honey samples. These findings are supported by the plot, which shows a low level of the selected metals in those districts that are at the origin of the coordinate axis. Fertilizer and pesticide residues in the environment are also due to an appropriate treatment of domestic and rural sewage, rural runoff and agricultural activities. Despite the fact that harmful metals like lead are present in trace amounts in fertilizers and agrochemicals, they can accumulate in soils and plants, which are transported by bees and finally appear in honey (Lambert et al. 2012). Pb from asphalt roads, tires, and brakes can be trapped in the atmosphere by particulate matter (Soriano et al. 2012). The greatest Pb level of all samples was found in Nowshera and Karak, which is likely due to the district's proximity to a major state highway, which results in higher concentrations of pollutants from car smoke, which contains suspended particles (Prestes et al. 2006).
Beekeepers who employ fumigants to keep bees away during honey harvesting, for example, may contaminate hives with metals contained in the smoke, which can impact metal concentrations in honeybees. Mining activities, industries such as brick kilns, as well as metals containing emissions also cause metal contamination (Clark and Knudsen 2013).
For the identification of similarity between different groups Cluster Analysis (CA) is used. These similarities are used to create dendogram. All the districts samples based on the metals concentration were classified into 2 broad clusters. The cluster 1 comprises of four districts, namely, Kohat, Bajaur, Dir lower and Khyber Agency. The second cluster comprised of four districts, namely Karak, Nowshera, Mohmand agency and Dir Upper (Fig. 3). Cluster analysis was also applied by Marchini et al. (2007) to the metals contents in honey samples from Brazil.
Human Health Risk Assessment via Consumption of Honey
Average Daily Dose (ADD)
Table 4 shows the computed values of average daily doses for metals for different categories of people due to consumption of honey based on food frequency questionnaire. A group's health hazard or risk is determined by the route and degree of exposure. As a result, determining the degree of exposure through identifying pollutant pathways to target groups is critical. Metal exposure to people occurs mostly through ingestion, inhalation, and skin contact. The food chain or ingestion is the most important of these channels. The intake data can be used to investigate a specific aspect of interest (Javed and Usmani 2016; Ahmed et al. 2015).
The average daily dose (ADD) of eight trace heavy metals (Lead, Chromium, Copper, Nickel, Cadmium, Zinc, Iron, and Manganese) was calculated based on the content of each heavy metal in honey and their respective consumption rate. A questionnaire survey was conducted to determine the average rate of consumption. A health risk assessment was carried out on adults, both male and female, as well as children. For adults, following was the decreasing order of estimated daily intakes (ADD) values of the examined metals in honey: Fe > Zn > Cu > Ni > Mn > Pb > Cd, suggesting that Fe and Zn had the biggest contribution among the heavy metals.
Health Risk Estimation
Health risk assessments are used to express the excess probability of acquiring cancer during a 70-year lifetime based on assumptions. THQ values for each metal have been calculated and are shown in Table 5. THQ has an acceptable guideline value of 1 according to the USEPA (von Stackelberg and Williams 2021). Single metal HQ values ingested by honey ingestion were all less than1 as show.
For both male and female adults HQ values of individual metals ingested via honey consumption followed the order; Cd > Pb > Cr > Mn > Zn > Fe > Cu > Ni. While for children the HQ value of individual metals were in the order: Pb > Cd > Cr > Mn > Zn > Fe > Cu > Ni. The average HI values of the eight heavy metals obtained from honey consumption by males, females, and children were 6.00E−03, 6.21E−03, and 4.46E−03, respectively, all of which were less than 1 (Fig. 4), indicating that there was no significant non carcinogenic risk from heavy metals.
Cancer Risk (CR) Assessment
CR values were calculated for male and female adults through consumption of Pb, Cr, Ni and Cd in honey as shown in Table 6. For adults male and female, the cancer risk of selected heavy metals varied from 9.27E−05 to 3.25E−02 and from 9.61E−05 to 3.38E−02, respectively.
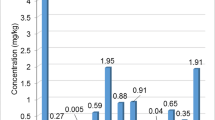
This parameter's values below 1E−6 are deemed tolerable, those between 1E−6 and 1E−4 are considered acceptable, and those beyond 1E−4 are considered intolerable (Mustatea et al. 2021). Table 5 shows that there is no discernible cancer risk for Pb because the CR value was within acceptable ranges. However, there is a little risk in the case of Cr for Kohat, Karak, Bajur, and Dir Upper, where the CR values were highest in the case of as Karak 3.49E−03 and 3.62E−03, respectively. Anthropogenic pollution may reach its current level in the future, creating a risk, because the CR values for Ni in male and female adults in Karak and Dir lower were higher than 1E−4. Similarly, the computed CR values for Cd, which was discovered exclusively in Kohat and Nowshera districts, show that it is important to pay attention to its concentration in Nowshera district. The overall comparison for both male and female adults in respect of CR value based on average values are also shown in supplementary Fig. 1.
