Abstract
Occurrence of traditional (PBDEs) and novel (HBB, PBEB, DBDPE) brominated flame retardants, as well as the natural compounds of MeO-PBDEs, were studied in a shellfish species (Hexaplex trunculus) sampled from Bizerte Lagoon. PBDE and MeO-PBDE mean concentrations in murex soft tissues were 187 and 264 ng g−1 lw respectively. The alternative flame retardants were not identified. The sum of PBDE and MeO-PBDE levels recorded in murex from the investigated aquatic ecosystem were comparable or a relatively lower than those reported for other organisms from other regions across the world. The amount of PBDE and MeO-PBDE concentrations from the Bizerte Lagoon recorded in murex were comparable or a relatively lower than those recorded from other areas across the world for other species. There is not a danger to the population health with regard to PBDE intakes associated with the consumption of murex in Bizerte city. We believe that this is the first study of the analysis of these pollutants in marine gastropod mollusks from Tunisian aquatic areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Brominated flame retardants (BFRs) have been highlighted for their potential environmental risks during these two past decades (Zhen et al. 2016). Among them, polybromodiphenyl ethers (PBDEs) are the most known used compounds. PBDEs are typically produced at three different levels of bromination: Penta-BDE, Octa-BDE, and Deca-BDE (Aznar-Alemany et al. 2019). PBDEs have been identified in several environmental samples (Aznar-Alemany et al. 2017), such as air (Aznar-Alemany et al. 2017), sediment (Aznar-Alemany et al. 2017), water (Aznar-Alemany et al. 2017), and sludge (Aznar-Alemany et al. 2017), in addition to biological matrices (Aznar-Alemany et al. 2018), such as seafood (Barón et al. 2013; Zaccaroni et al. 2018; Aznar-Alemany et al. 2017), bird eggs (Barón et al. 2014b), and human milk (Hassine et al. 2012; Aznar-Alemany et al. 2017). The accumulation of PBDEs in various environmental matrices and biota has been documented after decades of research (McGrath et al. 2017), whereas harmful effects, such as endocrine disturbance and developmental neurotoxicity, have been well proven (McGrath et al. 2017). Due to their persistence, bioaccumulation, long-distance transport, and negative health impacts (Tao et al. 2019), the three commercial PBDE mixtures were classified as persistent organic pollutants (POPs) in the Stockholm Convention (Liu et al. 2018). A new generation of BFRs have been emerged as an alternative to the banned compounds and were labeled new, emerging, or novel BFRs (NBFRs) (McGrath et al. 2017).
Decabromodiphenylethane (DBDPE), bis(2,4,6-tribromophenoxy)ethane (BTBPE), 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) are among the most known NBFRs. DBDPE replaced Deca-BDE formulas, BTBPE replaced Octa-BDE and bis(2-ethylhexyl) tetrabromophthalate, and EH-TBB replaced Penta-BDE mixtures (McGrath et al. 2017). Hexabromobenzene (HBB), 2,3,4,5,6-pentabromotoluene (PBT), and 2,3,4,5,6-pentabromoethylbenzene (PBEB) are used for the prevention of flame propagation instead of the banned BFRs in a various polymers (McGrath et al. 2017). HBB is used in the following fields: plastics, textiles, woods, paper, electrical, and manufactured products (Covaci et al. 2011). It is obtained from deca-BDEs and other PBDEs heat degradation (Trabalón et al. 2017). PBEB is a flame retardant additive, particularly used in polyester thermoset resins (circuit boards, textiles, adhesives, wire and cable coatings, polyurethane foam) (Ezechiáš et al. 2014). DBDPE is used in the same way as Deca-BDE, i.e., an additive to various polymeric materials, such as high-impact polystyrene (HIPS), acrylonitrile butadiene styrene (ABS), polypropylene, and textiles, such as cotton and polyester (Lee et al. 2013).
Like PBDEs, NBFRs are released from various origins, such as manufacturing, waste incineration, recycling facilities, and other overall industrial procedures via atmospheric emissions to the environment (McGrath et al. 2017). Their physicochemical properties, pollution, and toxicity patterns are generally comparable to those of PBDEs (McGrath et al. 2017). The detection of these new BFRs have been reported in different matrices (Trabalón et al. 2017; Li et al. 2019). The knowledge on their possible toxicities and environmental fate is still very limited; however, some NBFRs have already been shown to be endocrine disruptive (Li et al. 2019).
The presence of naturally produced compounds, such as methoxylated PBDEs (MeO-PBDEs), also has been taken into account due to their reported higher toxic potential compared with the parent PBDEs (Weijs et al. 2009; Ben Ameur et al. 2013; El Megdiche et al. 2017; Choo et al. 2018).
Most remarkably, in some marine organisms, such as bivalves, salmon, and whales, the structural analogs of PBDEs have wide geographic apportionments and slightly higher levels relative to PBDEs parent; they can disturb the homeostasis of the thyroid hormone, are toxic to nerve tissue, disturb oxidative phosphorylation, and alter the synthesis of estradiol (Choo et al. 2018). These substances are not known to have identified anthropogenic origins (El Megdiche et al. 2017). Biogenic production through the metabolism of PBDEs or natural synthesis through a biobromination process has been proposed as their origin (Kierkegaard et al. 2004; El Megdiche et al. 2017). MeO-PBDEs are synthesized naturally by sponges or algae in the marine ecosystem (Ben Ameur et al. 2011; El Megdiche et al. 2017). The primary sources of human exposure to POPs are skin uptake, inhalation, and ingestion of polluted food (El Megdiche et al. 2017).
Among all aliments, seafood is one of the primary sources of pollutants, although fish goods represent only approximately 10% or less of a standard diet (El Megdiche et al. 2017). Gastropod mollusks are mostly used as sentinel organisms of organic contamination in aquatic ecosystems, because they are recognized to bioaccumulate these substances, giving a time-integrated indication of environmental pollution, plus an accurate data on the prospective public health impacts of seafood ingestion (Grilo et al. 2013; El Megdiche et al. 2017; Govaerts et al. 2018; Liu et al. 2018).
The Lagoon of Bizerte (Northern Tunisia) is an environmentally important region (El Megdiche et al. 2017). Indeed, in this region, several local aquatic organisms live, feed, and die, and many of the pelagic species are breeding there (El Megdiche et al. 2017).
Furthermore, this lagoon is subjected to many anthropogenic stresses, including population growth, industrial operations (cement factory, metallurgical enterprise, shipyard, plants for tire production, etc.), plus marine and commercial harbors for shipping (El Megdiche et al. 2017). The banks of the lagoon also were used as waste open dump sites (El Megdiche et al. 2017). The direct and indirect releases of municipal and industrial waste and runoff contribute to the chemical pollution of the lagoon by multiple harmful chemicals (El Megdiche et al. 2017), such as organochlorine pesticides (OCPs) (El Megdiche et al. 2017), organohalogen compounds, such as polychlorinated biphenyls (PCBs) (El Megdiche et al. 2017), polycyclic aromatic hydrocarbons (PAHs) (El Megdiche et al. 2017), PBDEs and their methoxylated analogs in fish, mussel, clams, and urchins (Barhoumi et al. 2014; Ben Ameur et al. 2011, El Megdiche et al. 2017), and heavy metals (El Megdiche et al. 2017).
Despite the report of the existence of these pollutants, no studies focused on the accumulation of organobrominated pollutants originated from synthetic and natural sources in gastropod mollusks from the Bizerte Lagoon are available. In addition, recent data are very scarce on the occurrence of the non-PBDE BFRs in Tunisian coastal regions. In fact, only one study had interested on the evaluation of non-PBDE BFR levels in Bizerte Lagoon sea urchin (Mekni et al. 2019).
