Abstract
The study assessed the health risks associated with heavy metal ingestion and explored the use of honey bee products as a bio-indicator for heavy metal pollution. All honey bee products tested showed heavy metals, but some honey samples had concentrations exceeding permissible limits for Cd, Pb, Ni, and Cr. The mean concentrations of heavy metals (mg/kg) in the honey, propolis, bee wax, and bee pollen were Fe (1.32) > Zn (1.31) > Pb (0.46) > Ni (0.18) > Cr (0.16) > Cu (0.14) > Co (0.12) > Mn (0.05) > Cd (0.03), Fe (8) > Zn (1.13) > Mn (0.59) > Pb (0.13) > Ni (0.07) > Cu (0.06) > Co (0.05) > Cr (0.03) > Cd (0.02), Fe (1.31) > Pb (0.41) > Ni (0.407) > Zn(0.25) > Mn (0.12) > Co(0.10) > Cu (0.07) > Cr (0.05) > Cd (0.002), and Fe (2.2) > Zn (0.75) > Ni (0.25) > Pb (0.16) > Cu (0.05) > Mn (0.045) > Co (0.04) > Cr (0.01) > Cd(0.002), respectively. Similarly, the mean concentration of heavy metals (mg/kg) in the soil, flowers and pine pollen was Fe (539.08) > Zn (89.53) > Mn (66.91) > Ni (58.5) > Co (19.2) > Cr (11.42) > Pb (6.58) > Cu (5.71) > Cd (0.19), Fe (3.12) > Zn (0.95) > Mn (0.72) > Ni (0.29) > Cu (0.16) > Cr (0.14) > Pb (0.059) > Co (0.057) > Cd (0.003) and Fe (2.59) > Zn (1.75) > Mn (0.43) > Pb (0.34) > Co (0.1) > Cr (0.07) > Cu (0.06) > Cd (0.039) > Ni (0.03), respectively. The atomic absorption spectrophotometry procedure was validated through a recovery study and achieved accuracy through the limit of detection (LOD) and limit of quantification (LOQ). The mean Bio concentration factor (BCF) indicated that the transfer from soil to honey was higher than from soil to flower. The metal pollution index (MPI) of the selected indicators was in descending order: soil > honey > flowers > propolis > pine pollen > beeswax > bee pollen. The hazard quotient (HQ) and hazard index (HI) were below one, showing no chronic health risk. The carcinogenic risk (CR) of Cd, Cr, and Ni in honey for children, male and female adults for the consumers exceeds the acceptable level, making Cd, Cr, and Ni the most concerning heavy metals in honey. The study suggests that regular monitoring of heavy metal pollution is essential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decade, there has been a growing interest in bioindicator-based techniques for detecting and evaluating environmental contaminants. Honey bee products are ideal bio-indicators for atmospheric pollution bio-monitoring due to honey bee’s continuous exposure to contaminants during their foraging activity. Since honey bees forage across an area of around 7 km2, honeybee products can serve as useful markers of chemical pollution in the environment [1].
The mineral composition of honey is highly linked with its floral and geographical origin, its geochemical composition, and the pollution level at a location. The composition of the honey bee products can be considered to be indicative of a wide range of environmental factors in a region [2].
A suitable indicator is able to assess the presence of contaminants in the environment both qualitatively and quantitatively. An indicator also has the potential to identify the impact of harmful substances on biological functions [3]. Elemental content in the honeybees due to short-term spatiotemporal variations gets adjusted by the metabolism of the honeybees, which masks the variations. However, long-term environmental pollution can be assessed by honeybee products [4]. Honey bee products can be used to monitor various pollutants, depending on where the apiary or honey beehive is located. The interactions that honey bees have with the environment, especially the plants and soil, affect the quality of the honey. Bees’ bodies are covered with hairs, which pick up pollutants from the environment they interact with. These characteristics make honeybee products not only environmental indicators but also passive bioaccumulators. They are ideal for the monitoring of large areas, even in areas with poor infrastructure like mountains and villages [5].
Heavy metals, originating from natural and anthropogenic sources, are ubiquitous in the environment and are released recurrently, causing damage to biological and physical ecosystems [2]. Heavy metal contamination in plants, soil, and honey bee products is primarily caused by pollution from transport, industry, and transboundary sources [6]. The sources of heavy metals in soil are frequently industrial processes, fertilizers and pesticides used in agriculture, and air deposition from industrial and automobile emissions. Bees have the ability to gather polluted nectar, pollen, and water from these contaminated areas, which can lead to the contamination of honey and other bee products with heavy metals. Heavy metals are also present in soil and honey bee products as a result of inappropriate waste management and urban pollution [7].The location of apiary near pollution sources, such as factories, highways, mines, tailing ponds, volcanic activity and agrochemicals can results in heavy metal pollution in honey and other honeybee products [8]. The deposition of heavy metals from the source takes place in the form of dust or in gaseous form. Consumption of contaminated food with heavy metals can lead to metabolic abnormalities, headaches, nausea, vomiting, respiratory diseases, anaemia, renal, pulmonary, and heart failure [9]. The accumulative effects of metals can be threatening when only one metal such as, Cd causes cancer, badly affects liver and kidneys and also causes osteoporosis [10]. Heavy metals, prevalent in diverse environments, induce mutagenesis, teratogens, and carcinogenesis. They have been added to the list of priority pollutants by the USEPA since the 1970s [11]. This has been recognized as a global public health concern. Risk assessment assesses the potential hazards of contaminated sites and products, considering pollution sources, pathways, and receptors. Information on heavy metal concentrations in foodstuffs and dietary intake is crucial for human health risk assessment [12]. Emerging trends have led to the adaptation of advanced analytical methods for analyzing metals in honeybee products. These improved methods differ in nature of sample, sensitivity, complexity of method, type, speed of analysis, cost, and skill of the analyst. The common methods to determine heavy metals in honey include atomic absorption spectroscopy techniques like flame atomic absorption spectroscopy (FAAS), as well as inductively coupled plasma (ICP) methods such as ICP-MS, ICP-OES, and ICP-AES. Microwave plasma atomic emission spectrometry (MP-AES) and graphite furnace atomic absorption spectrometry (GFAAS) are also effectively used [13].
