Abstract
The occurrence and spatial distribution of 13 organophosphorus flame retardants (OPFRs), 11 polybrominated diphenyl ethers (PBDEs), and eight novel brominated flame retardants (NBFRs) were investigated in Jinjiang river water, sediment, crucian carp, and groundwater in Chengdu, China. OPFRs were predominant and ubiquitous contaminants in the Jinjiang river water, sediment, groundwater, fish muscle, fish gills, and viscera with concentrations ranging from 19.1 to 533 ng L−1, 12.5 to 253 ng g−1, 11.7 to 149 ng L−1, 114 to 2108 ng g−1 lipid weight (lw), 220 to 638 ng g−1 lw, and 116 to 1356 ng g−1 lw, respectively. The halogenated OPFRs were the primary pollutant in the Jinjiang river water samples, whereas nonhalogenated OPFRs were the dominant OPFRs in the sediments. Brominated flame retardants were not detected in the groundwater, whereas the NBFRs detected in aquatic environment at low frequency. The ΣPBDEs ranged from n.d. to 23.4 ng L−1 and n.d. to 48.7 ng g−1 in the Jinjiang river water and sediment, respectively. BDE-209 was dominant in the sediment samples with concentrations ranging from n.d. to 47.2 ng g−1. The PBDEs levels in the muscle, gills, and viscera of the crucian carp ranged from 10.6 to 90.6 ng g−1 lw, n.d. to 75.6 ng g−1 lw, and n.d. to 219 ng g−1 lw, respectively. BDE-47, chlorinated, and alkyl OPFRs were the main contaminants in the fish samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Flame retardants (FRs), such as polybrominated diphenyl ethers (PBDEs), novel brominated flame retardants (NBFRs), and organophosphorus flame retardants (OPFRs), are added to polymers in consumer products widely to prevent flame spreading (CEFIC 2007), which ensuring the safety of life enormously and boosting the development of human society. In addition to using as FRs in furniture, wood, wall coverings, and textiles, some of these organic compounds are used as plasticizers, antifoaming agents, stabilizers, as well as additives in products of lubricants and hydraulic fluids (Marklund et al. 2005; Fries and Puttmann 2001; Li et al. 2018). Because these chemicals are adsorbed to the surface of the polymer more weakly rather than chemically bounding, previous studies have noted that these compounds can escape into environment by volatilization, leaching, and abrasion (Marklund et al. 2005; Li et al. 2014; Sundkvist et al. 2010). Therefore, FRs had been found in diverse environmental matrices and even in human samples (Fries and Puttmann 2001; Chen et al. 2011).
Due to the hazard of PBDEs, which present risks of persistence, bioaccumulation, and potential endocrine disruptors (Stieger et al. 2014; Wu et al. 2012), pentaBDE and octaBDE have been phased out by EU Water Flamework Directive (BSEF 2011). As a result, PBDEs have been replaced by NBFRs and OPFRs. Nevertheless, NBFRs are considered emerging pollutants because of their accumulation in environmental matrices (Shi et al. 2016). Conversely, ubiquitous occurrence of OPFRs and their potential adverse effects to ecosystem and human health, such as carcinogens and potential neurotoxicants, have attracted much attention (Li et al. 2014; Cequier et al. 2015; Salamova et al. 2014a, b). Therefore, previous studies have been focused on the occurrence of PBDEs, NBFRs, and OPFRs in rivers and sediments (Li et al. 2014; Shi et al. 2016; Cristale et al. 2013a, Sun et al. 2012; Chen et al. 2013; Regnery and Puttmann 2010; Ding et al. 2015; Cristale et al. 2013b, Lee et al. 2016). The detailed information is shown in Table S1 in the Supplementary materials (SM). To date, only a few studies have been investigated the concentrations of PBDEs, NBFRs, and OPFRs in wild fish simultaneously (Chen et al. 2011; Widelka et al. 2016; Giulivo et al. 2016; Hallanger et al. 2015), as well as those in groundwater (Fries and Puttmann 2001; Ding et al. 2015; Khan et al. 2016).
To our knowledge, most investigations of FR levels have been concentrated on coastal cities in China. Little is known regarding the FR levels in paired water–sediment–fish samples in aquatic environments of inland China (Shi et al. 2016; Su et al. 2017), especially for fish samples. Therefore the primary objective of this study was to evaluate simultaneously the contamination of PBDEs, NBFRs, and OPFRs in the ground water, river (paired surfaces water and sediment), and field site fish (fish muscle, gills, and viscera) from Chengdu, China. To the best of our knowledge, this is the first study to investigate simultaneously the PBDEs, NBFRs, and OPFRs in the paired samples of surface water, sediments, and fish, which could help us to understand deeply FRs distribution in aquatic environment and biota.
Materials and Methods
Chemicals and Reagents
The details of chemicals and reagents used in this study are shown in Sect. 1 in the SM.
Samples Collection
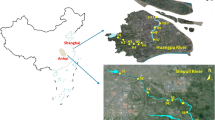
The paired surface water samples (S1 to S28, n = 28), sediment samples (n = 26, expect for S1 and S2, which are at the source of the Jinjiang river with high water velocity, and the sediment could not settle easily), and fish samples (at sites S2, S3, S4, S8, S12, S15, and S17, n = 7) were collected from Jinjiang river during fall 2016 in Chengdu, China (Fig. 1; Table S2 in the SM). 1 L of surface water was collected from 28 sites using amber glass bottles. Sediment samples were collected by a grab sampler, whereas fish samples (only crucian) were obtained from employed fishermen.
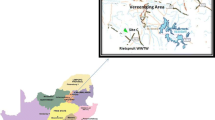
Groundwater samples (n = 29) were collected from established well of the suburb of Chengdu along the third ring road (Fig. 1; Table S3), and the depth of the selected well was at least 20 m. These sample sites located in five different districts, which are Wenjiang district, Pixian district, Xindu district, Longquan district, and Shuangliu district. All of the samples are shallow groundwater.