To provide more information on the pollution of the Bizerte Lagoon and to assess the potential hazards to shellfish consumers, this work evaluated the residue concentrations of persistent organobrominated compounds in a particular murex species (Hexaplex trunculus) among its edible marine species. The criteria of selection of this species are the following: previous use as sentinel species for chemical surveillance, broad distribution in the surveilled area, simple sampling, broad commercial spread, and thus a reliable indication of human exposure to the pollutants studied. In addition, this work is the first to be concerned in assessing the concentrations of PBDE, MeO-BDE, HBB, PBEB, and DBDPE in Tunisian aquatic ecosystem gastropods. In addition, this research is the first to be concerned in assessing the concentrations of PBDE, MeO-BDE, HBB, PBEB, and DBDPE in Tunisian aquatic ecosystem gastropods.
Materials and Methods
Study Area
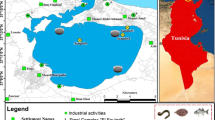
The lagoon of Bizerte is Tunisia’s second biggest lagoon. It is situated in a very considerable economic part of northern Tunisia (latitude, 37°80′–37°14′ N; longitude, 9°46′–9°56′ E) (El Megdiche et al. 2017). Its area and mean depth are respectively of 128 km2 and 7 m (El Megdiche et al. 2017). It connects with the Mediterranean Sea through a 7-km long channel to the north and through the Tinja River to the south with the Ichkeul Lake (El Megdiche et al. 2017). Wild murex samples were obtained from four different sites of the Bizerte Lagoon (Fig. 1). They were chosen based on potential variations in amounts of contamination and bivalve presence. Menzel Jemil Station (S1), 37°13′04 N, 9°54′46 E, is situated close a mussel farming zone and gets steady discharge from Menzel Jemil City’s urban runoff and sewerage; it also gets agricultural effluents from several manufacturing units (textile and electronic industries) situated in Menzel Jemil City (El Megdiche et al. 2017). Menzel Abderrahmen Station (S2), 37°13′43 N, 9°51′46 E, is situated close to a town of 10,000 residents, bordered by industrial units (textile, electronic, and metallurgical industries). The sampling site gets steady constant influx of untreated effluents (El Megdiche et al. 2017). The Chaara station (S3), 37°13′90 N, 9°49′49 E, is situated close the cement factory and is affected by the intensive traffic of fishing boats and urban wastewater from Zarzouna and Bizerte towns. (El Megdiche et al. 2017). The Chanel station (S4), 37°15′68 N, 9°51′54 E, is located in a highly populated region under intense sea traffic, with big cement works and oil refineries being grouped together (Lafabrie et al. 2013; El Megdiche et al. 2017).
Sample Collection
Murex (Hexaplex trunculus) were sampled during the month of February 2010 from the four investigated areas. According El Megdiche et al. (2017) and to reduce interindividual variability, each composite sample consisted of at least 150 murex of homogeneous size (53–35-mm shell length) collected from each sampling site (El Megdiche et al. 2017). After collection, samples were treated as described by El Megdiche et al. (2017). In fact, from each investigated area we prepared three pools of 50 murex. After deshelling murex and the homogenization of their soft tissues, they were stored at − 20 °C before the freeze drying process. The lyophilized murex samples were kept at − 20 °C before analysis.
Chemicals
The target alternative BFRs (HBB, PBEB, and DBDPE) were obtained from Wellington Laboratories Inc. (Guelph, ON, Canada). The standard mixture of 40 PBDE congeners (BDE-1, BDE-2, BDE-3, BDE-7, BDE-8, BDE-10, BDE-11, BDE-12, BDE-13, BDE-15, BDE-17, BDE-25, BDE-28, BDE-30, BDE-32, BDE-33, BDE-35, BDE-37, BDE-47, BDE-49, BDE-66, BDE-71, BDE-75, BDE-77, BDE-85, BDE-99, BDE-100, BDE-116, BDE-118, BDE-119, BDE-126, BDE-138, BDE-153, BDE-154, BDE-155, BDE-166, BDE-181, BDE-183, BDE-190 and BDE-209, the standard mixture of 8 MeO-PBDEs (5-MeO-BDE-47, 6-MeO-BDE-47, 4′-MeO-BDE-49, 2′-MeO-BDE-68, 5′-MeO-BDE-99, 5′-MeO-BDE-100, 4′-MeO-BDE-101 and 4′-MeO-BDE-103), as well as the 13C12-BDE-209 were provided by Wellington Laboratories Inc. (Guelph, ON, Canada). All necessary solvents for the analysis of the studied chemicals were provided by Merck (Darmstadt, Germany).
Sample Preparation
The analysis of the selected substances was performed following the method adopted by Barón et al. (2014a). One gram of lyophilized sample was spiked with 5 ng of 13C-PBDEs and 50 ng of 13C-BDE-209 and kept overnight for equilibration. After equilibration step, the sample was extracted by the pressurized liquid extraction (PLE) by loading it into an 11-mL extraction cell. Furthermore, the extract was evaporated and after the gravimetric determination of the lipid content, it was cleaned up in two steps: fat remove using H2SO4 (conc.), followed by purification with SPE using alumina cartridges (AL-N, 5 g). Finally, the eluate was concentrated to incipient dryness and reconstituted in 40 μL of toluene.
Instrumental Analysis
The determination of the investigated substances in extracts were performed using an Agilent 7890C gas chromatograph connected to an Agilent 5975A Network mass spectrometer, working in negative chemical ionization mode (NCI) using NH4+ as reagent gas (Barón et al. 2013). The instrument operating conditions were used as described by Barón et al. (2014a).
Quality Assurance and Quality Control
The quality assurance of samples was done as detailed by Barón et al. (2014a). Procedural blanks revealed the no detection of analytes of interest. The detection and quantification confirmation criteria for the studied compounds were performed according to those reported by Barón et al. (2014a).
The used adopted methodology leads to recoveries comprised between 46 and 90% for the studied compounds. Relative standard deviation values (RSD) were < 10% for all substances, which indiciates that method reproductibilty is satisfactory. For the quantification of the studied compounds, a multilevel calibration curves were done and good linearity was obtained (R2 > 0.995). The instrumental limits of detection (LODs) determined as three times the signal to noise ratio (Trabalón, et al. 2017), varied from 0.07 to 0.74 ng g−1 lipid weight (lw) for PBDEs and from 0.1 to 0.2 ng g−1 lw for MeO-PBDEs. Concerning HBB, PBEB, and DBDPE LODs were respectively 0.06, 0.06, and 1.06 ng g−1 lw. The instrumental limits of quantification (LOQs) calculated as ten times the signal to noise ratio (Ben Ameur, et al. 2011), varied between 0.23 and 2.50 ng g−1 lw for PBDEs and between 0.3 and 0.6 ng g−1 lw for MeO-PBDEs. For HBB, PBEB, and DBDPE, LOQs were respectively 0.20, 0.20, and 3.53 ng g−1 lw.
Estimated Daily Intake and Risk Evaluation
For the estimation of the risk for the local residents, through the consumption of murex in Bizerte, we have used the methodology reported by Ni et al. (2012) and Staskal et al. (2008) for the calculation of the estimated daily intake (EDI), hazard quotient (HQ), and cancer risk (CR) (El Megdiche et al. 2017).