Azad Kashmir, a mountainous, forested region in Pakistan, is renowned for its ideal conditions for beekeeping and natural honey production. To ensure food safety, honey should be free of any unwanted contaminants. Local honey products are sold at high prices due to their nutritional and medicinal benefits; however, the quality of honey is compromised due to poor beekeeping practices and lack of contamination monitoring. Honeybee products can serve as indicators of environmental health in terms of heavy metal contamination and the possible risk associated with the ingestion of heavy metals. Previous studies showed honey bee products, including honey, propolis, bee wax, and bee pollen, as good indicators for environmental changes around the apiaries [14, 15, 16, 17, 18].
However, to our knowledge, no research has been published reporting the concentration of heavy metals in the honeybee products of Azad Kashmir, Pakistan. Therefore, the current study aimed to assess the level of heavy metals in honey bee products and also assessed the metal pollution index (MPI), health risk assessment, and bioconcentration factor in selected areas of Azad Kashmir, Pakistan. In addition, this study investigated potential connections between honey bee products and certain environmental indicators.
Methodology
Study Site
Azad Jammu and Kashmir is located between 33° and 36° latitude in the north-eastern part of Pakistan, with an area of 13297 km2. The study site is mostly hilly, with elevations ranging from 360 m in the south to 6325 m in the north, and has a wide range of climates, from a dry-subtropical climate in the southern districts (Kotli, Mirpur, and Bhimber) to a moist temperate climate in the northern districts (Neelum, Muzzafarabad, Poonch, Sudhnoti, and Haveli) [19]. It offers a great variety of landscapes, ranging from plains to mountains, with diverse flora, from seasonal crops to wild floral species, having great potential for domesticated and wild honeybees. Apis cerana (Asian honeybee), Apis dorsata (giant honeybee), and Apis florea (little bee) are the four types of native honeybees, while Apis mellifera (western honeybee) is the exotic honeybee reported to participate in the production of honey in different locations of the study site [20,21,22,23]. Locals and farmers involved in honey beekeeping in the area generate economic revenue from honey production.
Sampling
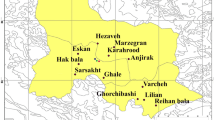
Sampling was carried out throughout the years 2020–2021. In total, 99 samples were collected for this study, of which sixty (60) were honey samples, six (6) were propolis samples, four (4) were bee pollen samples, five (5) were bee wax samples, three (3) were pine pollens, ten (10) were soil samples, and eleven (11) were flower samples. Honey bee products were collected from local beekeepers, households, and roadside vendors in glass containers and then stored in a refrigerator for further analysis. To explore the transfer of heavy metals from other mediums of the environment into honey, pine pollen, soil, and flowers were also collected from the selected sites (Fig. 1). Understanding the movement of heavy metals through various environmental matrices can enhance our comprehension of human exposure pathways. At each sampling point, about 1 kg of soil samples were taken around the apiaries within the vicinity of 7 km. Plant species that were commonly found around most of the sites were selected. Trifolium rapens, Berberis lycium, Brassica campestris, Bauhinia variegata, Rubinia pseudoacacia, Narcissus tazeta, Melia azedarach, Anethum graveolens, Coriandrum sativum, and Rosa brunonii were the representative plant species from which flower samples were collected.
Acid Digestion of Samples
Prior to acid digestion, the bee products were homogenised, flowers were air dried and crushed, soil samples were sun-dried, and then oven-dried at 80 ºC. The samples were subjected to acid digestion for further examination of heavy metals. Briefly, one (1 g) sample of honey bee products, pine pollen, soil, and crushed flowers was acid digested with aqua regia. A sample was taken in a teflon beaker and digested with 12 ml of aqua regia (12 ml HCl + 4 ml HNO3). Beakers were covered with watch glasses and heated on a hot plate for 2 h, and the temperature was gradually increased. Honeybee products, flowers, and pine pollen were heated at 120 ºC for 90 min, and soil samples were heated for 4.5 h. The suspension and solutions were allowed to cool and filtered through Whatman filter No. 42, and the volume was made up to 50 ml with deionized water. The concentration of heavy metals (Cd, Cr, Co, Cu, Pb, Ni, Zn, Fe, and Mn) was determined through a flame atomic absorption spectrophotometer (AA-7000). The instrument was calibrated with standard solutions. The operating conditions for FAAS are presented in Table 1
Quality Control and Quality Assurance
Every chemical utilized in the analysis was of the analytical grade. The results were presented as mean concentrations of triplicates along with the standard deviation. By diluting the reference solution of each metal with ionized water, a standard for all metals was created, and used to evaluate the accuracy of atomic absorption spectrophotometry. The instrument was calibrated after every ten samples.
LOD and LOQ were calculated based on the standard deviation of blanks [24].
LOD was determined by the following equation:
LOQ was determined by the following equation:
LOD values were 0.66, 0.06, 0.17, 0.09, 0.1, 0.13, 0.02, 0.02, 0.05 mg/kg for Fe, Zn, Mn, Co, Cu, Ni, Cr, Cd, and Pb. Fe, Zn, Mn, Co, Cu, Ni, Cr, Cd, and Pb had determined LOQ values of 1.95, 0.14, 0.45, 0.26, 0.31, 0.34, 0.05, 0.06, 0.14 mg/kg respectively.
A recovery was conducted in order to validate the analytical procedure. The samples were analysed like real samples after being spiked with the standards for known concentrations of heavy metals.
The recovery was calculated by the following equation:
The percent average recovery for Fe, Zn, Mn, Co, Cu, Ni, Cr, Cd, Pb were 90, 92, 90, 90, 87, 86,90, 88, 92% respectively.