All water samples were kept at 4 °C until extraction. The sediment samples were freeze-dried and sieved through a stainless steel 100-mesh sieve and kept at −20 °C until extraction. Fishes were dissected and then wrapped with filter paper and kept at −20 °C until extraction.
Sample Preparation
The water samples were extracted using the method described by Cristalea et al. (2012). Briefly, a portion of 500 mL of the water was spiked with the mixed internal standards and was extracted using OASIS HLB 200-mg cartridges (WATERS, USA). The target chemicals were eluted using 15 mL of dichloromethane/hexane (1:1), followed by 15 mL of dichloromethane/acetone (1:1), and then concentrated under soft N2 flow to almost dryness and reconstituted in 250 μL of toluene. Procedural blank was prepared using 500 mL Mill-Q water in the same manner as the water samples simultaneously.
For the sediment samples, the extraction methods were based on the description of lacorte (2013). Briefly, 1.5 g of freeze-dried sediment was transferred to 20 mL glass tubes, spiked with the mixed internal standards, and kept in contact overnight. After that, the sediment samples were extracted using ethyl acetate/cyclohexane (5:2) by vortex (1 min), followed by ultrasonic extraction (10 min). The extraction was centrifuged at 3000 rpm for 10 min. Then, the extract was concentrated to 1 mL under N2 flow. Approximately 200 mg of activated copper was added to each sample and kept in contact overnight. The clean-up was performed using 10 g of Florisil cartridges (CNW). The cartridges were conditioned using 60 mL of ethyl acetate/cyclohexane (5:2). After sample percolation, elution was performed by 60 mL of ethyl acetate/cyclohexane (5:2). Finally, the extract was concentrated under N2 flow to almost dryness and reconstituted in 500 μL of toluene.
In the case of the fish samples, 1 g of the sample (muscle, gill, or viscera) was weighted and extracted using Soxhlet extraction with hexane/DCM (1:1) for 24 h after spiking with the mixed internal standards. Lipid content was detected by gravimetric determination using 10% of the extraction with solvent evaporation method. Then, the residual extraction was divided into two parts; half of the extraction was used for analysis of OPFRs directly after purifying by the method described for the sediment samples above, and the other part was purified using the method described by Chen et al. (2011) with a little modification to determine PBDEs and NBFRs. Briefly, the extraction was treated with sulphuric acid to remove lipids, then followed by purifying on a 2-g silica gel (CNW). Elution was performed by 6.5 mL of hexane/DCM (3:2) and 8 mL of DCM. Finally, the extraction was concentrated under N2 flow to almost dryness and reconstituted in 200 μL of toluene. More physic-chemical data of the investigated FRs are displayed in Table S4 in the SM. Procedural blanks of the sediment and fish samples were performed in the same manner of the samples without addition of sediment and fish, respectively.
Analytical Methods
Analysis of the OPFRs, PBDEs, and NBFRs were performed using Shimadzu GC-EI-MS/MS equipped with an AOC-20i+s autosampler (Kyoto, Japan). Helium was used as the carrier gas and Ar as the collision gas. The column for analysis of the OPFRs was Rtx-5MS (30 m × 0.250 mm × 0.25 μm) (Shimadz, Japan). Injections (1 µL) were performed in the pulsed splitless mode. The injector, transfer line, and ion source were set at 300, 300, and 230 °C, respectively. The injection port was held at 300 °C. The flow rate of helium (99.999%) was 1.52 mL min−1. GC-EI-MS/MS parameters of the FRs for MRM detection in the positive ion mode are displayed in Table S5 in the SM.
Separations of the PBDEs and NBFRs were performed using a DB-5MS column (15 m × 0.250 mm × 0.1 μm) (J&W Scientific, USA). Injections (1 µL) were made in the pulse splitless mode. The injector, transfer line, and ion source were set at 300, 300, and 230 °C, respectively. The injection port was held at 285 °C. The gas chromatographic oven temperature program for separation of the OPFRs, PBDEs, and NBFRs are shown in Table S6 in the SM.
Quality Control and Quality Assurance
As shown in Table S7 in the SM, good linearity were obtained for the OPFRs (r = 0.9910–0.9999) and BFRs (R = 0.9863–0.9998). All of the investigated BFRs were not detected in the procedural blanks. Therefore, the method limits (MDLs) of detection of the BFRs were calculated as three times of the signal to noise ratio obtained with spiked samples, and ranging from 0.03 to 152 ng L−1 for water, from 0.02 to 101 ng g−1 for sediment, and from 0.02 to 118 ng g−1 for fish tissues. Most of the OPFRs were detected in the procedural blanks, and the MDLs of the OPFRs were calculated as the average blank concentrations plus three times the standard deviation. Recoveries were investigated using each type of the sample by triplicate after spiking the native FRs standards, and the results are presented in Table S9 in the SM. To ensure accuracy and precision during analysis, a group of the solutions, including 10, 20, and 100 μg L−1 FRs standards, were analyzed after every 30 injections, and the results are shown in Table S8 and S9.
Results and Discussion
Concentrations and Spatial Distribution of the FRs in the Investigated Matrices
River Water
Jinjiang river is the major river of Chengdu. Two tributaries of the upper reaches are Fu River and Nan River. HeJiang kiosk which located at the center of Chengdu (first-ring area) is the confluence of the two rivers. The two tributaries (Fu River and Nan River) and the interflow together are called Jinjiang River.
The levels of the ΣOPFRs, ΣPBDEs, and ΣNBFRs in the Jinjiang River are presented in Fig. 2. It is clear that the concentrations of the FRs followed the tendency in ∑OPFRs > ∑PBDEs > ∑NBFRs, which was in line with the previous report (Khan et al. 2016).
