Abstract
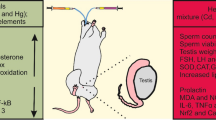
Few or no studies have measured the toxic effects of subchronic exposure to leachate using key markers linked with spermatogenesis and cellular adenosine triphosphate (ATP) in an experimental rat model. This study was undertaken to evaluate the toxic effects of leachate obtained from the Elewi Odo municipal battery-recycling site (EOMABRL) on male reproductive function using testicular hormones and biomarker of cellular ATP. EOMABRL was administered at 0, 20, 40, 60, 80, and 100 % concentrations to adult male rats for 60 days. After exposure, serum was collected for hormonal biochemistry assays, and testes were collected to determine the activity of xanthine oxidase (XO) and lactate dehydrogenase (LDH). Exposure of animals to EOMABRL resulted in a 519.7, 285.7, 569.1, 606.1, and 1,793.2 % increase in XO activity with a sequential decrease in LDH activity (marker of cellular ATP) by 44.1, 55.9, 61.4, 69.3, and 89.7 %, respectively, compared with the control. Furthermore, EOMABRL caused a significant inhibitory effect on serum luteinizing hormone, follicle-stimulating hormone, and testosterone levels. We conclude that some possible mechanisms by which EOMABRL elicits toxicity in male rat testes could be through inhibition of LDH activity and depletion of serum hormone levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The infertility rate among men is increasing. This may be linked to the accumulation of heavy metals in environmental soil results from anthropogenic activities, particularly from metal-containing recycling sites. In addition, the occurrence of high concentrations of these inorganic substances in groundwater used for drinking purposes has been recognized as a major public health concern in several parts of the world. Every day, millions of people are exposed to hazardous metals by way of drinking water (WHO 1993). This is one of the predominant causes of groundwater pollution. Heavy metals are problematic in soils at battery recycling sites. In addition, it had been discovered that adverse health effects were observed in locations where heaps of waste were dumped for recycling purposes (CDCP 2009). These water contaminants leach into well water or thermal springs from the recycling sites or percolate into surface water from soil-rich heavy metals (CEPA 2012). Other potential sources of metal toxicity include the use of metal-contaminating herbicides, insecticides, pesticides, rodenticides, preservatives, and by-products of fossil fuels (Flora et al. 2004).
Detailed studies on male reproduction with reference to mixed-metal exposures are scant, but the effects of individual heavy metals, such as lead (Pb), cadmium (Cd), cobalt (Co), and chromium (Cr), on male reproduction has been studied in several experiments. A number of epidemiological investigations indicated that occupational exposure to Pb and Cd significantly lowered average sperm counts, and a lesser proportion of sperm were found to be motile in exposed subjects compared with the controls (Matthies et al. 1989). The latest evidence suggests that Pb interferes with the ability of spermatozoa to undergo the acrosome reaction, thus leading to infertility (Winder 1989). Another metal that has been implicated in reproductive dysfunctions is Cr. A recent report suggested that exposure to Cr results into changes in semen quality and reproductive hormone levels. In addition, other metals—such as iron (Fe), nickel (Ni), and Co—have been implicated in reactive oxygen species (ROS) production resulting in oxidative damage of sperm DNA (Apostoli et al. 2007). Experimental studies further suggested that many metals have adverse effects on male reproductive cells (Chowdhury 2009). Inhalation or ingestion of these inorganic substances has been shown to cause cancer in humans resulting in tumours of the skin, lung, liver, urinary bladder, and testes, and it has been classified as a proven human carcinogen and antispermatogenic agent (IARC 2012; ECB 2010). As such, the United States Environmental Protection Agency now lists some 80,000 chemicals in human use, but only a small percentage have been tested for long-term safety. Among these, xenobiotics that are poisonous in nature have become of particular concern to male reproductive health (Robaire and Hales 2003; Aitken et al. 2004).
In Nigeria, developing a new product from waste batteries has become prolific. This is achieved when the metallic compositions contained in batteries are subjected to different recycling processes (Huang et al. 2010). This practice can be traced to innumerable adverse effects on the environment and mammals (CDCP 2009). This is due to the leaching of wastewater containing nondegradable materials, which percolate through the soil to pollute the water table (source of drinking water) for human use (CDCP 2009). Although several studies have reported the spermatotoxic effects of exposure to individual laboratory heavy metals on reproductive function, few or no studies have investigated the testicular effects when mammals are collectively exposed to these metals in nature. The purpose of the present study was undertaken to investigate the evidence whether leachate (natural mixed-metal exposure) from a battery-recycling site is capable of inducing/initiating reproductive dysfunctions using some key biomarkers linked with spermatogenesis and cellular ATP and to possibly elucidate the underlying mechanisms of toxicity during long-term exposure in an experimental rat model.