Health Risk Assessment
Risk assessment evaluates the potential adverse health effects of hazardous contaminants to determine the rate of exposures through assessing carcinogenicity or non-carcinogenicity. It is conducted using scientific knowledge to understand the behaviour, exposure, dose, and contamination levels of pollutants and their toxicity. In general, it depends on the amount of pollutant in the environment, the exposure duration, and its toxicity.
Hazard Quotient (HQ)
The HQ was used to estimate the chronic toxic risks of individual metals using the following equation:
where ADD is the average daily metal intake (mg/kg/day) and RFD is the daily intake reference dose (mg/kg/day) interchangeably termed as TDI which is tolerable daily intake suggested by USEPA, RFDs for Cd, Cr, Ni, Zn, Pb, Cu, Co, Mn, and Fe are 0.001, 0.003, 0.02, 0.3, 0.04, 0.001, 0.14, and 0.7 mg/kg/day respectively, and TDI for Pb is 0.0036 mg/kg/day [25].
Average Daily Dose (ADD)
ADD was estimated by following equation:
where C is the mean heavy metal concentration in mg/kg, IR is the honey consumption rate in kg/person/day, which is 0.005, and BW is the average bodyweight (67 kg, 62.3 kg, and 15 kg for adult males, females, and children, respectively) [26]
The total chronic toxic risk of multiple metals was estimated by the Hazard Index (HI) using the following equation:
The important point to remember about the dose additive is that every component is supposed to have the same critical target, irrespective of the mechanism.
Cancer Risk (CR)
The carcinogenic effects of Pb, Cd, Cr, and Ni were estimated using the following equation.
where CR is the lifetime cancer risk known as Cancer Risk (CR), EDI is the estimated daily intake, and CSF is the Cancer Slope Factor (mg/kg/day). CSF determines the probability of developing cancer from the consumption of honey containing metals above permissible limits. The slope factors for Pb, Cd, Cr, and Ni are 0.0085, 0.5, 0.38, and 1.7, respectively [27]. Cancer risk associated with multiple carcinogenic metals is expressed as cumulative cancer risk. A CR value greater than 1 × 10–4 is considered an unacceptable risk of developing cancer. Where the range between 1 × 10–6 and 1 × 10–4 is an acceptable range for carcinogenic risk [28].
Bio-concentration Factor (BCF)
BCF is a measure of an organism's ability to accumulate specific substances, such as heavy metals, compared to its environment. It is crucial in environmental investigations, providing insights into potential risks and assisting in understanding the upward movement of pollutants in the food chain. Vigilant monitoring is essential for pollution management and ecosystem integrity.
BCF was calculated by the following equation:
where C is the concentration of trace metals in honey and flowers (mg/kg) and Cw is the concentration of trace metals in the soil [29].
Metal Pollution Index (MPI)
MPI is based on the geometric mean of the heavy metal concentration and is a common practice for different environmental matrices, including foods like honey [30]. The MPI (mg/kg) was determined using the following equation:
where Cfn is the concentration of metal in the sample.
Statistical Analysis
The basic data from the current study was evaluated through MS-Excel. ANOVA was used to assess statistical spatial differences in the heavy metals in the honey bee products. The results were significant at p < 0.05. Moreover, multivariate statistical techniques, such as principal component analysis (PCA) was applied and performed with the XLSTAT 2023 software for Microsoft Excel.. PCA was used to categorize heavy metals into natural and anthropogenic origins by minimising variables by varimax rotation, focusing mainly on factors with eigenvalues exceeding 1 [15].
Results and Discussion s
Heavy Metals in Honey Bee Products
Table 2 represents the level of studied heavy metals in the analysed honey bee products. The concentration of Cr in all the indicators followed the trend: Honey > Bee wax > propolis > bee pollen. Ni followed the trend (bee wax > bee pollen > honey > propolis), and Co followed the trend: Honey > bee wax > Propolis > Pollen. The acquired data showed that Fe and Zn were the most abundant heavy metals in the honey bee products, except the bee wax, which also showed a high concentration of Pb. Zinc is a vital mineral essential for maintaining a healthy immune system, protein production, and other bodily processes. However, high zinc concentrations can be toxic, causing plants to exhibit symptoms similar to those found in other heavy metal toxicities. The results of heavy metals in the current study were compared with research studies conducted in other parts of the world (Table 3). It has been found that the Cr concentration (0.16 ± 0.003 mg/kg) in the honey of the study area was higher as compared to that reported in the honey of Algeria (0.05 mg/kg), while the concentration of Cr was lower than other studies conducted in Romania (1.16 mg/kg), Nigeria (5.40–6.67 mg/kg), and Ethiopia (6.66 mg/kg). The Ni concentration in the honey was 0.18 ± 0.03 mg/kg, which was higher than reported in Romania (0.07 mg/kg). Zn (1.31 ± 0.9), Cu (0.14 ± 0.05), and Mn (0.05 ± 0.014) concentrations were less than reported in Romania and Algeria. Fe (1.32 mg/kg) is less than the reported concentration in Algeria. The heavy metal ranges observed in bee wax were in line with the study in Egypt. The Cd and Pb concentrations in beeswax in the current study were lower than those in Vietnam. Ni showed less concentration in bee wax as compared to Pakistan and Croatia. Zn, Cu, Pb, and Cd in propolis exhibited less concentration than reported in Poland. The Fe concentration observed in propolis was higher, while the Cd and Mn concentrations were lower than the reported concentrations in Turkey. Cd and Pb in bee pollen were less than compared to the concentrations observed in Vietnam. Cr, Fe, Zn, Cu, and Mn in bee pollen exhibited less concentration as compared to Turkey (Table 3).