The OPFRs were detected in all of the river water samples, whereas the PBDEs and NBFRs were found in 39.2% and 46.4% of the surface water samples, and the details of the FRs levels are shown in Fig. 2 and Table 1. The concentrations of the ΣOPFRs in the river water ranged from 19.1 to 533 ng L−1, with a mean value of 204 ng L−1. TCPP and TCEP were the most abundant contaminants in the surface water samples, respectively, followed by TEP and TBEP. The higher levels of TCPP and TCEP mainly caused by the widely use of them in FRs, rubber, and textile coatings (Marklund et al. 2005). On the other hand, low biodegradability and low octanol–water coefficient of TCPP (logKow = 2.59) and TCEP (logKow = 1.47) make them more abundant in water, which was in line with previous study conducted in urban surface water from Beijing (Shi et al. 2016). It is worth noting that the concentrations of TCPP were significantly higher (P < 0.01) than those of TCEP in the surface water samples. TCEP was gradually replaced by TCPP on the industrial application in Europe from 1990s due to the carcinogenic and neurotoxic to animals (Quednow and Puttmann 2009), which might be emulated by China or around the world.
OPFRs can be divided into three main groups: halogenated OPFRs, aryl OPFRs, and alkyl OPFRs. TCEP, TCPP, and TDCIPP belong to halogenated OPFRs, TPHP, EHDPP, TCP, and TCrP belong to aryl OPFRs, whereas TEP, TPrP, TiBP, TnBP, TBEP, and TEHP are a part of alkyl OPFRs. Based on the current study, the halogenated phosphate were the dominant OPFRs in the surface water, and the concentrations of the chlorinated OPFRs were significantly higher (P < 0.01) than those of the alkyl OPFRs and aryl OPFRs in the water samples. In addition, the concentrations of the alkyl OPFRs were significantly higher (P < 0.01) than those of the aryl OPFRs. The higher solubility of the chlorinated OPFRs compared with the alkyl OPFRs and aryl OPFRs might partially explain the higher levels of the halogenated phosphate in the investigated water. The levels of halogenated phosphate showed positive correlation corresponding with those in alkyl OPFRs and aryl OPFRs, suggesting that nonhalogenated phosphate was combined using with halogenated phosphate in the study area. No significant difference of the OPFRs concentrations was observed in the upper reaches (S1-S4), middle reaches (S5-S23), and the lower reaches (S24-S28) of Jinjiang river (P > 0.05). The levels of the OPFRs increased obviously at sampling sites S10, S15, and S25. The sampling site S15, which located near the furniture factories and sofa stores, exhibited the highest level of the OPFRs. In addition, the sampling sites S10 and S25 located around densely populated holiday resort, indicating that anthropogenic activities might have contribution to OPFRs pollution. The ratio of chlorine phosphate to alkyl phosphate (Rchlorine phosphate/alkyl phosphate) were greater than 1 in most of the river samples, and ranged from 1.07 to 21.1 except for the S3 (0.10), S5 (0.41), and S12 (0.92), suggesting that there might be other point sources of alkyl phosphate around these three sites. Therefore, the OPFRs levels were related to industrial and human activity at each sample site (Santín et al. 2013).
The average concentration of the ΣBFRs in the river was 3.59 ng L−1, and it was significantly lower than the level of the ΣOPFRs (P < 0.01). As shown in Fig. 2, the composition of the PBDEs exhibited great variations among each sampling site. In general, BDE-47 and BDE-28 were the most frequently detected compounds with detection frequency of 28.6% equally, followed by BDE-99 (14.3%), BDE-100 (14.3%), and BDE-209 (10.7%). BDE-153, BDE-138, BDE-183, and BDE-197 were only found at S9, whereas BDE-154 was detected at S9 and S28. Relatively higher levels of the PBDEs were found at S7, S8, and S9, and BDE-209 was only detected at the three sites. There are three waste water treatment plants (WWTPs) around the sites S7, S8, and S9 (Fig. 1). Previous studies have documented that PBDEs with higher degree of bromination have slower biodegradation rate (Zhao et al. 2018) and whether WWTPs contributed higher PBDEs levels in Jinjiang River water need further study. Generally, PBDEs pollution was mainly concentrated in the middle reaches of the Jinjiang River.
In the case of the NBFRs, PBT was the predominant component. The detection frequency of PBT was 21.4%, followed by HBB (14.3%), whereas the concentrations of ATE, PBEB, TBB, BTBPE, and DBDPE were below the MDLs in the Jinjiang River water based on the current study. PBT was mostly used in textile, rubbers, polyethylene, polypropylene, and polystyrene. The relatively higher PBT detection frequency and concentration in the river water samples might be caused by its lower biodegradability. Like PBDEs and OPFRs, relatively higher concentration of NBFRs were found in the middle reaches of the Jinjiang River water, which has a high population density, and around the furniture factories, sofa stores, and WWTPs. The discharge of wastewater from furniture factories and sofa stores and WWTPs might contaminate the surface water.
The correlation coefficient matrix of the investigated compounds in the Jinjiang River water are displayed in Table S10. Briefly, significant positive correlation were found among the different FRs compounds. For instance, the isomeric compounds TiBP and TnBP, BDE-99 and BDE-100, and BDE-153 and BDE-154 were significantly positive correlated (P < 0.01), suggesting that these isomeric compounds might have similar pollution resource (Khan et al. 2016; Su et al. 2017). Positive correlation existed in some of the OPFRs and BFRs compounds, such as PBT and TCEP (0.419), PBT and TCPP (0.739), PBT and TEHP (0.323), PBT and TCP (0.416), HBB and TEHP (0.350), and BDE-28 and TCPP (0.328). In addition, significant positive correlation were presented in some of the NBFRs and PBDEs, such as BDE-28 and PBT (0.389), BDE-28 and HBB (0.373), BDE-47 and PBT (0.337), BDE-47 and HBB (0.443), BDE-99 and BTBPE (0.340), BDE-153 and BTBPE (0.720). The significant correlation among the different FRs congeners revealed that these compounds might come from similar sources, and the results were in line with the previous study (Su et al. 2017). Besides, significantly positive correlation (P < 0.05) were observed in the total concentrations of the OPFRs and PBDEs, NBFRs, and BFRs; however, the levels of the OPFRs and PBDEs showed insignificant correlation.