Conclusions
In all samples, the metal concentration was distributed randomly. In decreasing order, the mean concentrations of nine elements were Fe > Zn > Ca > Cu > Ni > Mn > Pb > Cr > Cd. Based on the mineral content the classification of honey samples also reflected the geographical origin of the honey. Metals ingested via honey consumption in male and female adults the HQ values followed this order, Cd > Pb > Cr > Mn > Zn > Fe > Cu > Ni, while for children the HQ values of individual metals were in the order; Pb > Cd > Cr > Mn > Zn > Fe > Cu > Ni, indicating that the contribution of Cd and Pb was the highest among the daily intakes of heavy metals. The computed HI values for all of the examined metals, for various honey samples, were all less than 1, implying that honey consumption poses little or no non carcinogenic risk. In case of Karak and Mohmand agency Ni, while in case of Kohat and Nowshera districts Cd showed high probable cancer risk. As a result, regulatory bodies in all countries should enact necessary regulations and legislation to regulate honey production, handling, and quality control in order to ensure its safety.
Data Availability
The data will be available upon proper request to the corresponding author.
References
Achudume AC, Nwafor BN (2010) The ecological assessment of metals in local brands of honey in Southwest Nigeria. Afr J Agric Res 5:2608–2610
Aghamirlou HM, Khadem M, Rahmani SM, Mahvi AH, Akbarzadeh A, Nazmara S (2015) Heavy metals determination in honey samples using inductively coupled plasma-optical emission spectrometry. J Environ Health Sci Eng 13:1–8
Ahmed MK, Baki MA, Islam MS, Kundu GK, Habibullah-Al-Mamun M, Sarkar SK, Hossain MM (2015) Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ Sci Pollut Res 22:15880–15890
Alimentarius C (2001) Revised codex standard for honey. Codex Stan 12:1982
Alvarez-Suarez JM, Tulipani S, Romandini S, Bertoli E, Battino M (2010) Contribution of honey in nutrition and human health: a review. Med J Nutr Metab 3:15–23
Belouali H, Bouaka M, Hakkou A (2008) Determination of some major and minor elements in the east of Morocco honeys through inductively coupled plasma optical emission spectrometry. Apiacta 43:17–24
Bilandžić N, Đokić M, Sedak M, Kolanović BS, Varenina I, Končurat A, Rudan N (2011) Determination of trace elements in Croatian floral honey originating from different regions. Food Chem 128:1160–1164
Bogdanov S (2006) Contaminants of bee products. Apidologie 37:1–18
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Čelechovská O, Vorlova L (2001) Groups of honey-physicochemical properties and heavy metals. Acta Vet Brno 70:91–95
Clark JJ, Knudsen AC (2013) Extent, characterization, and sources of soil lead contaminations in Small-Urban residential neighborhoods. J Environ Qual 42:1498–1506
Conti ME (2000) Lazio region (central Italy) honeys: a survey of mineral content and typical quality parameters. Food Control 11:459–463
Dhahir SA, Hemed AH (2015) Determination of heavy metals and trace element levels in honey samples from different regions of Iraq and compared with other kind. Am J Appl Chem 3:83–92
Djama AAD, Goffri MK, Koua AA, Ofosu FG, Aboh IJK (2011) Trace elements analysis of some antiparasitic medicinal plants in Côte d’Ivoire using energy-dispersive X-ray fluorescence (EDXRF) technique. Curr Res J Biol Sci 3:209–215
Dobrinas S, Soceanu A, Birghila S, Belc M (2006) Determination of some essential elements in different types of Romanian floral and non-floral bee honeys and propolis. EEMJ 2006:5
Dourado GS, Gomes VV, Maia MTV, Vasconcelos AA, Costa KS, Faial KDCF, Taube PS (2019) Determination of macro and trace element levels in honey from the lower Amazonian region, Brazil
El Sohaimy SA, Masr SHD, Shehata MG (2015) physicochemical characteristics of honey from different origins. Ann Agric Sci 60:279–287
Fallahi F, Alijani B (2012) Locating fertile lands, with emphasis on the development of fruit orchards climatic elements-farming approach. GIS RS Geogr Environ Sustain 2:54–65