The heavy metal concentrations in the analysed honey samples were compared with the maximum permissible limits (MPLs) of 0.2 mg/kg for Cd, 1.5 mg/kg for Cr, 10 mg/kg for Zn, 5.5 mg/kg for Mn, 3 mg/kg for Cu, 1 mg/kg for Pb, 0.5 mg/kg for Ni, and 15 mg/kg for Fe set by FAO/WHO (1993) [42] The concentration of Cd, Cr, Pb, and Ni was exceeding the limits. The exceeding Cd, Cr, Pb, and Ni concentrations might be attributed to the location of the apiary near a roadside or traffic, exposure to fertilizers, the use of animal dung to smoke the hive for honey extraction, and the use of galvanized containers. High exposure to Cr can cause nose irritation, ulcers, and breathing problems, while long-term exposure can damage the kidney, liver, and circulatory systems, leading to nerve diseases and skin irritation. Ni in trace amounts is beneficial to humans for activating biological systems, while higher amounts can cause enzyme inactivation and nasal cavity cancer. A study in Ethiopia also detected heavy metals like Pb, Cr, Cd, and Ni in the honey and linked the addition of these heavy metals from storage containers, poor processing and storage, and the nearness of honey bee hives to highways, metal workshops, and the use of fertilisers in the crops [34]
There may be natural and anthropogenic sources of Ni in honeybee products, including the weathering of rocks, wind-blown dust, and stainless steel utensils used for handling and storage [43]. Honeybee combs' age significantly impacts their Ni concentrations, ranging from 0.8 to 2.5 mg/kg from one to five-year-olds [35]. The nectar and pollen from hyperaccumulative plants can contribute to the high concentration of Ni [44].
It is also evident from the results that the presence of heavy metals in all honey bee products was detected. However, the concentration of metals in honey was relatively higher than other products, indicating the transfer of metals to end products as well as contamination during handling. The nutrients and elements found in honey bee products play a significant role in biochemical processes and environmental pollution assessment [45]
Heavy metals in honey cause toxicity, risking human health. The accumulation of heavy metals in honey bee products and environmental compartments is an essential contributor to environmental health [46]. Some metals are naturally present in the environment, e.g., Cu, from volcanic eruptions, forest fires, and windblown dust. Co occurs naturally through the breakdown of organic matter and the weathering of local minerals into soil particles [47].
At low concentrations some metals are micronutrients but they may cause toxicity at higher concentration. Cu and Zn are essential elements for human health but high doses of Cu can cause liver and kidney damage, anemia and gastrointestinal problems. Likewise, high levels of Zn can damage pancreas and affect protein metabolism negatively. On the other hand, Heavy metals like Cd and Pb cause toxicity at a very low concentration leading to many diseases including high risk of cancer [48, 49].
Results of One-way ANOVA are displayed in the Table 4. Significant (p < 0.05) spatial variations in the level of heavy metals in the honey bee products were also exhibited except in case of Fe, Zn and Mn. Similarly, variations among the honey bee products and other components like soil, pine pollen and flowers were also statistically significant except Ni (soil), Fe (pollen), Zn (pollen) and Mn (pollen). These variations might be attributed to the different geography, in different districts as well as plant sources. For example the contribution of hyper accumulative metal flora can contribute to the pollution of bee products [50]. Furthermore, the types of honeybees, location of apiary, forage behavior and handling techniques are also determining factors in the variation of pollution [51, 52].
Heavy Metals in Soil, Pine Pollen and Flowers
There is a close link between the environmental conditions of the area and the quality of honeybee products. Table 5 shows the mean concentration of selected heavy metals in the analysed pine pollen, flowers, and soil. The trend of variations of heavy metals in soil was Fe > Zn > Mn > Ni > Co > Cr > Pb > Cu > Cd, and pine pollen and flowers were Fe > Zn > Mn > Pb> Co > Cr > Cu > Cd > Ni and Fe > Zn > Mn > Ni > Cu > Cr > Pb > Co > Cd, respectively. Results have indicated that Fe, Zn, and Mn are the most dominant heavy metals, like in honey bee products. Cd was the least abundant metal in soil and flowers, while Ni was the least abundant heavy metal in the pine pollen. The study found that Cu and Pb concentrations remained consistent in all compartments, with Zn concentrations decreasing from soil to flowers. Soil exhibited the highest concentration of Co, followed by pine pollen and then flowers (Table 5).
The analyzed plant species showed variations in the accumulation of heavy metals (Fig. 2). For instance, A. graveolens has the highest quantities of Cr, Cd, and Pb. The species B. variegata displayed the lowest levels of Cd, Cr, and Fe. M. azedarach and N. tazeta showed the highest Ni concentrations. Moreover, the Cu concentration was best represented by N. tazeta while C. sativum exhibited the highest concentration of Zn. B. lyceum had the highest concentration of Mn and Co, whereas B. campestris had the highest concentration of Fe.
The results clearly show that soil contains more of all metals than pine pollen and flowers. It is evident that, the mean Ni concentration (58.5 mg/kg) was higher than the allowable limit. The weathering of rocks and inappropriate waste disposal are two sources of Ni pollution. As a result, there may be a transfer in the food chain that results in life-threatening conditions such as illnesses, allergies, and cancer [53]. High metal concentrations near apiaries can cause toxicity in honeybee products, passing through the food chain and indicating environmental health risks associated with heavy metal ingestion [16]. In the current study, soil accumulated the most heavy metals as compared to pine pollen and flowers, which makes it a sink for pollutants. Soil from different geographical regions can impact the heavy metals in honeybee products. Although the main sources of heavy metals in the soil are natural rock materials present in the composition of the soil, anthropogenic activities can also affect the level of heavy metals [16]. Changes in the quality of the soil in a certain area result in the contamination of other mediums in the environment. Even non-hyperaccumulating plants can passively absorb heavy metals from the soil and absorb them into their nectar. Since nectar is primarily valued for its sugar content, even a small amount of toxicity can have an impact on honeybee behavior [54].