To determinate the EDI, the International Institute of Nutrition and Food Technology (http://www.institutdenutrition.rns.tn) provided us the CR value (27 g day−1) (El Megdiche et al. 2017).
Statistical Analysis
SPSS software (SPSS 10.0 for Windows, SPSS Inc.) was used for the statistical treatment of the obtained data. Spearman rank correlation was used for the examination of the relationship between parameters.
Results and Discussion
Table 1 shows a summary of the concentration range and arithmetic means for PBDEs and MeO-BDEs in soft tissue homogenates of murex. These investigated compounds were detected in all the samples from the four studied sites, showing their ubiquity in the studied coastal area.
Seven BDEs (BDE-28, 47, 99,154, 153, 183, and 209) were present as predominant pollutants among the 40 studied PBDE congeners, with detection frequencies of 100%, 100%, 17%, 56%, 83%, 83%, and 50% respectively. Only five congeners with a detection frequency of 100% for 6-MeO-BDE-47, 2′-MeO-BDE-68, and 5-MeO-BDE-100 and 83% for 4-MeO-BDE-103 were detected for MeO-PBDE compounds.
Concerning NBFRs, no compounds from the three analyzed compounds was detected in any of the study shellfish species, which is in accordance with current results from other studies (Papachlimitzou et al. 2012; Barón et al. 2013, 2014a, b; Mekni et al. 2019). Indeed, when non-PBDE BFRs were detected in biota, their levels were lower than those of PBDEs (Munschy et al. 2011; Trabalón et al. 2017). Mean concentrations of the sum of PBDEs and sum of the MeO-PBDEs were 187 ng g−1 lipid weight (lw) and 264 ng g−1 lw respectively.
The pollution level of PBDEs and MeO-PBDEs depends of the localization of investigated sites. In fact, murex from S2 have the highest concentration of PBDEs (269 ng g−1 lw), whilst samples from this site showed the lowest MeO-PBDE levels (129 ng g−1 lw). The concentrations of PBDE in S2 were significantly higher than in S3 and S4 (Mann–Whitney, p < 0.05), whereas the concentrations of MeO-PBDE in S4 were significantly higher than in S1, S2, and S3 (Mann–Whitney, p < 0.05). Consequently, it can be hypothesized that murex from station S2 were more subjected to PBDEs exposition than those obtained from S1, S2, and S3 stations. Moreover, the existence of significant variations in the sum of MeO-BDE concentrations between S4 and the other three stations may lead to the hypothesis that murex from station S4 were more impacted by MeO-BDEs than those sampled from S1, S2, and S3.
The low sum of PBDE concentrations in S4 compared with those from S2 may be explained by its proximity to the sea (El Megdiche et al. 2017), where this area is characterized by significant water exchanges and by the sediment resuspension phenomena (El Megdiche et al. 2017). The industrial development and population growth could be responsible of the high PBDE levels in S2 compared with the other stations. Furthermore, the important variations in PBDE concentrations between S2 and the remaining stations could be clarified by sources variation, hydrodynamic circumstances, turbidity, or the existence of organic matter (river inputs) at various sampling locations (El Megdiche et al. 2017). Effluents from textile and electronic mills and from local wastewater discharges are the most evident sources of PBDEs (El Megdiche et al. 2017). In addition, some urban agglomeration releases from untreated effluents into rivers, particularly from the cities of Mrezig and Guenich, situated respectively in the northwest and southeast could be another possible origins of PBDEs contamination in the Bizerte Lagoon (El Megdiche et al. 2017). In addition, other towns located near the lagoon, Bizerte, Zarzouna, Menzel Jemil, Menzel Abderrahmen, and Menzel Bourguiba, have sewage facilities, but the treated wastewater from the treatment plants (Bizerte and Menzel Bourguiba) are released to the lagoon and this may lead to its contamination by PBDEs (El Megdiche et al. 2017).
The high MeO-PBDE concentrations in station S4 compared to the remaining sites could be due to its proximity to the Mediterranean Sea (El Megdiche et al. 2017). Indeed, MeO-PBDEs did not have a recognized anthropogenic origin, so the spatial distribution of these chemicals was not associated with industrial operations and the population in the towns near the studied stations (El Megdiche et al. 2017). The sampling sea area is a potential influencing factor (El Megdiche et al. 2017). Stations S1 and S2 are situated in the inner part of the lagoon, far from the ocean and severely influenced by the surrounding continent (El Megdiche et al. 2017). Consequently, their water temperature and salinity differ significantly from that of oceans (El Megdiche et al. 2017). Station S4 is a point of return between the lagoon of Bizerte and the Mediterranean Sea (El Megdiche et al. 2017). Because most MeO-PBDEs in oceans have been considered as marine natural products, various marine environments may lead to distinct concentrations of MeO-PBDEs in biota (El Megdiche et al. 2017). Therefore, the samples collected from S4 station were influenced by the Mediterranean Sea (El Megdiche et al. 2017). This reason could explain why the sum of MeO-PBDEs concentration value in samples obtained from this site was higher than those from S1, S2, and S3 stations.
PBDE Pattern
In Fig. 2, in each studied site, the contribution of each PBDE congener to the sum of PBDEs is provided. In the four investigated sites, very comparable PBDE patterns were observed for murex samples. In all the analyzed samples, among the seven detected congeners, BDE-47 was the prevalent. It contributed 56%, 63%, 34%, and 40% of ∑PBDEs respectively for S1, S2, S3, and S4. The prevalence of this congener in the current study was in accordance with other results reported in seafood samples in other studies (El Megdiche et al. 2017).
For the other remaining congeners, their corresponding abundance was the following: BDE-183 > BDE-28 > BDE-154 > BDE-153 > BDE-99 in S1. The relative prevalence of PBDEs in S2 was as follows: BDE-183 > BDE-28 > BDE-153/BDE-154 > BDE-99 > BDE-209. The relative prevalence of the other principal congeners in S3 was the following: BDE-183 > BDE-154 > BDE-153 > BDE-28 > BDE-209. Finally, their relative abundance in S4 station was BDE-183 > BDE-28 > BDE-153 > BDE-28/BDE-154 > BDE-209.
In the Bizerte Lagoon murex, the lack of BDE-99 and -100 detection is consistent with prior research performed in this aquatic environment and showing their absence in mussels and clams as well (Barhoumi et al. 2014; El Megdiche et al. 2017). In addition, this finding is similar to those reported in Senegal and Norway gastropods (Bodin et al. 2011; Govaerts et al. 2018) and in Chilean clams (Barón et al. 2013). Indeed, these two congeners were identified in the tissues of gastropods from China, Japan, Korea, and North Carolina (Hu et al. 2010; La Guardia et al. 2012; Byun et al. 2013; Kobayashi et al. 2015).
Usually, PBDEs were manufactured at three distinct levels of bromination, i.e., mixtures of Penta-BDE, Octa-BDE, and Deca-BDE (El Megdiche et al. 2017). The formulation of Penta-BDE consists of 41–42% tetra-BDEs (principally BDE-47) and 44–45% penta-BDEs (principally BDE-99 and BDE-100), whereas the formulation of Deca-BDE consists largely of BDE-209 (97–98%), with a low quantity of nona-BDEs (0.3–3%) (El Megdiche et al. 2017).