Farooq KZ, Maqbool T (2008) Physical and spectroscopic characterization of Pakistani honey. Ciencia e Investigaciónagraria 35:199–204
Fatimah B, Abubakar G, Aliyu S (2013) Analysis of biochemical composition of honey samples from North-East Nigeria. Biochem Anal Biochem 2:1000139
Fredes C, Montenegro G (2006) Heavy metal and other trace elements contents in honey bee in Chile. Int j Agric Nat Resour 33:50–58
Golob T, Doberšek U, Kump P, Nečemer M (2005) Determination of trace and minor elements in Slovenian honey by total reflection X-ray fluorescence spectroscopy. Food Chem 91:593–600
Gruszecka-Kosowska A, Mazur-Kajta K (2016) Potential health risk of selected metals for Polish consumers of oolong tea from the Fujian Province, China. Hum Ecol Risk Assess Int J 22:1147–1165
Harmanescu M, Popovici D, Gergen I (2007) Mineral micronutrients composition of bee’s pollen. J Agroaliment Process Technol 13:175–182
Iqbal J, Carney WA, LaCaze S, Theegala CS (2010) Metals determination in biodiesel (B100) by ICP-OES with microwave assisted acid digestion. Open Anal Chem J 4:18
Javed M, Usmani N (2016) Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelusarmatus inhabiting, thermal power plant effluent loaded canal. Springerplus 5:1–8
Ji XF, Zheng N, Wang Y, Liu Q, Zhang JJ (2015) Accumulation of mercury in soil-maize system of non-ferrous metals smelting area and its related risk assessment. Huanjingkexue 36:3845–3851
Kebebe D (2019) Study on pesticide residues and heavy metals levels in honey samples collected from Walmara District of Oromia special zone, Ethiopia. Sci J Food Sci Nutri 5:1–5
Khalil MI, Sulaiman SA, Boukraa L (2010) Antioxidant properties of honey and its role in preventing health disorder. Open Nutraceuticals J 3:6–16
Lambert O, Piroux M, Puyo S, Thorin C, Larhantec M, Delbac F, Pouliquen H (2012) Bees, honey and pollen as sentinels for lead environmental contamination. Environ Pollut 170:254–259
Lanjwani MF, Channa FA (2019) Minerals content in different types of local and branded honey in Sindh, Pakistan. Heliyon 5:e02042
Zeliha L, Ahmet A (2008) Determination of heavy metals in honey samples from central anatolia using plasma optical emission spectrofotometry (ICP-OES). Pol J Environ Stud 17:549
Leita L, Muhlbachova G, Cesco S, Barbattini R, Mondini C (1996) Investigation of the use of honey bees and honey bee products to assess heavy metals contamination. Environ Monit Assess 43:1–9
Mao X, Hua Y, Chen G (2014) Amino acid composition, molecular weight distribution and gel electrophoresis of walnut (Juglansregia L.) proteins and protein fractionations. Int J Mol Sci 15:2003–2014
Marchini LC, Moreti ACDCC, Otsuk IP (2007) Physicochemical composition of Apis mellifera honey samples from S~ao Paulo State, Brazil. Quim Nova 30:1653–1657
Markert BA, Breure AM, Zechmeister HG (2003) Definitions, strategies and principles for bioindication/biomonitoring of the environment. In: Trace metals and other contaminants in the environment, vol 6, pp 3–39
Marquès M, Correig E, Capdevila E, Gargallo E, González N, Nadal M, Domingo JL (2021) Essential and non-essential trace elements in milks and plant-based drinks. Biol Trace Element Res 2021:1–10
Meo SA, Al-Asiri SA, Mahesar AL, Ansari MJ (2017) Role of honey in modern medicine. Saudi J Biol Sci 24:975–978
Miller JN, Miller JC (2005) Statistics and chemometricsfor analytical chemistry. Pearson Education Ltd, Harlow, pp 213–240
Molan PC (2002) Re-introducing honey in the management of wounds and ulcers theory and practice. Ostomy Wound Manage 48:28–40
Mustatea G, Ungureanu EL, Iorga SC, Ciotea D, Popa ME (2021) Risk assessment of lead and cadmium in some food supplements available on the romanian market. Foods 10:581
Nega A, Mulugeta E, Abebaw A (2020) Physicochemical analysis and determination of the levels of some heavy metals in honey samples collected from three district area of east Gojjam zone of Amhara region, Ethiopia. J Agric Sci Food Res 11:279
Oluyemi EA, Feujit G, Oyekunle JAO, Ogunfowokan AO (2008) Seasonal variations in heavy metal concentrations in soil and some selected crops at a landfield in Nigeria. Afr J Environ Sci Technol 8:138–147