The concentrations of the investigated FRs reported by previous studies are shown in Table S1. Obviously, the ΣPBDEs level in the Jinjiang River was relatively lower than those in Pakistan and the United Kingdom (Cristale et al. 2013b, Mahmood et al. 2015). However, it was much higher than those in surface water from the United States, Spain, and Canada (Cristalea et al. 2012; Venier et al. 2014; Law et al. 2006a, b). In the case of the domestic river, the concentration of the ΣPBDEs in the Jinjiang River was relatively higher than those in Jiulong River estuary in Fujian (Wu et al. 2015). Limited data are available on NBFRs in surface water. Based on the current study, the ΣNBFRs level was approximately three orders of magnitude higher than that in the United States and Canada (Venier et al. 2014; Law et al. 2006a, b). However, the ΣNBFRs concentration was much lower than that in Liuyang River in China (Ting et al. 2009). The level of the ΣOPFRs was comparable to that obtained in Arga (urban area in Spain) and Elbe (Germany) (Cristalea et al. 2012; Bollmann et al. 2012) but relatively higher than those in the United States (Venier et al. 2014) and relatively lower than that in Rhine River (Germany), United Kingdom, Besòs (industrial area in Spain), Beijing, and Bohai Sea in China (Shi et al. 2016; Cristale et al. 2013b, Cristalea et al. 2012; Bollmann et al. 2012; Wang et al. 2015).
Sediment
As shown in Fig. 3, the OPFRs were the dominant FRs in the sediment samples, which was similar to that in the water samples. The concentrations of the ΣOPFRs in the sediment samples ranged from 32.3 to 171 ng g−1 of dry weight (dw), with an average concentration of 79.3 ng g−1. The lipophilic properties of the FRs make them bind well to particles and have a tendency to accumulate in sediments (Cristale et al. 2013a), and sediments are important sinks in aquatic ecosystems for FRs (Iqbal et al. 2017). TiBP was the most dominant contaminant in the sediments (Fig. 3; Table 1). TEP, TEHP, TBEP, TCPP, and TCEP presented intermediate average concentrations up to 7.04 ng g−1 dw, whereas TnBP, TPHP, TDCIPP, TPrP, TCrP, and TCP exhibited relatively lower concentrations and ranged from n.d. to 24.3 ng g−1 dw. The most frequently detected compounds were TCPP, TCEP, TDCIPP, TEP, and TiBP (detected frequency 100%), followed by TBEP (97%) and TnBP (86%). Unlike the river water, the nonhalogenated phosphate were more abundant than the halogenated phosphate in the sediments. Nonhalogenated phosphate are mostly used as plasticizers, antifoaming agents, and additives (Quintana et al. 2008). Therefore, the large consumption of nonhalogenated phosphate might result in high levels of contamination. On the other hand, despite of lower biodegradability and relative persistent of halogenated OPFRs, periodic dredging of the sediments in Jinjiang River by the government of Chengdu might result in low levels of halogenated OPFRs due to their relatively low consumption compared with nonhalogenated OPFRs.
To our knowledge, limited data have been reported on OPFRs contamination in freshwater sediment, especially for China (Table S1) (Iqbal et al. 2017). The concentration of the ΣOPFRs in this study was relatively higher than that in Greece, Yugoslavia, and Netherlands (Brandsma et al. 2015; Giulivo et al. 2017) and slightly lower than that in Italy (Giulivo et al. 2017).
The alkyl phosphate were the dominant OPFRs and the concentrations of alkyl phosphate were significantly higher (P < 0.01) than those of chlorine phosphate and aryl phosphate in the sediment samples. In addition, the concentrations of alkyl phosphate shows significantly positive correlated (P < 0.01) with chlorine phosphate and aryl phosphate, suggesting that different phosphate have similar sources. Relatively higher concentrations of the OPFRs were found between S10 and S17 in the middle reaches of the Jinjiang River sediment samples. The OPFRs concentrations decreased at S18 might due to the fact that the OPFRs have been partitioned into the sediments in the upper reaches, such as the sampling sites S15 to S17, which were influenced by WWTPs as shown in Fig. 1. In addition, the OPFRs concentrations at sampling site S13 was approximately equal to the sum of the OPFRs levels of S8 and S12, which was located at the confluence of two tributaries (Fu River and Nan River).
The concentrations of the BFRs ranged from n.d. to 51.6 ng g−1 with a mean value of 10.7 ng g−1 in the sediments, with average concentrations of 9.03 and 1.69 ng g−1 for ΣPBDEs and ΣNBFRs, respectively. In the case of the PBDEs, unlike the river water, BDE-209 was the main compound (accounted for 92.8%) in the sediments, which was in accordance with previous studies from the United Kingdom, Italy, Yugoslavia, Korea, and China (Chen et al. 2013; Cristale et al. 2013b, Giulivo et al. 2017; Lee et al. 2012), reflecting the fact that the use of the deca-BDE technical formulation accounted for 75% of the overall BDE consumption (Martellini et al. 2016). As regards to spatial distribution of BDE-209, higher BDE-209 concentrations were found at the sampling sites S8 to S14, which were surrounded by WWTPs, indicating that the discharge of WWTPs influenced the BDE-209 concentration in the sediment. Based on the current study, BDE-47 was the secondary main PBDE congener (3.22%) in the sediments. In addition, BDE-28, BDE-154, BDE-153, BDE-138, and BDE-196 were below the MDLs in the sediments. Obviously, relatively higher PBDEs pollution was found in the middle reaches (S8 to S18; Fig. 3), implying that the PBDEs levels and distribution were affected by the human activity. The concentration of the ΣPBDEs in the sediment in Jinjiang river was lower than most foreign countries, such as South Africa, Italy, Greece, Spain, and Yugoslavia (Cristalea et al. 2012; Giulivo et al. 2017; Sühring et al. 2015; Olukunle et al. 2015). The ΣPBDEs level in this study was at least ten times lower than that in Dongjiang and Zhujiang Rivers (Chen et al. 2013) and relatively lower than that in Dayanhe and Pearl Rivers in China (Chen et al. 2013).