In soft tissues of the Bizerte Lagoon murex, percentages of tri-, tetra-, penta-, hexa-, hepta-, and deca-BDE congeners in soft tissues of the Bizerte Lagoon murex were 7%, 48%, 1%, 18%, 25%, and 0.4% respectively. The cause for the composition of found PBDE congeners is unknown in this study. Hinge on the detection of BDE-99, BDE-183, BDE-209 in addition to the high percentage of BDE-47 and on the basis of the PBDE congeners percentage in each commercial mixture, it might be explained by commercial Penta-BDE, Octa-BDE, and Deca-BDE formulations (El Megdiche et al. 2017). Ben Ameur et al. (2011) and Mekni et al. (2019) demonstrated the detection of BDE-100 in fish muscle and sea urchin sampled from Bizerte Lagoon, which is a component of the Penta-BDE formulation structure. The comparatively higher concentration of BDE-47 found in this study could explain the reduced amount of BDE-99 due to its debromination, caused by microorganisms and/or endogenous enzymatic structures, and the widespread use of the mixture of Penta-BDE rather than the others (El Megdiche et al. 2017). In this work, the comparatively elevated percentage of BDE-28 in this study may be due to the debromination of higher brominated congeners (El Megdiche et al. 2017). However, it also might have been affected by conversion and debromination (El Megdiche et al. 2017). For example, the conversion of BDE-99 into BDE-47 and BDE-183 into BDE-154 has been demonstrated in common carp (El Megdiche et al. 2017).
Other studies have demonstrated that microbial reductive debromination or photochemical degradation may contribute to BDE-47 formation (El Megdiche et al. 2017). There is a lack of data on the PBDE metabolism and transformation with regard to shellfish.
An absence of accumulation of BDE-99 by a few field-collected fish species also has been shown (El Megdiche et al. 2017). PBDE debromination has been proposed within fish tissues leading to significant accumulation of less brominated congeners (El Megdiche et al. 2017). This could indicate that body burdens of PBDE may reflect both direct uptake from more extremely brominated congeners exposure and debromination (El Megdiche et al. 2017). The most probable reason for no BDE-100 congeners identified in the shellfish species of this work is no uptake from exposure (El Megdiche et al. 2017). A previous study performed by Jin et al. (2008) has shown that BDE-99, -100, -154, -153, and -183 congeners are not contained in the manufacturing Deca-BDE mixtures from the Shangdong province (El Megdiche et al. 2017). Results from this test show that BDE congeners in some shellfish tissues undergo a previously unknown pathway of biotransformation (El Megdiche et al. 2017).
The no identification of BDE-100 could be occur from the absence of release from the products, which they contain and from the absence of their photodegradation added to their metabolization by murex and the absence of their uptake from exposure (El Megdiche et al. 2017). In fact, in samples of sediment and bivalve, PBDEs distribution, ensembles with commercial formulations of Penta- and Octa-BDE, can be entered into the coastal ecosystem through atmospheric deposition and shipment operations (El Megdiche et al. 2017). It has been shown that major congeners of commercial Penta- and Octa-BDE mixtures (BDE-47, -99, -100, -154, -153, and -183) have been revealed to be susceptible to simple discharge and disintegration of goods, such as polyurethane foams, paints, and clothing, which could contribute to their elevated emission levels into the surrounding area (El Megdiche et al. 2017). In addition, PBDE congeners are distributed between the gaseous and particle phases after their release into the ecosystem (El Megdiche et al. 2017). They then undergo atmospheric degradation and/or long-range transport to regions outside the dismantling location for e-waste (El Megdiche et al. 2017). Furthermore, it stays unclear whether the main reason for the no identification of BDE-99 is it accumulations, the removal side of the bioaccumulation phase, the product’s release and disintegration capabilities, or physiochemical degradation (El Megdiche et al. 2017).
Despite the variations in PBDE concentrations, tetra- and penta-congeners appear to prevail globally in environmental samples (El Megdiche et al. 2017). The principal congener in the Deca-BDE formulation (BDE-209) was identified in samples from the four studied stations. BDE-209 murex levels from the investigated lagoon were lower than the other congeners of PBDEs, whereas high BDE-209 levels were revealed in aquatic seafood (Barón et al. 2013; Moon et al. 2007; Munschy et al. 2015). This outcome is similar to those showed by Sudaryanto et al. (2009), Bartrons et al. (2012), Liu et al. (2014), and El Megdiche et al. (2017). It has been noted that higher brominated congeners are mainly linked to particulate organic carbon due to their high log Kow values (El Megdiche et al. 2017). Murex trunculus, as a carnivorous, and feed on bivalves and other gastropods, could accumulate these contaminants from these preys. In this work, the presence of the higher brominated congeners may be due to their presence from the preys ingestion in their gut due to nondepuration of murex before shucking (El Megdiche et al. 2017).
The BFR concentrations in literature in seafood samples is sparse, and most of the published data displayed PBDE concentrations in ng/g wet weight (ww), leading to a difficulty for the comparison with our outcomes, expressed in ng/g lw (El Megdiche et al. 2017).
In Table 2 are indicated the BDE concentrations reported throughout the world in some gastropod samples. In murex from the Bizerte Lagoon, the measured concentrations of PBDE congeners are lower than those recorded in gastropods from other marine ecosystems throughout the world, such as China (Wang et al. 2011) and North of Carolina (La Guardia et al. 2012). The concentrations are higher than those determined in seafood from Congo (Verhaert et al. 2013), China (Hu et al. 2010; She et al. 2013; Du et al. 2017; Yin et al. 2017), Korea (Byun et al. 2013), and Japan (Kobayashi et al. 2015). The obtained levels were within the same range as those recorded in other Norwegian (Govaerts et al. 2018) and North China (Hu et al. 2010) gastropods.
MeO-BDE Pattern
Only four congeners among the eight studied MeO-PBDEs were identified in the murex specimens at quantifiable concentrations: 6-MeO-BDE-47, 2′-MeO-BDE-68, 5′-MeO-BDE-100, and 4′MeO-BDE-103 (Table 1). The main abundant MeO-BDE compounds represented by 6-MeO-BDE-47 and 2′-MeO-BDE-68 were identified in all the examined samples with a mean total level of 196, 121, 246, and 431 ng g−1 lw respectively in S1, S2, S3, and S4.
2′-MeO-BDE-68 congener percentage contribution to the total MeO-PBDE concentrations was 63, 62, 58, and 62 respectively in S1, S2, S3, and S4 stations (Fig. 3). Although the 6-MeO-BDE-47 congener contribution to the total MeO-PBDE concentrations was 29%, 31%, 35%, and 34% respectively in in S1, S2, S3 and S4. The total amount of 5′-MeO-BDE-100 and 4′-MeO-BDE-103 was 8%, 6%, 7%, and 4% of the sum of all MeO-PBDEs detected in S1, S2, S3, and S4, respectively.
MeO-BDE-47 and 2-MeO-BDE-68 are the principal predominant MeO-PBDEs identified based on the literature (El Megdiche et al. 2017). Indeed, it has been reported that these two congeners have the higher contributions in Canadian, Chinese, and Chilean mollusk species (Kelly et al. 2008; Zhang et al. 2010; Barón et al. 2013; Sun et al. 2013; Yin et al. 2015). Nevertheless, other congeners have been sometimes reported (El Megdiche et al. 2017). MeO-tetra-PBDE concentrations generally appear to be higher than MeO-tri or MeO-penta-PBDE concentrations (El Megdiche et al. 2017). MeO-PBDE profiles revealed in this study were comparable to those reported in other works assessing these compounds in bivalves and aquatic invertebrates (Barón et al. 2013; Zhang et al. 2010; El Megdiche et al. 2017; Mekni et al. 2019).