Orisakwe OE, Nduka JK, Amadi CN, Dike DO, Bed O (2012) Heavy metals health risk assessment for population via consumption of food crops and fruits in Owerri, South Eastern, Nigeria. Chem Cent J 6:77
Oroian M, Prisacaru A, Hretcanu EC, Stroe SG, Leahu A, Buculei A (2016) Heavy metals profile in honey as a potential indicator of botanical and geographical origin. Int J Food Prop 19:1825–1836
Osman KA, Al-Doghairi MA, Al-Rehiayani S, Helal MI (2007) Mineral contents and physicochemical properties of natural honey produced in Al-Qassim region, Saudi Arabia. J Food Agric Environ 5:142
Pisani A, Protano G, Riccobono F (2008) Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem 107:1553–1560
Porrini C, Sabatini AG, Girotti S, Ghini S, Medrzycki GF (2003) Honey bees and bee products as monitors of the environmental contamination. Apiacta 38:63–70
Rashed MN, Soltan ME (2004) Major and trace elements in different types of Egyptian mono-floral and non-floral bee honeys. J Food Compos Anal 17:725–735
Samarghandian S, Farkhondeh T, Samini F (2017) Honey and health: a review of recent clinical research. Pharmacogn Res 9:121
Saxena S, Gautam S, Sharma A (2010) Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem 118:391–397
Soriano A, Pallarés S, Pardo F, Vicente AB, Sanfeliu T, Bech J (2012) Deposition of heavymetals fromparticulate settleablematter in soils of Na industrialised área. J Geol Explo 113:36–44
Tomczyk M, Zaguła G, Puchalski C, Dżugan M (2020) Transfer of some toxic metals from soil to honey depending on bee habitat conditions. Acta Universitatis Cinbinesis Ser E Food Technol 24(1):1
Tuzen M (2002) Determination of some metals in honey samples for monitoring environmental pollution
Tuzen M, Sibel S, Mendi D, Soylak M (2007) Trace element levels in honeys from different regions of Turkey. Food Chem 103:325–330
Tustafa M, Soylak M (2005) Trace heavy metal levels in microwave digested honey samples from Middle Anatolia, Turkey. J Food Drug Anal 13:13
Vettorazzi A, López de Cerain A, Sanz-Serrano J, Gil AG, Azqueta A (2020) European regulatory framework and safety assessment of food-related bioactive compounds. Nutrients 12:613
von Stackelberg K, Williams PR (2021) Evolving science and practice of risk assessment. Risk Anal 41:571–583
Wang J, Kliks MM, Jun S, Li QX (2010) Residues of organochlorine pesticides in honeys from different geographic regions. Food Res Int 43:2329–2334
Wieczorek J, Pietrzak M, Pomianowski J, Wieczorek Z (2014) Honey as a source of bioactive compounds. Pol J Nat Sci 29(3):275–285
World Health Organization (2000) Environment health criteria 221: zinc. Environmental Health Criteria (221)
Yang F, Massey IY (2019) Exposure routes and health effects of heavy metals on children. Biometals 32(4):563–573
Yaqub G, Khalid M, Ikram A, Sohai A (2020) Monitoring and risk assessment due to presence of metals and pesticides residues in honey samples from the major honey producing forest belts and different brands. Food Sci Technol 40:331–335
Yılmaz H, Yavuz Ö (1999) Content of some trace metals in honey from south-eastern Anatolia. Food Chem 65:475–476
You SH, Wang SL, Pan WH, Chan WC, Fan AM, Lin P (2018) Risk assessment of methylmercury based on internal exposure and fish and seafood consumption estimates in Taiwanese children. Int J Hyg Environ Health 221:697–703
Zhong WS, Ren T, Zhao LJ (2016) Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal 24:46–55
Funding
The authors are very thankful to Higher Education Commission (HEC) of Pakistan for funding the entire study under National Research Projects for Universities (NRPU) program, Project No. 8914.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ullah, R., Jan, F.A., Gulab, H. et al. Metals Contents in Honey, Beeswax and Bees and Human Health Risk Assessment Due to Consumption of Honey: A Case Study from Selected Districts in Khyber Pakhtunkhwa, Pakistan. Arch Environ Contam Toxicol 82, 341–354 (2022). https://doi.org/10.1007/s00244-021-00910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-021-00910-7