With respect to the NBFRs, BTBPE was the predominant contamination. Higher level of BTBPE was found at S26, and the PBDEs and OPFRs also exhibited relatively higher concentrations, suggesting that there might have point pollution source. The sediment concentration of the ΣNBFRs from Jinjiang River was much lower than that in Besòs in Spain (Cristalea et al. 2012) and relatively lower than that in Yugoslavia (Giulivo et al. 2017). In addition, it was approximately two orders of magnitude lower than that in the Dongjiang, Zhujiang, and Dayanhe Rivers in China (Chen et al. 2013) and relatively lower than that in the Pearl River (Chen et al. 2013). However, the ΣPBDEs and ΣNBFRs levels were relatively higher than that in the Yellow River in China (Su et al. 2017).
The correlation coefficient matrix of the selected FRs, ΣOPFRs, ΣPBDEs, ΣNBFRs, and ΣBFRs in the sediments are displayed in Table S11. Obviously, most of the PBDEs and OPFRs congeners levels were significantly correlated with each other, especially for BDE-47 and other PBDE congeners, indicating that debromination could release lower brominated compounds (Law et al. 2014). Part of the OPFRs and BFRs compounds were positively correlated with each other [such as BTBPE and TCEP (0.404); BTBPE and TEHP (0.432); BDE-47 and TCPP (0.569); BDE-47 and TEHP (0.662); BDE-99 and TCPP (0.405)]. Similar results were observed for some of the NBFRs and PBDEs compounds [e.g., BDE-47 and BTBPE (0.714); BDE-99 and BDE-100 (0.601); BDE-100 and BTBPE (0.446); BDE-209 and TBB (0.393)]. The significant correlation among the different FRs compounds in the sediments samples revealed that these compounds might come from similar sources.
In addition, the total organic contents (TOC) of the investigated sediment samples are shown in the Fig. S1. The TOC levels of the sediments in Jinjiang river ranged from 0.69 to 5.55%. Significant correlation was found between TOC and the PBDEs and OPFRs congeners (P < 0.01), indicating that TOC level influenced the distribution of the OPFRs and PBDEs (Giulivo et al. 2017).
Fish
The concentrations of the OPFRs and BFRs in the fish muscle, gills, and viscera samples of the collected crucian carp from the Jinjiang river are given in Table 1 and Fig. 4. All of the OPFRs compounds were detected in the fish samples expect for TCrP and TCP, and the total mean concentrations of the OPFRs in the fish muscle, gill, and viscera were 1161, 375, and 692 ng g−1 lipid weight (lw), respectively. No significant difference in the OPFRs levels was found among the fish muscle, gill, and viscera samples. TCPP was the most predominant compound, which contributed 33.1% of the total OPFRs, followed by TBEP (20.4%) and TiBP (19.5%).
Generally, higher fish OPFRs concentrations were found in the middle reaches, such as the fish sampling sites S12, S15, and S17, implying anthropogenic activities and FRs environmental levels could influence OPFRs level in aquatic biota. Considering swimming range of the fish, it is reasonable that the mean concentrations of the sediment and river water samples from three upper reaches sampling sites and three lower reaches sampling sites around each fish sampling site were used to calculate bioaccumulation factor (BAF, ratio of the FRs level in the fish tissue to those in the water sample) and biota-sediment accumulation factor (BSAF, ratio of the FRs level in the fish tissue to those in the sediment sample) of the fish muscle, gills, and viscera samples. Apparently, the BAF and BSAF of the OPFRs in the fish samples were greater than 1 around the fish sampling site, indicating that the OPFRs have bioaccumulation potential in fish. As for chlorine OPFRs, the mean concentration of TCPP was higher than that of TCEP and TDCIPP in the fish. It could be partially explained by the huge consumption of TCPP nowadays and even the higher bioaccumulation potential of TCPP, which needs further study.
Limited data were reported on OPFRs contamination in the fish samples. The ΣOPFRs level in the muscle, gills, and viscera of crucian carp were relatively higher than those in Italy, Greece, and Yugoslavia (Giulivo et al. 2017).
The mean ΣBFRs concentrations in the fish muscle, gills, and viscera were 33.4, 22.2, and 305 ng g−1 lw, respectively, of which ΣPBDEs were 31.5, 22.2, and 55.8 ng g−1 lw, and ΣNBFRs were 1.91, n.d., and 5.42 ng g−1 lw, respectively. Significant differences were found between the OPFRs and BFRs levels in the fish muscle, gills, and viscera samples (P < 0.01) based on the current study.
Based on the current study, the mean BAF and BSAF values of the ΣBFRs in the fish muscle, gills, and viscera samples were greater than 1, suggesting the BFRs have bioaccumulation potential. Like the OPFRs in the fish, the middle reaches sampling sites S8 and S12 displayed higher concentrations of the PBDEs levels, and the PBDEs contributed 94.1% of the total BFRs contamination in the fish samples. BDE-47 and BDE-209 were the most abundant compounds and accounted for 34.4% and 29% of the ΣPBDEs, respectively. Followed by BDE-154 and BDE-28, which respectively contributed 14.1% and 13.1% of the ΣPBDEs. BDE-47 was the most frequently detected compounds (81%), probably due to its higher bioaccumulation capacity compared with BDE-209, which has a higher degree of bromination (Eljarrat et al. 2007). Interestingly, the concentration of BDE-209 in the crucian was relatively higher compared with those in Hyco River fish (Chen et al. 2011), and BDE-209 was only detected in the viscera samples at concentrations up to 154 ng g−1, indicating that BDE-209 could reduce by metabolism and debromination in the fish. The average ratio of BDE-47 to ΣPBDEs was 0.50 and 0.42 in the fish muscle and gill samples, respectively, which were much greater than that in the water (0.12) and sediment (0.03). It indicated that the composition of PBDEs were rather different in aquatic environment and biota due to the accumulation and metabolism, especially for debromination in biota. As regarding to the NBFRs, only BTBPE, DPTE, and TBB were found in the viscera or fish muscle samples, indicating that most investigated NBFRs would not accumulate in the fish or have a fast metabolism velocity based on the current study.