Published data on MeO-BDE concentration in gastropod specimens is rare, so the comparison of the obtained results with those cited in literature is difficult. The sum of MeO-PBDEs detected levels in murex from the lagoon of Bizerte (mean: 264 ng g−1 lw, range 129–449) were higher than those in bivalves from Chinese coastal areas (3.63 ng g−1 lw) (Sun et al. 2013), from Liaodong Bay (15.9 ng g−1 lw) (Zhang et al. 2010), from the coast of Concepcion (Chile) (3.5–49 ng g−1 lw), from the Canadian Arctic (mean: 14 ng g−1 lw) (Kelly et al. 2008), and in Baltic Sea bivalve sampled in 2011 (87.8 ng g−1 lw) (Dahlberg et al. 2016). Our levels were in the same range also with those in Baltic Sea bivalve collected in 2008 (160–420 ng g−1 lw) (Löfstrand et al. 2011) and lower than those of the Bizerte Lagoon urchins (364 ng g−1 lw) (Mekni et al. 2019).
Potential Source of MeO-PBDEs
In this present study, murex sampled from S3 and S4 and located near the Mediterranean Sea contained higher concentration of MeO-BDEs than those from sites located far from the Mediterranean (S1 and S2), moreover concentrations of PBDEs were higher in stations S1 and S2. According to these observations, it is suggested that, rather than PBDEs biotransformation, at least some of the MeO-BDEs in the studied biota are originated from natural sources (Wang et al. 2011).
In fact, the fact that the two congeners of MeO-BDEs which are prevalent (2′-MeO-BDE-68 and 6-MeOBDE-47) in Bizerte Lagoon murex have been shown to be natural products produced by marine organisms rather than metabolism also supports this hypothesis (Wang et al. 2011).
As reported by Vetter (2006), a higher percentage of 2′-MeO-BDE-68 to that of the sum of MeOBDEs might reveal sponges as the dominant source of MeO-PBDEs, whereas a higher contribution of 6-MeO-BDE-47 would indicate algae as the main source of MeO-PBDEs (Ben Ameur et al. 2013; El Megdiche et al. 2017). Therefore, in samples of each studied area the ratio between these two chemical compounds which have natural origin (2′-MeOBDE-68/6-MeO-BDE-47) was determined. Mean values of this quotient were 2.15, 1.98, 1.66, and 1.86 respectively in S1, S2, S3, and S4. Thus, and based to data reported by Vetter (2006), the murex obtained from Bizerte Lagoon would be contaminated MeO-PBDEs predominantly from sponges.
In order to have more ideas about the potential origins of MeO-PBDEs in murex, the statistical link between PBDEs and MeO-PBDEs were investigated. In this study, no significant correlations between the sum of MeO-PBDEs and of PBDEs for the four investigated sites was revealed (S1: rs = 0.23, p > 0.05; S2: rs = 0.18, p > 0.05; S3: rs = 0.30, p > 0.05; S4: rs = 0.28, p > 0.05), which indicates that MeO-PBDEs did not originate principally from PBDEs. In addition, there was no correlation between BDE-47 and 6-MeO-BDE-47 concentrations in samples for the four areas (S1: rs = 0.12, p > 0.05; S2: rs = 0.20, p > 0.05; S3: rs = 0.15, p > 0.05; S4: rs = 0.25, p > 0.05). The lack of significant correlation between these two congeners also was revealed in Chinese freshwater fish (Zhou et al. 2016), in the Bizerte Lagoon fish and clams (Ben Ameur et al. 2011, 2013; El Megdiche et al. 2017), in the Baltic Sea mussels (Dahlberg et al. 2016) and from Swedish water pikes (Kierkegaard et al. 2004). Based on this outcome, it can be hypothesized that the 6-MeO-BDE-47 accumulation does not originate from step I of BDE-47 conversion (Ben Ameur et al. 2013; El Megdiche et al. 2017). These obtained results were in accordance to those published for bivalves from other studies (Kelly et al. 2008; Ben Ameur et al. 2013; Sun et al. 2013; El Megdiche et al. 2017).
At last, all the outcomes recorded in this study contribute to the assumption that MeO-PBDEs detected in the investigated shellfish were originated primarily from accumulation by natural sources in marine environments rather than from PBDE biotransformation (Ben Ameur et al. 2013; El Megdiche et al. 2017).
Risk Assessment of Human Exposure
The EDI of PBDEs and MeO-PBDEs were estimated from the consumption of the selected shellfish species for the general population in Bizerte (Northern Tunisia) (Table 3) to give an indication of the magnitude of exposure through Murex trunculus intake to the distinct pollutants. In addition, to assess the prospective health danger linked with the ingestion of this gastropod species from the Lagoon of Bizerte, HQ, and CR also were calculated.
The sum of EDI of BDE-47, BDE-99, BDE-153, BDE-209, total PBDEs, and MeO-PBDEs are presented in Table 3. The assessed EDI values of PBDEs for residents at S1, S2, S3, and S4 was respectively of 0.28, 0.40, 0.25, and 0.19 ng/kg /day based on the average body weight of native citizens. Concerning MeO-PBDEs, based on EDI values in S1, S2, S3 and S4, the EDI could be considerate as the main human exposure route to PBDEs in the town of Bizerte, which is consistent with results recorded by prior works investigated on fish, clams, and urchins from the Bizerte Lagoon (Ben Ameur et al. 2013; El Megdiche et al. 2017; Mekni et al. 2019).
For the studied pollutants in this work, data on literature about their dietary intakes are very limited. In this present study, the calculated dietary intake values of PBDEs, with an average value of 19.7 ng/day, were higher than those recorded in fish and shellfish from Catalonia, Spain (30.7 ng/day) (Bocio et al. 2003), in fish and shellfish from Belgium (59.5 ng/day) (Sioen et al. 2008), in Japan fish from South Korea (65.9 ng/day) (Lee et al. 2013), and in fish and shellfish from Shanghai (41 ng/day) (Yu et al. 2011). EDI values of PBDEs from Bizerte murex is in the same range with those obtained for fish and seafood from Dutch (van Leeuwen and de Boer 2008), for urchins from Bizerte Lagoon (24.6 ng/day) (Mekni et al. 2019), for Italian fish and seafood (20.9 ng/day) (Martellini et al. 2016), for Chinese seafood (15.9–17.6 ng/day) (Guo et al. 2010), for Finlandese fish (23 ng/day) (Kiviranta et al. 2004), for Swedish fish (23 ng/day) (Darnerud et al. 2006), for fish and fish products from Sweden (19.3 ng/day) (Törnkvist et al. 2011), and for Italian fish and mollusk (20.3 ng/day) (Martellini et al. 2016). Nevertheless, the value of their dietary intake was higher than those determined in Spain’s clam (0.02 ng/day) and mussels (0.34 ng/day) (Domingo et al. 2006), and Bizerte Lagoon’s clams (7.04 ng/day) (El Megdiche et al. 2017).
Concerning MeO-PBDEs, the mean value of dietary intake calculated in the present study (19.8 ng/day; range 8.03–27.5 ng/day) was in the same range to those reported in Spanish seafood (15.4 ng/day) (Trabalón et al. 2017) and in fish from the Bizerte Lagoon (28.6 ng/day) (Ben Ameur et al. 2013). However, it is higher than those recorded in the Bizerte Lagoon clams (2 ng/day) (El Megdiche et al. 2017), in Hong Kong fish (35–301 ng/day) (Wang et al. 2011), and lower than assessed in sea urchin from the Bizerte Lagoon (227 ng/day) (Mekni et al. 2019).