Metal Pollution Index (MPI)
MPI is an authentic tool to differentiate the combined influence of metals geographically. This indicates the cumulative impacts of different sources of metals in a region [55]. The MPI of honey showed that district Sudhnoti showed the highest MPI (0.36 mg/kg), whereas Kotli showed the lowest MPI (0.03 mg/kg) (Fig. 3). It was also observed that among all the selected indicators, soil indicated the highest MPI, followed by honey, which is 9.25 and 1.51 mg/kg, respectively (Fig. 4). Soil plays an important role in food quality and safety because it affects the composition of food at the root of the food chain [56]. The differences between the sites might be due to the different topography and respective agricultural and farming practices. It is evident that the mountainous northern districts displayed a higher MPI in comparison to the areas in the south. The soil of Azad Jammu and Kashmir is divided into three categories geographically, including the soils of the mountain plateau, mountain slope, and inter-mountainous valley, which differ on the basis of organic matter and agricultural practices [57] This difference in the formation of soil impacts the metal pollution index in different districts. MPI also depends on the climatic conditions and the types of honeybees in the area. Soil indicated the highest MPI, which makes it a possible source of heavy metal transfer in other compartments and the food chain as well.
Human Health Risk Assessment
Hazard Quotient (HQ)
The result of the health risk assessment is displayed in Table 6. The study computed the ADD, THQ, and HI of heavy metals for males, females, and children in selected divisions of Azad Kashmir through honey consumption. As illustrated in Table 7, the highest ADD was recorded for children depending upon their body weight less than the adult male and female. The daily intake of heavy metals in honey was compared with the Rfd or provisional tolerable weekly intake (PTWI) for Pb as set by the US EPA to assess the potential health risk [58]. This ratio between two doses is called the health quotient (HQ). There is no chance of chronic toxic effects if the HQ is less than one. HI is the combined or cumulative chronic toxic effects of multiple metals in honey. The mean HQ values for heavy metals occupied the following decreasing order for non-carcinogenic risk: Pb > Co > Cr > Cd > Ni > Zn > Cu > Fe > Mn. The total health risk index values varied from 0.01 to 0.86 for children, from 0.001 to 0.21 for female adults, and from 0.001 to 0.19 for male adults, which were within the safe limits. Results showed that HQ and HI were below one, thus indicating no chronic toxic effects on health from the consumption of honey. A previous study in Pakistan also found that HI and HQ for honey consumption were below 1, showing no chronic toxic health effects to consumers [26]
Assessment of Cancer Risk (CR)
CR values were calculated for male, female adults and children through the consumption of Cd, Cr, Ni and Pb. (Table 8). CR values between 1 × 10–6—1 × 10–4 are considered acceptable, those below than 1 × 10–6 are tolerable and more than 1 × 10–4 are intolerable [26]. The CR values for selected metals decreased as Ni > Cr > Cd > Pb. In children the maximum CR values for Cd, Cr, and Ni were intolerable posing cancer risk. The mean CR value for children was within acceptable range. Maximum CR value for Cd, Cr and Ni were intolerable for children. Maximum CR value of Ni was also intolerable for male and female adults. The ∑CR values for adult male, female and children varied as 6.55 × 10–7-2.90 × 10–4, 7.04 × 10–7-3.12 × 10−4and 2.93 × 10–6-1.30 × 10–3, respectively. At estimated daily intake, children are at risk of cancer more than the male and female adults. Ni and Cr are two heavy metals responsible for higher CR. Ni and Cr in honey can be attributed to the mishandling during processing including use of steel instruments and corrosion [59, 60]. Our findings are in agreement to the previous study in Nigeria which reported carcinogenic risk from Pb and Cr due to consumption of honey [11]
Bioconcentration Factor (BCF)
The results of BCF are presented in Tables 9 and 10 revealing the sequential transfer of the studied heavy metals from soil to honey and soil to flower of plants. The trend of transfer for Bagh was in the sequence; Cd > Pb > Cu > Zn > Co > Cr > Ni > Fe > Mn, while for Haveli, Poonch, Sudhnoti, Mirpur it was: Cd > Pb > Cr > Zn > Cu > Co > Ni > Fe > Mn;Pb > Cu > Cr > Zn > Cd > Co > Ni > Fe > Mn;Cu > Pb > Ni > Co > Fe > Cd > Zn > Cr > Mn; Cd > Cu > Pb > Co > Zn > Fe > Cr > Ni > Mn respectively. Overall, among all heavy metals Cd was recorded to have highest BCF in three sites followed by Pb and Cu whereas, Mn had the least BCF values in all sites. The BCF from soil to flowers was lower than the soil to honey. The transfer of Cd, Pb and Cu in the study area from soil to honey showed health concern. In a study carried out in Romania, the highest BCF values were reported for Cd in linden honey was 0.023 while polyfloral honey had the highest BCF value of Pb which was less than the recorded highest values of Cd and Pb in the present study [12]. Plants exhibited variations in the BCF values for instance, A. graveolens showed the highest BCF for Cd (0.07), N. tazeta indicated the highest BCF for Ni, B. campestris indicated highest BCF for Fe (0.01), T. officinale and R. pseudoacacia indicated the highest BCF for Co whereas,the highest BCF for Zn and Mn was recorded in C. sativum and Berberis lyceum, respectively. Overall, Cd had highest bioaccumulation rate as compared to other heavy metals in all selected flowers. This can attributed to the accumulation of Cd in plants from both soil and atmospheric deposition. However, the accumulation dynamics in plants are influenced by the type of metal present in the environment, the amount present in the organ, and the type of plant. Soil is also one of the vital compartment for the heavy metals movement. The migration of heavy metals in the soil–plant-honey bee food chain is greatly influenced by soil properties. The findings of our study are in agreement to the study conducted in North-eastern Bulgaria, reported that the investigated heavy metal content in honey, plant and soil depend on geography of the area, soil composition and different cultivars of melliferous flora [61]. This study also corroborates the findings of research in Romania, who reported highest BCF values for Cr and linked this Cr contamination with phytosanitary activities carried out in the agricultural land near beehives [8].