Materials and Methods
Sampling Site and Leachate Preparation
Leachate was obtained from the Elewi Odo municipal battery recycling site (EOMABRL), which is located at Ibadan North of Oyo State, Nigeria (latitude 7°25.08′N and 7°25.11′N; longitude 3°56.45′E and 3°56.42′E). It is close to a stream and situated in a residential estate. It covers approximately 1,080 m2 and 200 × 120 m of land. A randomized sampling technique (Houk 1992; Siddique et al. 2005) was used to collect the first-horizon solid soils (0 to 15 cm deep) from five different points in the municipal automobile battery recycling site. Five randomly collected samples from each point were pooled to make a single representative sample.
Leachate (100 %) was prepared from a homogenous mixture according to a standard procedure (Ferrari et al. 1999) by adding 100 g of sample to 100 ml of distilled water (w/v) and shaken for 48 h at 32 °C. Thereafter, the sample was left to sediment for 30 min, and the supernatant was filtered with a 2.5-μm filter paper; the filtrate was stored at 4 °C for further use. Sample waters were collected from wells around the site. These were designated as WL-A and WL-B. The stream water collected near the site was regarded as STREAM; the drinking water sample (8 km far from recycling site) was collected and used as reference control (designated as CDW).
Chemicals and Reagents
Xanthine was purchased from Sigma (St. Louis, Missouri, USA). Kits for serum hormone levels and LDH were purchased from Random Laboratory Limited, United Kingdom. Unless stated otherwise, all other chemicals and reagents were of analytical grades and were obtained from British Drug Houses grades (Poole, Dorset, UK), and the water used was glass distilled.
Heavy-Metal Analysis
The nine metals—namely, copper (Cu), Pb, Cd, Co, Cr, zinc (Zn), Fe, Ni, and manganese (Mn)—were analyzed in the leachate samples, and all of the water samples were collected. Briefly, 100 ml each of leachate and water sample was digested by heating with concentrated HNO3, and the volume was reduced to 2–3 ml. This volume was made up to 10 ml with 0.1 N HNO3, and the concentrations of the metals were estimated using atomic absorption spectrophotometry (Association of Official Analytical Chemists 2005). The levels of these metals were assessed because of their reported occurrences in both solid and liquid wastes in Nigeria (Nduka et al. 2007; Longe and Enekwechi 2007; Longe and Balogun 2010; Farombi et al. 2011; Akintunde and Oboh 2013a).
Experimental Protocol
Subchronic Exposure
Thirty adult male Wistar rats, weighing approximately 160–220 g and obtained from the Department of Biochemistry, Federal University of Technology, Akure, Nigeria. were randomly assigned into six groups of five animals per group. They were housed in a plastic suspended cage placed in a well-ventilated rat house, provided rat pellets and water ad libitum, and subjected to a natural photoperiod of 12 h of light and 12 h of darkness. The rats were selected from the colony maintained under the controlled conditions of temperature (35 ± 2 °C) and humidity (50 ± 5 %). The rats in group 1 served as control and were administered 1 ml of distilled water by gastric intubation. Animals in groups 2 through 6 received 1 ml each of 20, 40, 60, 80, and 100 % of leachate, respectively, per kilogram of body weight by gastric intubation. The experiment lasted for 60 days (subchronic exposure). Blood samples were allowed to clot and centrifuged at low speed (3,000 g) at room temperature for 15 min. The supernatant (serum) was removed and used for the determination of serum hormone levels. The testes were processed for biochemical analysis. Animal care and handling was performed according to the institutional guidelines of Nigeria Academy. The study was approved by the Institutional Animal Ethical Committee.
Xanthine Oxidase Assay
Xanthine oxidase was assessed in the testicular supernatant using xanthine as substrate (De Lamirande 1992).
Lactate Dehydrogenase (LDH) Assay
The testicular homogenate was assayed for LDH activity using a commercially available kit (Randox Laboratories UK). Assay was performed according to the manufacturer’s instructions (Weisshaar 1975).
Radioimmunoassay (RIA) of LH and FSH
Serum levels of LH and FSH were measured using available radioimmunoassay (RIA) kit. They were determined by a double-antibody RIA using 125I-labelled radio ligand as described by Watanabe et al. (1985). Intra-assay and interassay coefficients of variation were 5.4 and 6.9 % for LH and 4.8 and 11.4 % for FSH, respectively. The sensitivity of LH and FSH assays were 1.9 and 9.8 pg/tube, respectively.