Due to the extremely limited data about toxicological and epidemiological information related to PBDEs, assessing the prospective health risk of PBDEs remains a defiance (Ben Ameur et al. 2013; El Megdiche et al. 2017). Actually, just the lowest observed adverse effect level (LOAEL) of 1 mg/kg/day, hinge on the most delicate endpoints of toxic impacts of PBDEs (Bocio et al. 2003; Darnerud et al. 2001) and Agency for Toxic Substances and Disease Registry (ATSDR)-derived Minimal Risk Levels (MRLs) with respective values of 0.007 and 10 mg/day for lower brominated congeners and Deca-BDE (El Megdiche et al. 2017) were proposed. Thus, the hazard assessment related to PBDEs was conducted in this study just for PBDE congeners (BDE-47, -153, and -209) having an available reference dose value.
In the current study, the sum of PBDEs dietary intake for adults (0.19–0.4 ng/kg bw/day) is considerably lower than the proposed LOAEL and the oral MRLs (El Megdiche et al. 2017). The possible PBDEs health hazard from murex intake in Bizerte is thus regarded to be limited based on present toxicological data (El Megdiche et al. 2017). This outcome is in line with those stated by Ben Ameur et al. (2013), Meng et al. (2007), Guo et al. (2010), and by El Megdiche et al. (2017).
As far as the hazard quotient is concerned, values superior to 1 reveal possible hazard of exposure for humans (El Megdiche et al. 2017). Based on the HQ values recorded in the present work (Table 3) and which are all well below 1.0, it could be suggested that the exposition hazard of citizens to PBDEs via the ingestion of murex in the region of Bizerte is limited (El Megdiche et al. 2017). This result is in accordance with those stated in Italian fish and mussels (El Megdiche et al. 2017), in sea food from China (El Megdiche et al. 2017), in Hong Kong fish (El Megdiche et al. 2017), and in Bizerte Lagoon seafood (El Megdiche et al. 2017; Mekni et al. 2019).
In this study, the cancer hazard related to the presence of BDE-209 was also evaluated using the method described by Staskal et al. (2008). The obtained CR values varied from 0.00 to 1.90 × 10−9, with an average value of 6.38 × 10−10. These outcomes were a little lower than those stated for Bizerte clams and urchins (Mekni et al. 2019) and higher than those reported in China and in Italy (Mekni et al. 2019).
Finally, it may be hypothesized that no negative health impacts are related to the exposition of the resident population to PBDE bioaccumulated in Bizerte Lagoon murex soft tissues through murex consumption based on the obtained results (El Megdiche et al. 2017; Mekni et al. 2019).
Conclusions
This study is the first reporting BFR levels in gastropod mollusks in Tunisian aquatic ecosystems. Both classic PBDEs and novel BFRs in addition to the natural compounds of MeO-PBDEs were investigated in Bizerte lagoon using a biological matrix which is murex (Hexaplex trunculus).
Polybromodiphenyl ethers and their methoxylated analogs were found in all murex specimens collected from the Bizerte Lagoon. The obtained levels were in the same range to or relatively higher than those stated for other organisms from other aquatic ecosystems across the world. However, emerging BFRs were not detected in any of the samples.
No hazards for the citizen’s health were related to the accumulation of PBDEs via the murex ingestion based on several available guidelines. Overall, this work presents a regional data evaluation of the incidence and concentrations of organohalogen flame retardants in murex and proves the role of Hexaplex trunculus as bioindicator species able to differentiate between numerous amounts of environmental pollution, which confirms its use in monitoring strategies.
References
Aznar-Alemany Ò, Aminot Y, Vilà-Cano J et al (2018) Halogenated and organophosphorusflame retardants in European aquaculture samples. Sci Total Environ 612:492–500. https://doi.org/10.1016/j.scitotenv.2017.08.199
Aznar-Alemany Ò, Sala B, Plön S et al (2019) Halogenated and organophosphorus flame retardants in cetaceans from the southwestern Indian Ocean. Chemosphere 226:791–799. https://doi.org/10.1016/j.chemosphere.2019.03.165
Aznar-Alemany Ò, Trabalón L, Jacobs S et al (2017) Occurrence of halogenated flame retardants in commercial seafood species available in European markets. Food Chem Toxicol 104:35–47. https://doi.org/10.1016/j.fct.2016.12.034
Barhoumi B, Menach KL, Clérandeau C et al (2014) Assessment of pollution in the Bizerte lagoon (Tunisia) by the combined use of chemical and biochemical markers in mussels, Mytilus galloprovincialis. Mar Pollut Bull 84:379–390. https://doi.org/10.1016/j.marpolbul.2014.05.002
Barón E, Rudolph I, Chiang G et al (2013) Occurrence and behavior of natural and anthropogenic (emerging and historical) halogenated compounds in marine biota from the Coast of Concepcion (Chile). Sci Total Environ 461–462:258–264. https://doi.org/10.1016/j.scitotenv.2013.05.006
Barón E, Eljarrat E, Barceló D (2014a) Gas chromatography/tandem mass spectrometry method for the simultaneous analysis of 19 brominated compounds in environmental and biological samples. Anal Bioanal Chem 406:7667–7676. https://doi.org/10.1007/s00216-014-8196-7
Barón E, Máñez M, Andreu AC et al (2014b) Bioaccumulation and biomagnification of emerging and classical flame retardants in bird eggs of 14 species from Doñana Natural Space and surrounding areas (South-western Spain). Environ Int 68:118–126. https://doi.org/10.1016/j.envint.2014.03.013
Bartrons M, Grimalt JO, de Mendoza G, Catalan J (2012) Pollutant dehalogenation capability may depend on the trophic evolutionary history of the organism: PBDEs in freshwater food webs. PLoS ONE 7:e41829. https://doi.org/10.1371/journal.pone.0041829
Ben Ameur W, Ben Hassine S, Eljarrat E et al (2011) Polybrominated diphenyl ethers and their methoxylated analogs in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) from Bizerte Lagoon, Tunisia. Mar Environ Res 72:258–264. https://doi.org/10.1016/j.marenvres.2011.09.009
Ben Ameur W, El Megdiche Y, Eljarrat E et al (2013) Organochlorine and organobromine compounds in a benthic fish (Solea solea) from Bizerte Lagoon (northern Tunisia): implications for human exposure. Ecotoxicol Environ Saf 88:55–64. https://doi.org/10.1016/j.ecoenv.2012.10.021
Bocio A, Llobet JM, Domingo JL et al (2003) Polybrominated diphenyl ethers (PBDEs) in foodstuffs: human exposure through the diet. J Agric Food Chem 51:3191–3195. https://doi.org/10.1021/jf0340916
Bodin N, N’Gom Ka R, Le Loc’h F et al (2011) Are exploited mangrove molluscs exposed to persistent organic pollutant contamination in Senegal, West Africa? Chemosphere 84:318–327. https://doi.org/10.1016/j.chemosphere.2011.04.012