Principal Component Analysis (PCA)
PCA is an effective tool for identifying patterns in a huge dataset of correlated variables. In the current study PCA was applied on whole dataset (Table 11). The PCA resulted in many components, only those components were considered having Eigen values >1. Analyzing data from PCA it can be seen that three components were resulted responsible for 86.23% of variance in the data. PC1 was responsible for 48.74% variations and was dominated by the positive loading of Ni, Co, Cd, Fe, Zn and Cu whereas, the PC2 was dominated by loading of Cr and Pb and PC3 was dominated by the loading of Mn. It is evident from the results of the PCA analysis that elements in the PCI were the most important in influencing the quality of the honey which might be due to natural as well as anthropogenic activities carried out in the study area. For instance, smoking of the beehive for the extraction of honey, corrosion of steel containers due to acidic nature of honey after prolonged storage, rock formation, galvanized steel used in the tools, wall paints, batteries and leaded gasoline are the sources for these particular heavy metals in the study area [59]. In the second component the positive loading of Pb and Cr show their anthropogenic source. The sources of Pb and Cr might be agricultural activities and cross boarder ammunition is also source of Pb. As the study area is near to the line of control (LOC), the firing from the other side frequently take place. In the PC3, Mn might be related to its accumulation in plants from the environment.
Conclusion
This study attempts to assess the quality of the environment by using honey bee products as an indicator of heavy metal pollution and also investigates the health risks associated with the ingestion of heavy metals through honey. The result of the study showed that heavy metals' existence was reported in all analyzed honey bee products. The concentration of Cd, Cr, Pb, and Ni exceeded the maximum permissible limits in honey samples. Fe and Zn were common and abundant for their essential nature in all the products. Soil showed maximum concentration of all heavy metals however the detected concentration was below the permissible limits. The honey samples and the analyzed indicators showed spatial variations in MPI. Similarly, BCF showed variations in the metal accumulation tendencies in the soil-honey-flower food chain. Health risk analysis showed that that HQ and HI values were below the recommended limit (< 1) however carcinogenic risk was observed due to Ni, Cd, and Cr in honey to population. It is concluded that honey bee products can be used as reliable indicators of heavy metal pollution in the region and carcinogenic risk was associated with the consumption of honey by population. For more realistic cancer risk associated with heavy metals, Honey samples should also be assessed for Arsenic (As) where there are potential natural and anthropogenic sources due to its toxicity. Regular monitoring of heavy metals in honey is recommended to ensure the quality of honey for the health and safety of consumers.
Data Availability
Data will be available on request.
References
Conti ME, Astolfi ML, Mele G, Ristorini M, Vitiello G, Massimi L, Finoia MG (2022) Performance of bees and beehive products as indicators of elemental tracers of atmospheric pollution in sites of the Rome province (Italy). Ecol Indic 140:109061. https://doi.org/10.1016/j.ecolind.2022.109061
Girotti S, Ghini S, Ferri E, Bolelli L, Colombo R, Serra G, Sangiorgi S (2020) Bioindicators and biomonitoring: honeybees and hive products as pollution impact assessment tools for the Mediterranean area. EuroMed J Envir Integr 5(3):1–16. https://doi.org/10.1007/s41207-020-00204-9
Chropeňová M, Gregušková EK, Karásková P, Přibylová P, Kukučka P, Baráková D, Čupr P (2016) Pine needles and pollen grains of Pinus mugo Turra–A biomonitoring tool in high mountain habitats identifying environmental contamination. Ecol Indic 66:132–142. https://doi.org/10.1016/j.ecolind.2016.01.004
Sager M (2017) The honey as a bioindicator of the environment. Ecol Chem Engr 24(4):583. https://doi.org/10.1515/eces-2017-0038
Herrero-Latorre C, Barciela-García J, García-Martín S, Peña-Crecente RM (2017) The use of honeybees and honey as environmental bioindicators for metals and radionuclides: a review. Environ Rev 25(4):463–480. https://doi.org/10.1139/er-2017-0029
Gliga O (2016) The content of heavy metals in the bees’ body depending on location area of hives. Sci Pap Anim Sci Ser 65:169–175
Tomczyk M, Zaguła G, Puchalski C, Dżugan M (2020) Transfer of some toxic metals from soil to honey depending on bee habitat conditions. Acta Universitatis Cibiniensis Series E: Food Technology 24(1):49–59
Bora FD, Babeș AC, Călugăr A, Jitea MI, Hoble A, Filimon RV, Bunea CI (2023) Unravelling heavy metal dynamics in soil and honey: A case study from Maramureș Region. Romania Foods 12(19):3577. https://doi.org/10.3390/foods12193577
Ebubekir İZOL, Enes KAYA, Karahan D (2021) Investigation of some metals in honey samples produced in Different Regions of Turkey’s Bingöl province by ICP-MS. Mellifera 21(1):1–17
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Enviro Res Public Health 17(11):3782
Orisakwe OE, Ozoani HA, Nwaogazie IL, Ezejiofor AN (2019) Probabilistic health risk assessment of heavy metals in honey, Manihot esculenta, and Vernonia amygdalina consumed in Enugu State, Nigeria. Enviro Monit Assess 191:1–14. https://doi.org/10.1007/s10661-019-7549-2
Mititelu M, Udeanu DI, Docea AO, Tsatsakis A, Calina D, Arsene AL, Ghica M (2023) New method for risk assessment in environmental health: The paradigm of heavy metals in honey. Enviro Res 236:115194. https://doi.org/10.1016/j.envres.2022.115194