The levels of the FRs in fish samples reported in the previous studies around the world are shown in Table S1. Briefly, the levels of the ΣPBDEs in the fish viscera presented in this study was higher than those in Italy, Greece, Yugoslavia, Netherlands, South Africa, and Pearl River in China (Brandsma et al. 2015; Giulivo et al. 2017; Olukunle et al. 2015; Sun et al. 2016). However, it was approximately three times lower than that in the Dan and Roanoke Rivers in United States (Chen et al. 2011). As for the ΣPBDEs concentrations in fish muscle and gill, the results of the presented study were relatively lower than that in Italy, Greece, Yugoslavia, North Spain (Ebro River) (Chen et al. 2011; Widelka et al. 2016; Santín et al. 2013; Giulivo et al. 2017; Luigi et al. 2015), the reason might be the Adige and Evrotas Rivers were deeply influenced by agriculture and industrial activities. In addition, Chub, brown trout, marble trout, bullhead, and grayling that collected from the Adige and Evrotas Rivers are omnivorous and might accumulate more contamination from food chain. It is worth noting that the ΣPBDEs concentration of U.S. common carp in 2006–2007 was approximately two orders of magnitude higher than that in the current study. Because there were large usage of PBDEs in the United States before the prohibition of PBDEs, indicating that the large use of PBDEs and industrial activity were deeply influenced by the concentration of PBDEs in biota (Chen et al. 2011). However, the ΣPBDEs concentrations in fish muscle and gill was relatively higher than those in South Spain (Santín et al. 2013; Brandsma et al. 2015; Olukunle et al. 2015). Limited data are available on NBFRs levels in fish. The mean concentrations of the ΣNBFRs in fish muscle, gills, and viscera samples were relatively lower than that in Spain based on the current study (Santín et al. 2013, 2016).
The correlation coefficient matrix of ΣOPFRs and ΣBFRs among the river water, sediment, fish muscle, viscera, and gill samples are given in Table S12 in the SM. Briefly, significant positive correlation in ΣOPFRs and ΣBFRs were found between viscera and sediment samples, and muscle and sediment samples, indicating similar pollution source existed in these samples. The FRs levels in the river water and fish samples existed insignificant correlation. In addition to bioaccumulation, biomagnification via food ingestion through food chain is a key factor influence contaminants levels in fish (Greaves and Letcher 2017; Pittinger and Pecquet 2018; Gu et al. 2018) and needs further study.
Concentration and Spatial Variation of the FRs in the Groundwater
The investigated OPFRs were detected in all of the groundwater samples, whereas the BFRs were below the MDLs. The levels of the OPFRs in the groundwater are presented in Fig. 5 and Table 1. The concentrations of the ΣOPFRs in the groundwater ranged from 12.5 to 253 ng L−1, with a mean value of 51.8 ng L−1. TCPP and TCEP were the most abundant contaminants in most of the groundwater samples. In addition, TCEP and TCPP were detected at a frequency of 100%, followed by TDCIPP (97%) and TEHP (90%), whereas TPrP, TPHP, TCrP, and TCP were detected at the lowest concentration. Significant differences (P < 0.01) were found in the OPFRs levels between the river water and groundwater, indicating that sorption or degradation might be happen during the OPFRs infiltration. Previous study found that groundwater pollution was mainly caused by the discharge of domestic and industrial sewage (Khan et al. 2016). Similar results were found in our study, for instance, exceeding high OPFRs concentrations were measured in Xindu district, and there were some electromechanical equipment factories and furniture factories around XD2, XD4, and XD5. Besides, the relatively higher concentration of the OPFRs was found at SL8, located at the area with building materials factories and plastic packaging factories. It also might partially explain the phenomenon that TiBP presented the highest concentrations at SL8, as nonhalogenated phosphate are mostly used as plasticizers. The sampling sites WJ1 and LQ5 also showed relatively higher OPFRs concentration due to the area located at intensive residential area with high population density and more human activity. The results revealed that the OPFRs contamination in the groundwater samples was deeply influenced by industrial activity and human activity.
It is worth noting that the ratio of TCEP to TCPP (RTCEP:TCPP) in most of the groundwater samples was greater than 1. However, the RTCEP:TCPP in all of the Jinjiang river samples was less than 1. In addition, approximately a half of the groundwater samples with Rchlorine phosphate:alkyl phosphate less than 1, but most of the Jinjiang river samples with Rchlorine phosphate:alkyl phosphate greater than 1. Soil OPFRs pollution level, the solubility and degradability of the OPFRs are the key factors that influence the transfer of the investigated OPFRs from the upper soil layers into groundwater and needs further study.
The correlation coefficient matrix of the selected OPFRs in the groundwater are given in Table S13. In general, the isomeric compounds (TiBP and TnBP) were significantly positive correlated (P < 0.01), and some of the OPFRs compounds were also positive correlated (P < 0.01 or P < 0.05), suggesting these compounds have similar pollution source or leaching process.