Byun G-H, Moon H-B, Choi J-H et al (2013) Biomagnification of persistent chlorinated and brominated contaminants in food web components of the Yellow Sea. Mar Pollut Bull 73:210–219. https://doi.org/10.1016/j.marpolbul.2013.05.017
Choo G, Kim DH, Kim UJ et al (2018) PBDEs and their structural analogues in marine environments: fate and expected formation mechanisms compared with diverse environments. J Hazard Mater 343:116–124. https://doi.org/10.1016/j.jhazmat.2017.09.026
Covaci A, Harrad S, Abdallah MA-E et al (2011) Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int 37:532–556. https://doi.org/10.1016/j.envint.2010.11.007
Dahlberg A-K, Bignert A, Legradi J et al (2016) Anthropogenic and naturally produced brominated substances in Baltic herring (Clupea harengus membras) from two sites in the Baltic Sea. Chemosphere 144:2408–2414. https://doi.org/10.1016/j.chemosphere.2015.10.134
Darnerud PO, Eriksen GS, Jóhannesson T et al (2001) Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect 109:49–68. https://doi.org/10.1289/ehp.01109s149
Darnerud PO, Atuma S, Aune M et al (2006) Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, e.g. DDT) based on Swedish market basket data. Food Chem Toxicol 44:1597–1606. https://doi.org/10.1016/j.fct.2006.03.011
Du X, Chang H, Zhou Y et al (2017) Polybrominated diphenyl ethers and its methoxylated analogues in biota and sediment samples from two freshwater lakes in Yangtze River delta. Environ Earth Sci 76:171. https://doi.org/10.1007/s12665-017-6499-7
Domingo JL, Bocio A, Falcó G, Llobet JM (2006) Exposure to PBDEs and PCDEs associated with the consumption of edible marine species. Environ Sci Technol 40:4394–4399. https://doi.org/10.1021/es060484k
El Megdiche Y, Ameur WB, Bèchir H et al (2017) Anthropogenic (PBDE) and naturally-produced (MeO-PBDE) brominated compound levels in Bizerte Lagoon clams (Ruditapes decussatus): levels and human health risk assessment. Mar Pollut Bull 125:176–185. https://doi.org/10.1016/j.marpolbul.2017.08.005
Ezechiáš M, Covino S, Cajthaml T (2014) Ecotoxicity and biodegradability of new brominated flame retardants: a review. Ecotoxicol Environ Saf 110:153–167. https://doi.org/10.1016/j.ecoenv.2014.08.030
Govaerts A, Verhaert V, Covaci A et al (2018) Distribution and bioaccumulation of POPs and mercury in the Ga-Selati River (South Africa) and the rivers Gudbrandsdalslågen and Rena (Norway). Environ Int 121:1319–1330. https://doi.org/10.1016/j.envint.2018.10.058
Guo J, Wu F, Shen R, Zeng EY (2010) Dietary intake and potential health risk of DDTs and PBDEs via seafood consumption in South China. Ecotoxicol Environ Saf 73:1812–1819. https://doi.org/10.1016/j.ecoenv.2010.08.009
Hassine SB, Ameur WB, Gandoura N, Driss MR (2012) Determination of chlorinated pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human milk from Bizerte (Tunisia) in 2010. Chemosphere 89:369–377. https://doi.org/10.1016/j.chemosphere.2012.05.035
Hu G, Dai J, Xu Z et al (2010) Bioaccumulation behavior of polybrominated diphenyl ethers (PBDEs) in the freshwater food chain of Baiyangdian Lake, North China. Environ Int 36:309–315. https://doi.org/10.1016/j.envint.2010.01.002
Jin J, Liu W, Wang Y, Yan Tang X (2008) Levels and distribution of polybrominated diphenyl ethers in plant, shellfish and sediment samples from Laizhou Bay in China. Chemosphere 71:1043–1050. https://doi.org/10.1016/j.chemosphere.2007.11.041
Kelly BC, Ikonomou MG, Blair JD, Gobas FAPC (2008) Bioaccumulation behaviour of polybrominated diphenyl ethers (PBDEs) in a Canadian Arctic marine food web. Sci Total Environ 401:60–72. https://doi.org/10.1016/j.scitotenv.2008.03.045
Kierkegaard A, Bignert A, Sellström U et al (2004) Polybrominated diphenyl ethers (PBDEs) and their methoxylated derivatives in pike from Swedish waters with emphasis on temporal trends, 1967–2000. Environ Pollut 130:187–198. https://doi.org/10.1016/j.envpol.2003.12.011
Kiviranta H, Ovaskainen M-L, Vartiainen T (2004) Market basket study on dietary intake of PCDD/Fs, PCBs, and PBDEs in Finland. Environ Int 30:923–932. https://doi.org/10.1016/j.envint.2004.03.002
Kobayashi J, Imuta Y, Komorita T et al (2015) Trophic magnification of polychlorinated biphenyls and polybrominated diphenyl ethers in an estuarine food web of the Ariake Sea, Japan. Chemosphere 118:201–206. https://doi.org/10.1016/j.chemosphere.2014.08.066
Grilo TF, Cardoso PG, Pato P et al (2013) Organochlorine accumulation on a highly consumed bivalve (Scrobicularia plana) and its main implications for human health. Sci Total Environ 461–462:188–197. https://doi.org/10.1016/j.scitotenv.2013.04.096
La Guardia MJ, Hale RC, Harvey E et al (2012) In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima). Environ Sci Technol 46:5798–5805. https://doi.org/10.1021/es3004238
La Nutrition en Tunisie - Informations générales [WWW Document] (n.d.) http://www.institutdenutrition.rns.tn/. Accessed 6 Aug 2019
Lafabrie C, Hlaili AS, Leboulanger C et al (2013) Contaminated sediment resus-pension induces shifts in phytoplankton structure and function in aeutrophic Mediterranean lagoon. Knowl Manag Aquat Ecosyst 410(16):5. https://doi.org/10.1051/kmae/2013060
Lee S, Kannan K, Moon H-B (2013) Assessment of exposure to polybrominated diphenyl ethers (PBDEs) via seafood consumption and dust ingestion in Korea. Sci Total Environ 443:24–30. https://doi.org/10.1016/j.scitotenv.2012.10.099
Li X, Dong S, Wang R et al (2019) Novel brominatedflame retardant (NBFR) concentrations and spatial distribu-tions in globalfishmeal. Ecotoxicol Environ Saf 170:306–313. https://doi.org/10.1016/j.ecoenv.2018.11.112
Liu X, Jiao Y, Lin C et al (2014) PBDEs, hydroxylated PBDEs and methoxylated PBDEs in bivalves from Beijing markets. Chemosphere 110:97–103. https://doi.org/10.1016/j.chemosphere.2014.02.019
Liu Y, Liu J, Yu M et al (2018) Hydroxylated and methoxylated polybrominated diphenyl ethers in a marine food web of Chinese Bohai Sea and their human dietary exposure. Environ Pollut 233:604–611. https://doi.org/10.1016/j.envpol.2017.10.105
Martellini T, Diletti G, Scortichini G et al (2016) Occurrence of polybrominated diphenyl ethers (PBDEs) in foodstuffs in Italy and implications for human exposure. Food Chem Toxicol 89:32–38. https://doi.org/10.1016/j.fct.2015.12.026
McGrath TJ, Ball AS, Clarke BO (2017) Critical review of soil contamination by polybrominated diphenyl ethers (PBDEs) and novel brominated flame retardants (NBFRs); concentrations, sources and congener profiles. Environ Pollut 230:741–757. https://doi.org/10.1016/j.envpol.2017.07.009
Mekni S, Barhoumi B, Aznar-Alemany Ò et al (2019) Occurrence of halogenated flame retardants in sediments and sea urchins (Paracentrotus lividus) from a North African Mediterranean coastal lagoon (Bizerte, Tunisia). Sci Total Environ 654:1316–1325. https://doi.org/10.1016/j.scitotenv.2018.11.139