Bora FD, Andrecan AF, Călugăr A, Bunea CI, Popescu M, Petrescu-Mag IV, Bunea A (2024) Comprehensive elemental profiling of Romanian Honey: Exploring regional variance, honey types, and analyzed metals for sustainable apicultural and environmental practices. Foods 13(8):1253
Bayir H, Aygun, A (2022) Heavy metal in honey bees, honey, and pollen produced in rural and urban areas of Konya province in Turkey. Enviro Sci Pollut Res 1–10. https://doi.org/10.1007/s11356-022-21017-z
Ćirić J, Spirić D, Baltić T, Lazić IB, Trbović D, Parunović N, Đorđević V (2021) Honey bees and their products as indicators of environmental element deposition. BiolTrace Elem Res 199(6):2312–2319. https://doi.org/10.1007/s12011-020-02321-6
Murashova EA, Tunikov GM, Nefedova SA, Karelina OA, Byshova NG, Serebryakova OV (2020) Major factors determining accumulation of toxic elements by bees and honey products. Int Trans J Eng Mgmt App Sci Tech 11(3):11A03N. https://doi.org/10.14456/ITJEMAST.2020.54
Awan A (2017) The Production of Propolis, Bee Bread, Bees wax and Honey of Stingless Bee (Trigona Spp) on Various Hive’s Mediums in West District of Seram. In Proceeding International Seminar on Education (Vol. 1)
González-Martín MI, Escuredo O, Revilla I, Vivar-Quintana AM, Coello MC, Palacios Riocerezo C, Wells Moncada G (2015) Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sens 15(11):27854–27868. https://doi.org/10.3390/s151127854
Ullah R, Asghar R, Tanveer Z, Aziz S, Babar M, Ali S, Mahmood A (2018) Determination of heavy metals levels in water of River Jhelum in the State of Azad Jammu and Kashmir, Pakistan. Int Biosci 12:267–274. https://doi.org/10.12692/ijb/12.2.267-274
Faiz AH, Faiz LZ, Khan FM (2018) Biodiversity of insects in some areas of District Rawalakot, Azad Jammu and Kashmir (Pakistan). J Biores Mgmt 5(2):8–15
Niaz K, Faiz AUH, Ahmad A, Shabaz M (2019) Biodiversity of Invertebrates in some Selected Areas of District Bagh, Azad Jammu and Kashmir, Pakistan. Proc Pakistan Congress Zool 39:00–00
Hussain M, Liaqat H, Malik MF, Aftab K, Batool M, Iqbal R, Liaqat S (2023) Distribution Patterns of Insect Pollinator Assemblages at Deva Vatala National Park, Bhimber, Azad Jammu and Kashmir. https://doi.org/10.17582/journal.pjz/20221004171007
Bashir S, Malik MF, Hussain M (2023) Spatiotemporal occurrence of beehives of genus Apis in Northern Punjab and Azad Jammu and Kashmir. Pakistan Kuwait J Sci 50(2):40–46. https://doi.org/10.1016/j.kjs.2023.02.007
Little T (2015) Method validation essentials, limit of blank, limit of detection, and limit of quantitation. BioPharm Int 28(4)
Ru QM, Feng Q, He JZ (2013) Risk assessment of heavy metals in honey consumed in Zhejiang province, southeastern China. Food Chem Toxicol 53:256–262. https://doi.org/10.1016/j.fct.2012.12.015
Ullah R, Jan FA, Gulab H, Saleem S, Ullah N, Wajidullah, (2022) Metals contents in honey, beeswax and bees and human health risk assessment due to consumption of honey: a case study from selected districts in Khyber Pakhtunkhwa. Pakistan Arch Enviro Contam Toxicol 82(3):341–354. https://doi.org/10.1007/s00244-021-00910-7
EPA U (2018) Regional screening levels (RSLs)-generic tables. Last updated september
Scivicco M, Squillante J, Velotto S, Esposito F, Cirillo T, Severino L (2022) Dietary exposure to heavy metals through polyfloral honey from Campania region (Italy). J Food Compos Anal 114:104748
Czipa N, Diósi G, Phillipz C, Kovács B (2017) Examination of honeys and flowers as soil element indicators. Environ Monit Assess 189:1–12. https://doi.org/10.1007/s10661-017-6121-1
Zergui A, Boudalia S, Joseph ML (2023) Heavy metals in honey and poultry eggs as indicators of environmental pollution and potential risks to human health. J Food Compos Anal 119:105255. https://doi.org/10.1016/j.jfca.2023.105255
Bereksi-Reguig D, Bouchentouf S, Allali H, Adamczuk A, Kowalska G, Kowalski R (2022) Trace elements and heavy metal contents in West Algerian Natural Honey. J Anal Methods Chem. https://doi.org/10.1155/2022/7890856
Voica C, Iordache AM, Ionete RE (2020) Multielemental characterization of honey using inductively coupled plasma mass spectrometry fused with chemometrics. J Mass Spectrom 55(7):e4512. https://doi.org/10.1002/jms.4512
Ernest E, Onyeka O, Ozuah AC, Benard N (2018) Comparative assessment of the heavy and trace metal levels in honey produced within Nsukka and Enugu Metropolis. Food Public Health 8(2):42–46. https://doi.org/10.5923/j.fph.20180802.02
Adugna E, Hymete A, Birhanu G, Ashenef A (2020) Determination of some heavy metals in honey from different regions of Ethiopia. Cogent Food Agri 6(1):1764182. https://doi.org/10.1080/23311932.2020.1764182
Hassona NM, El-Wahed AAA (2023) Heavy metal concentrations of beeswax (Apis mellifera L.) at different ages. Bull Environ Contam Toxicol 111(3):26. https://doi.org/10.1007/s00128-023-03779-5
Ngat TT, Lam TX, Minh HG, Lien NTP (2020) Honeybee products as potential bioindicators of heavy metals contamination from Northern Vietnam. Vietnam J Biotechnol 18(2):373–384. https://doi.org/10.15625/1811-4989/18/2/15086
Gajger IT, Kosanović M, Oreščanin V, Kos S, Bilandžić N (2019) Mineral content in honeybees wax combs as a measurement of the impact of environmental factors. Bull Environ Contam Toxicol 103:697–703. https://doi.org/10.1007/s00128-019-02713-y
Roman A, Madras-Majewska B, Popiela-Pleban E (2011) Comparative study of selected toxic elements in propolis and honey. J Apic Sci 55(2):97–106
Şahinler N, Gül A, Şahinler S (2017) Heavy metals and trace elements content of different propolis types. Eur Int J Sci Tech 6(3):21–25