Conclusions
This is the first report on simultaneous analysis of BFRs (PBDEs and NBFRs) and OPFRs in groundwater, paired surface water, sediment, and fish (muscle, gills, and viscera) in China. The investigated OPFRs were dominant FRs and existed extensively in aquatic environment. PBDEs were main contributors to BFRs in the sediments and the fish samples. NBFRs were seldom detected in aquatic environment. The investigated OPFRs were detected in all of the groundwater samples with concentrations ranging from n.d. to 68.1 ng L−1, while all of the BFRs were blow the MDLs. Relatively higher concentrations of the FRs were found in the middle reaches of Jinjiang River, indicating that industrial and human activity could influence the distribution and composition of the FRs. The halogenated OPFRs were more abundant than the nonhalogenated OPFRs in the Jinjiang River water samples, whereas nonhalogenated OPFRs were the dominant OPFRs in the sediments. BDE-209 was the predominant PBDEs congener in the sediments. BDE-47 was the main PBDEs congener in the fish samples and BDE-209 only detected in the viscera samples. Chlorinated and alkyl OPFRs were dominant pollutants in the fish samples.
References
Bollmann UE, Moller A, Xie Z et al (2012) Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res 46:531–538
Brandsma SH, Leonards PE, Leslie HA, De BJ (2015) Tracing organophosphorus and brominated flame retardants and plasticizers in an estuarine food web. Sci Total Environ 505:22–31
BSEF (2011) Bromine science and environmental forum (BSEF). http://www.bsef.com/
CEFIC (2007) European Flame Retardants Association (EFRA). What are FRs? Flame retardant market statistics. www.cefic-efra.eu
Cequier E, Sakhi AK, Marce RM (2015) Human exposure pathways to organophosphate triesters: a biomonitoring study of mother–child pairs. Environ Int 75:159–165
Chen D, La Guardia MJ, Luellen DR et al (2011) Do temporal and geographical patterns of HBCD and PBDE flame retardants in U.S. fish reflect evolving industrial usage? Environ Sci Technol 45:8254–8261
Chen SJ, Feng AH, He MJ et al (2013) Current levels and composition profiles of PBDEs and alternative flame retardants in surface sediments from the Pearl River Delta, southern China: comparison with historical data. Sci Total Environ 444:205–211
Cristale J, Garcia Vazquez A, Barata C, Lacorte S (2013a) Priority and emerging flame retardants in rivers: occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ Int 59:232–243
Cristale J, Katsoyiannis A, Sweetman AJ et al (2013b) Occurrence and risk assessment of organophosphorus and brominated flame retardants in the River Aire (UK). Environ Pollut 179:194–200
Cristalea J, Quintanab J, Chalera R (2012) Gas chromatography mass spectrometry comprehensive analysis of organophosphorus, brominated flame retardants, byproducts and formulation intermediates in water.pdf. J Chromatogr A 1241:1–12
Ding J, Shen X, Liu W et al (2015) Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci Total Environ 538:959–965
Eljarrat E, Labandeira A, Marsh G et al (2007) Decabrominated diphenyl ether in river fish and sediment samples collected downstream an industrial park. Chemosphere 69:1278–1286
Fries E, Puttmann W (2001) Occurrence of organophosphate esters in surface water and ground water in Germany. J Environ Monit 3:621–626
Giulivo M, Capri E, Eljarrat E, Barcelo D (2016) Analysis of organophosphorus flame retardants in environmental and biotic matrices using on-line turbulent flow chromatography-liquid chromatography-tandem mass spectrometry. J Chromatogr A 1474:71–78
Giulivo M, Capri E, Kalogianni E et al (2017) Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European river basins. Sci Total Environ 586:782–791
Greaves AK, Letcher RJ (2017) A review of organophosphate esters in the environment from biological effects to distribution and fate. Bull Environ Contam Toxicol 98:2–7
Gu YG, Ning JJ, Ke CL, Huang HH (2018) Bioaccessibility and human health implications of heavy metals in different trophic level marine organisms: a case study of the South China Sea. Ecotoxicol Environ Saf 163:551–557
Hallanger IG, Sagerup K, Evenset A et al (2015) Organophosphorous flame retardants in biota from Svalbard, Norway. Mar Pollut Bull 101:442–447
Iqbal M, Syed JH, Katsoyiannis A et al (2017) Legacy and emerging flame retardants (FRs) in the freshwater ecosystem: a review. Environ Res 152:26–42
Khan MU, Li J, Zhang G, Malik RN (2016) First insight into the levels and distribution of flame retardants in potable water in Pakistan: an underestimated problem with an associated health risk diagnosis. Sci Total Environ 565:346–359
Lacorte JCS (2013) Development and validation of a multiresidue method for the analysis of polybrominated diphenyl ethers, new brominated and organophosphorus flame retardants in sediment, sludge and dust.pdf. J Chromatogr A 1305:267–275
Law K, Halldorson T, Danell R et al (2006a) Bioaccumulation and trophic transfer of some brominated flame retardants in a Lake Winnipeg (Canada) food web. Environ Toxicol Chem 25:2177
Law RJ, Allchin CR, Boer JD et al (2006b) Levels and trends of brominated flame retardants in the European environment. Chemosphere 64:209
Law RJ, Covaci A, Harrad S et al (2014) Levels and trends of PBDEs and HBCDs in the global environment: status at the end of 2012. Environ Int 65:147–158
Lee IS, Kim KS, Kim SJ et al (2012) Evaluation of mono- to deca-brominated diphenyl ethers in riverine sediment of Korea with special reference to the debromination of DeBDE209. Sci Total Environ 432:128
Lee S, Jeong W, Kannan K, Moon HB (2016) Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res 103:182–188
Li J, Yu N, Zhang B (2014) Occurrence of organophosphate flame retardants in drinking water from China. Water Res 54:53–61
Li X, Liu HL, Jia XS (2018) Novel approach for removing brominated flame retardant from aquatic environments using Cu/Fe-based metal-organic frameworks: a case of hexabromocyclododecane (HBCD). Sci Total Environ 621:1533–1541