Meng X-Z, Zeng EY, Yu L-P et al (2007) Assessment of human exposure to polybrominated diphenyl ethers in china via fish consumption and inhalation. Environ Sci Technol 41:4882–4887. https://doi.org/10.1021/es0701560
Moon H-B, Kannan K, Lee S-J, Choi M (2007) Polybrominated diphenyl ethers (PBDEs) in sediment and bivalves from Korean coastal waters. Chemosphere 66:243–251. https://doi.org/10.1016/j.chemosphere.2006.05.025
Munschy C, Héas-Moisan K, Tixier C et al (2011) Classic and novel brominated flame retardants (BFRs) in common sole (Solea solea L.) from main nursery zones along the French coasts. Sci Total Environ 409:4618–4627. https://doi.org/10.1016/j.scitotenv.2011.07.021
Munschy C, Olivier N, Veyrand B, Marchand P (2015) Occurrence of legacy and emerging halogenated organic contaminants in marine shellfish along French coasts. Chemosphere 118:329–335. https://doi.org/10.1016/j.chemosphere.2014.09.106
Ni H-G, Ding C, Lu S-Y et al (2012) Food as a main route of adult exposure to PBDEs in Shenzhen, China. Sci Total Environ 437:10–14. https://doi.org/10.1016/j.scitotenv.2012.07.056
Papachlimitzou A, Barber JL, Losada S et al (2012) A review of the analysis of novel brominated flame retardants. J Chromatogr A 1219:15–28. https://doi.org/10.1016/j.chroma.2011.11.029
She Y-Z, Wu J-P, Zhang Y et al (2013) Bioaccumulation of polybrominated diphenyl ethers and several alternative halogenated flame retardants in a small herbivorous food chain. Environ Pollut 174:164–170. https://doi.org/10.1016/j.envpol.2012.11.024
Sioen I, Bilau M, Verdonck F et al (2008) Probabilistic intake assessment of polybrominated diphenyl ethers and omega-3 fatty acids through fish consumption. Mol Nutr Food Res 52:250–257. https://doi.org/10.1002/mnfr.200700109
Staskal DF, Scott LLF, Haws LC et al (2008) Assessment of polybrominated diphenyl ether exposures and health risks associated with consumption of southern mississippi catfish. Environ Sci Technol 42:6755–6761. https://doi.org/10.1021/es800613k
Sudaryanto A, Setiawan IE, Ilyas M et al (2009) Levels of brominated flame retardants in sediments and their bioaccumulation potential inbiota from Jakarta Bay and its surroundings, Indonesia. In: Obayashi Y, Isobe T, Subramanian A, Suzuki S, Tanabe S (eds) Interdisciplinary studies on environmental chemistry—environmental research in Asia. TERRAPUB, Tokyo, pp 125–131
Sun J, Liu J, Liu Y, Jiang G (2013) Hydroxylated and methoxylated polybrominated diphenyl ethers in mollusks from Chinese coastal areas. Chemosphere 92:322–328. https://doi.org/10.1016/j.chemosphere.2013.03.042
Tao L, Zhang Y, Wu JP et al (2019) Biomagnification of PBDEs and alternative brominated flame retardants in a predatory fish: using fatty acid signature as a primer. Environ Int 127:226–232. https://doi.org/10.1016/j.envint.2019.03.036
Törnkvist A, Glynn A, Aune M et al (2011) PCDD/F, PCB, PBDE, HBCD and chlorinated pesticides in a Swedish market basket from 2005—levels and dietary intake estimations. Chemosphere 83:193–199. https://doi.org/10.1016/j.chemosphere.2010.12.042
Trabalón L, Vilavert L, Domingo JL et al (2017) Human exposure to brominated flame retardants through the consumption of fish and shellfish in Tarragona County (Catalonia, Spain). Food Chem Toxicol 104:48–56. https://doi.org/10.1016/j.fct.2016.11.022
van Leeuwen SPJ, de Boer J (2008) Brominated flame retardants in fish and shellfish—levels and contribution of fish consumption to dietary exposure of Dutch citizens to HBCD. Mol Nutr Food Res 52:194–203. https://doi.org/10.1002/mnfr.200700207
Verhaert V, Covaci A, Bouillon S et al (2013) Baseline levels and trophic transfer of persistent organic pollutants in sediments and biota from the Congo River Basin (DR Congo). Environ Int 59:290–302. https://doi.org/10.1016/j.envint.2013.05.015
Vetter W (2006) Marine halogenated natural products of environmental relevance. Rev Environ Contam Toxicol 188:1–57. https://doi.org/10.1007/978-0-387-32964-2_1
Wang HS, Du J, Ho KL et al (2011) Exposure of Hong Kong residents to PBDEs and their structural analogues through market fish consumption. J Hazard Mater 192:374–380. https://doi.org/10.1016/j.jhazmat.2011.05.036
Weijs L, Das K, Neels H et al (2009) Levels and profiles of persistent organic polluants in several tissues of harbor porpoises (Phoconea phoconea) from the Black Sea. Organohalogen Compd 71:432–436
Yin G, Asplund L, Qiu Y et al (2015) Chlorinated and brominated organic pollutants in shellfish from the Yellow Sea and East China Sea. Environ Sci Pollut Res 22:1713–1722. https://doi.org/10.1007/s11356-014-3198-8
Yin G, Zhou Y, Strid A et al (2017) Spatial distribution and bioaccumulation of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in snails (Bellamya aeruginosa) and sediments from Taihu Lake area, China. Environ Sci Pollut Res 24:7740–7751. https://doi.org/10.1007/s11356-017-8467-x
Yu Y-X, Huang N-B, Zhang X-Y et al (2011) Polybrominated diphenyl ethers in food and associated human daily intake assessment considering bioaccessibility measured by simulated gastrointestinal digestion. Chemosphere 83:152–160. https://doi.org/10.1016/j.chemosphere.2010.12.049
Zaccaroni A, Andreini R, Franzellitti S et al (2018) Halogenated flame retardants in stranded sperm whales (Physeter macrocephalus) from the Mediterra-nean Sea. Sci Total Environ 635:892–900. https://doi.org/10.1016/j.scitotenv.2018.04.147
Zhang K, Wan Y, An L, Hu J (2010) Trophodynamics of polybrominated diphenyl ethers and methoxylated polybrominated diphenyl ethers in a marine food web. Environ Toxicol Chem 29:2792–2799. https://doi.org/10.1002/etc.334
Zhen X, Tang J, Xie Z et al (2016) Polybrominated diphenyl ethers (PBDEs) and alternative brominated flame retardants (aBFRs) in sediments from four bays of the Yellow Sea, North China. Environ Pollut 213:386–394. https://doi.org/10.1016/j.envpol.2016.02.042
Zhou Y, Chen Q, Du X et al (2016) Occurrence and trophic magnification of polybrominated diphenyl ethers (PBDEs) and their methoxylated derivatives in freshwater fish from Dianshan Lake, Shanghai, China. Environ Pollut 219:932–938. https://doi.org/10.1016/j.envpol.2016.09.043
Acknowledgements
The Spanish Ministry of Science and Innovation (CEMAGUA-CGL2007-64551/HID) and the Tunisian Ministry of Higher Education and Scientific Research supported this study. The authors are thankful to R. Chaler, D. Fangul, and M. Comesaña for their help at the mass spectrometry work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ameur, W.B., Annabi, A., El Megdiche, Y. et al. Legacy and Emerging Brominated Flame Retardants in Bizerte Lagoon Murex (Hexaplex Trunculus): Levels and Human Health Risk Assessment. Arch Environ Contam Toxicol 78, 337–349 (2020). https://doi.org/10.1007/s00244-019-00694-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00694-x