Erdoğan A, Şeker ME, Kahraman SD (2023) Evaluation of environmental and nutritional aspects of bee pollen samples collected from East Black Sea region, Turkey, via elemental analysis by ICP-MS. Biol Trace Elem Res 201(3):1488–1502. https://doi.org/10.1007/s12011-022-03217-3
Gonzalez-Martin MI, Revilla I, Betances-Salcedo EV, Vivar-Quintana AM (2018) Pesticide residues and heavy metals in commercially processed propolis. Microchem J 143:423–429. https://doi.org/10.1016/j.microc.2018.08.040
Joint FAO (1993) Codex Alimentarius: sugars, cocoa products and chocolate and miscellaneous products. In Codex Alimentarius: sugars, cocoa products and chocolate and miscellaneous products. FAO
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020) Nickel: Human health and environmental toxicology. Int j Environ Res Public Health 17(3):679. https://doi.org/10.3390/ijerph17030679
Salihaj M, Bani A (2017) The nickel content in honey derived from serpentine and non-serpentine areas of Kosovo. Albanian J Agric Sci 557–563
Spirić D, Ćirić J, Đorđević V, Nikolić D, Janković S, Nikolić A, Teodorović V (2019) Toxic and essential element concentrations in different honey types. Int J Environ Anal Chem 99(5):474–485. https://doi.org/10.1080/03067319.2019.1593972
Zafeiraki E, Kasiotis KM, Nisianakis P, Manea-Karga E, Machera K (2022) Occurrence and human health risk assessment of mineral elements and pesticides residues in bee pollen. Food Chem Toxicol 161:112826. https://doi.org/10.1016/j.fct.2022.112826
De Matos BM, Fernandes ACC, Alves RSC, Alves DA, Junior FB, Batista BL, Carneiro MFH (2021) Effects of native forest and human-modified land covers on the accumulation of toxic metals and metalloids in the tropical bee Tetragonisca angustula. Ecotoxicol Environ Saf 215:112147. https://doi.org/10.1016/j.ecoenv.2021.112147
Yan X, Zhang F, Zeng C, Zhang M, Devkota LP, Yao T (2012) Relationship between heavy metal concentrations in soils and grasses of roadside farmland in Nepal. Int J Enviro Res Public Health 9(9):3209–3226. https://doi.org/10.3390/ijerph9093209
James VR, Panchal HJ, Shah AP (2023) Estimation of selected elemental impurities by inductively coupled plasma-mass spectroscopy (ICP-MS) in commercial and fresh fruit juices. Environ Monit Assess 195(11):1390
Bărbulescu A, Barbeș L, Dumitriu CŞ (2022) Impact of soil pollution on Melliferous plants. Toxics 10(5):239. https://doi.org/10.3390/toxics10050239
Beshaw T, Demssie K, Leka I (2022) Levels and health risk assessment of trace metals in honey from different districts of Bench Sheko Zone, Southwest Ethiopia. Heliyon 8(9). https://doi.org/10.1016/j.heliyon.2022.e10535
Manzoor M, Shah GN, Mathivanan V, Mir GM, Dar SA (2013) Estimation of mineral contents of Apis Cerana Indica, Apis Dorsata and Apis Mellifera honey from plains, Hills and Western Ghats of Tamil Nadu and Jammu & Kashmir. Int J Appl Nat Sci 2(3):45–52
El-Naggar A, Ahmed N, Mosa A, Niazi NK, Yousaf B, Sharma A, Chang SX (2021) Nickel in soil and water: Sources, biogeochemistry, and remediation using biochar. J Hazad Mater 419:126421. https://doi.org/10.1016/j.jhazmat.2021.126421
Xun E, Zhang Y, Zhao J, Guo J (2018) Heavy metals in nectar modify behaviors of pollinators and nectar robbers: Consequences for plant fitness. Environ Pollut 242:1166–1175. https://doi.org/10.1016/j.envpol.2018.07.128
Giri S, Singh AK (2019) Heavy metals in eggs and chicken and the associated human health risk assessment in the mining areas of Singhbhum copper belt. India Arch Environ Occup Health 74(4):161–170. https://doi.org/10.1080/19338244.2017.1407284
Tóth G, Hermann T, Da Silva MR, Montanarella LJEI (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Env Int 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
Abbasi MK, Rasool G (2005) Effects of different land-use types on soil quality in the hilly area of Rawalakot Azad Jammu and Kashmir. Acta agric Scand B Soil plant sci 55(3):221–228. https://doi.org/10.1080/09064710510008720
Winiarska-Mieczan A, Wargocka B, Jachimowicz K, Baranowska-Wójcik E, Kwiatkowska K, Kwiecień M (2021) Evaluation of consumer safety of Polish honey—the content of Cd and Pb in multifloral, monofloral and honeydew honeys. Biol Trace Elem Res 199:4370–4383. https://doi.org/10.1007/s12011-020-02535-8
Islam MN, Khalil MI, Islam MA, Gan SH (2014) Toxic compounds in honey. J Appl Toxicol 34(7):733–742. https://doi.org/10.1002/jat.2952
Tutun H, Kahraman HA, Aluc Y, Avci T, Ekici H (2019) Investigation of some metals in honey samples from West Mediterranean region of Turkey. Vet Res Forum 10(3):181–186
Atanasov AZ, Hristakov IS, Kuncheva GS, Koszel M, Dochev VY (2023) Assessment of heavy metals in soil, oilseed rape (Brassica napus L.) and honey. Plant Soil Env 69(8). https://doi.org/10.17221/265/2023-PSE
Funding
The article's author(s) stated that there is no funding linked to the work mentioned.
Author information
Authors and Affiliations
Contributions
Study design and conceptualisation (AM, SK, MJ) laboratory work (AM), statistical analysis (K) and Roles/Writing—original draft; and Writing—review & editing ( AM, K). The authors have all contributed to this paper and have given their approval for the published version.
Corresponding author
Ethics declarations
Competing Interest
The authors report there are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mushtaq, A., Khalid, S., Noor, M.J. et al. Honey Bee Products as Bio Indicator of Heavy Metals Pollution and Health Risk Assessment Through the Consumption of Multifloral Honey Collected in Azad Kashmir, Pakistan. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04313-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04313-2