Luigi V, Giuseppe M, Claudio R (2015) Emerging and priority contaminants with endocrine active potentials in sediments and fish from the River Po (Italy). Environ Sci Pollut Res Int 22:14050–14066
Mahmood A, Malik RN, Li J, Zhang G (2015) Distribution, congener profile, and risk of polybrominated diphenyl ethers and dechlorane plus in water and sediment from two tributaries of the Chenab River, Pakistan. Arch Environ Contam Toxicol 68:83–91
Marklund A, Andersson B, Haglund P (2005) Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants. Environ Sci Technol 39:7423–7429
Martellini T, Diletti G, Scortichini G et al (2016) Occurrence of polybrominated diphenyl ethers (PBDEs) in foodstuffs in Italy and implications for human exposure. Food Chem Toxicol 89:32–38
Olukunle OI, Sibiya IV, Okonkwo OJ, Odusanya AO (2015) Influence of physicochemical and chemical parameters on polybrominated diphenyl ethers in selected landfill leachates, sediments and river sediments from Gauteng, South Africa. Environ Sci Pollut Res Int 22:2145–2154
Pittinger CA, Pecquet AM (2018) Review of historical aquatic toxicity and bioconcentration data for the brominated flame retardant tetrabromobisphenol A (TBBPA): effects to fish, invertebrates, algae, and microbial communities. Environ Sci Pollut Res 25:14361–14372
Quednow K, Puttmann W (2009) Temporal concentration changes of DEET, TCEP, terbutryn, and nonylphenols in freshwater streams of Hesse, Germany: possible influence of mandatory regulations and voluntary environmental agreements. Environ Sci Pollut Res Int 16:630–640
Quintana JB, Rodil R, Reemtsma T et al (2008) Organophosphorus flame retardants and plasticizers in water and air II. Analytical methodology. TrAC Trends Anal Chem 27:904–915
Regnery J, Puttmann W (2010) Occurrence and fate of organophosphorus flame retardants and plasticizers in urban and remote surface waters in Germany. Water Res 44:4097–4104
Salamova A, Ma Y, Venier M, Hites RA et al (2014a) High levels of organophosphate flame retardants in the great. Environ Sci Technol Lett 1:8–14
Salamova A, Hermanson MH, Hites RA (2014b) Organophosphate and halogenated flame retardants in atmospheric particles from a European Arctic site. Environ Sci Technol 48:6133–6140
Santín G, Barón E, Eljarrat E, Barceló D et al (2013) Emerging and historical halogenated flame retardants in fish samples from Iberian rivers. J Hazard Mater 263:116–121
Santín G, Eljarrat E, Barceló D (2016) Simultaneous determination of 16 organophosphorus flame retardants and plasticizers in fish by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1441:34–43
Shi Y, Gao L, Li W (2016) Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ Pollut 209:1–10
Stieger G, Scheringer M, Ng CA, Hungerbuhler K (2014) Assessing the persistence, bioaccumulation potential and toxicity of brominated flame retardants: data availability and quality for 36 alternative brominated flame retardants. Chemosphere 116:118–123
Su X, Li Q, Feng J et al (2017) Legacy and emerging halogenated flame retardants in the middle and lower stream of the Yellow River. Sci Total Environ 601–602:1619–1627
Sühring R, Barber JL, Wolschke H et al (2015) Fingerprint analysis of brominated flame retardants and Dechloranes in North Sea sediments. Environ Res 140:569
Sun J, Liu J, Liu Q et al (2012) Sample preparation method for the speciation of polybrominated diphenyl ethers and their methoxylated and hydroxylated analogues in diverse environmental matrices. Talanta 88:669–676
Sun R, Luo X, Tang B et al (2016) Persistent halogenated compounds in fish from rivers in the Pearl River Delta, South China: geographical pattern and implications for anthropogenic effects on the environment. Environ Res 146:371
Sundkvist AM, Olofsson U, Haglund P et al (2010) Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit 12:943–951
Ting R, Wang YW, Chang W et al (2009) Identification and evaluation of a novel heterocyclic brominated flame retardant tris(2,3-dibromopropyl) isocyanurate in environmental matrices near a manufacturing plant in Southern China. Environ Sci Technol 43:3080
Venier M, Dove A, Romanak K et al (2014) Flame retardants and legacy chemicals in Great Lakes’ water. Environ Sci Technol 48:9563
Wang R, Tang J, Xie Z et al (2015) Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, north China. Environ Pollut 198:172–178
Widelka M, Lydy MJ, Wu Y, Chen D (2016) Statewide surveillance of halogenated flame retardants in fish in Illinois, USA. Environ Pollut 214:627–634
Wu J, Zhang Y, Luo X et al (2012) A review of polybrominated diphenyl ethers and alternative brominated flame retardants in wildlife from China: levels, trends, and bioaccumulation characteristics. J Environ Sci 24(2):183–194
Wu Y, Wang X, Li Y et al (2015) Polybrominated diphenyl ethers, organochlorine pesticides, and polycyclic aromatic hydrocarbons in water from the Jiulong River Estuary, China: levels, distributions, influencing factors, and risk assessment. Environ Sci Pollut Res 24:1–13
Zhao CH, Yan M, Zhong H et al (2018) Biodegradation of polybrominated diphenyl ethers and strategies for acceleration: a review. Int Biodeterior Biodegrad 129:23–32
Acknowledgements
This study was supported by the Natural Science Foundation of China (No. 21607108), MOE key laboratory of pollution processes and environmental criteria (Nankai University) open fund project (No. KL-PPEC-2013-7), and Tianjin fishery development service center youth science and technology innovation project (No. 18cz08).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hou, L., Jiang, J., Gan, Z. et al. Spatial Distribution of Organophosphorus and Brominated Flame Retardants in Surface Water, Sediment, Groundwater, and Wild Fish in Chengdu, China. Arch Environ Contam Toxicol 77, 279–290 (2019). https://doi.org/10.1007/s00244-019-00624-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00624-x