Serum Testosterone Assay
Serum-free testosterone was determined using an enzyme immunoassay (EIA) kit (Immunometrics, London, UK). This method is based on the competition of serum testosterone and alkaline phosphatase-labeled testosterone in binding to a limited amount of flourescein-labeled polyclonal antitestosterone antibody (Biswas et al. 2001). The reaction was terminated by the addition of EIA stop buffer (glycine buffer [pH 10.4] containing sodium hydroxide and a chelating agent), and the optical density was measured at 550 nm. The testosterone concentration of the test sample was interpolated from a calibration curve using testosterone EIA standards.
Histopathological Examination
The testes were fixed in Bouin’s fluid for 24 h before they were cut longitudinally into two equal halves and again post-fixed in fresh Bouin’s fluid for an additional 24 h. The tissues were dehydrated in ascending strengths of alcohol and cleared in xylene. Infiltrated and embedded in paraffin wax, tissue blocks were made by cutting them into 5-μm-thick sections with the help of a rotatory microtome. The sections were mounted on albumenized glass slides and stained with eosin and hematoxylin. Morphological study of testes was performed with the help of ocular micrometer scale under a light microscope.
Statistical Analysis
The results of the replicates were pooled and expressed as mean ± SD. One-way analysis of variance was used to analyze the results, and Duncan multiple test was used for post hoc analysis (Zar 1984). Statistical Package for Social Science (SPSS) 17.0 for Windows was used for the analysis, and the least significance difference was accepted at P < 0.05.
Results
The concentration of mixed metals in EOMABRL, the water samples from the wells, and the stream obtained at EOMABRL were detected, and the results are listed in Table 1. The metal mixture in the EOMABRL and water samples were higher than the drinking water sample, which was used as reference control (Table 1). First, in the EOMABRL, Cd (0.006 mg/L), Cr (0.068 mg/L), Fe (2.667 mg/L), Ni (0.05 mg/L), Pb (0.015 mg/L), and Mn (7.842 mg/L) exceeded WHO permissible limits by 100, 36 789, 150, 50, and 1,861 %, respectively (Table 1). In the STREAM, Fe (1.076 mg/L), Ni (0.048 mg/L), and Pb (1.548 mg/L) were greater than WHO permissible limits by 259, 140, and 15,380 % respectively (Table 1). Last, in the WL-A, Ni (0.044 mg/L), and Pb (0.068 mg/L) exceeded the WHO permitted limits in the drinking water by 120 and 580 %, respectively. A similar trend was observed in WL-B as Ni (0.049 mg/L) and Pb (0.306 mg/L) exceeded WHO permissible limits in drinking water by 145 and 2,960 %, respectively. This result ascertains that the major possible primary source of pollutants in the ambient drinking water comes from the battery recycling site.
Testicular Serum Hormone Levels
The levels of serum LH, FSH, and testosterone in rats treated with EOMABRL are shown in Figs. 1, 2 and 3. EOMABRL administration significantly (P < 0.05) decreased serum LH concentration compared with the control group by 32, 22, 48, 40, and 44 % respectively (Fig. 1). In addition, the level of FSH was significantly decreased (P < 0.05) by 31.3, 25, 40.6, and 31.3 %, respectively, relative to the control animals (Fig. 2). Similarly, there was significant inhibition of serum testosterone production (P < 0.05) compared with the control group by 20, 40, 20, 30, and 80 %, respectively, after EOMABRL exposure in the experimental rats (Fig. 3).
Effect of EOMABRL on luteinizing hormone level in rat treated. EOMABRL significantly (P < 0.05) decreased LH level in treated rats. Group 1 received 0 %, group 2 received 20 %, group 3 received 40 %, group 4 received 60 %, group 5 received 80 %, and group 6 received 100 %. Values represent mean ± SD. Values with different superscript are significantly (P < 0.05) different
Effect of EOMABRL on Follicle stimulating hormone level in rat treated. EOMABRL significantly (P < 0.05) decreased FSH level in treated rats. Group 1 received 0 %, group 2 received 20 %, group 3 received 40 %, group 4 received 60 %, group 5 received 80 %, and group 6 received 100 %. Values represent mean ± SD. Values with different superscript are significantly (P < 0.05) different
Effect of EOMABRL on testosterone level in rat treated. EOMABRL significantly (P < 0.05) decreased testosterone level in treated rats. Group 1 received 0 %, group 2 received 20 %, group 3 received 40 %, group 4 received 60 %, group 5 received 80 %, and group 6 received 100 %. Values represent mean ± SD. Values with different superscript are significantly (P < 0.05) different
Activity of Xanthine Oxidase
Exposure of animals to EOMABRL showed a significant (P < 0.05) increase in the activity of XO by 519.7, 285.7, 569.1, 606.1, and 1,793.2 %, respectively, in EOMABRL-treated rats (Fig. 4).
Effect of EOMABRL on the activity of xanthine oxidase (XO) in treated rats. EOMABRL significantly (P < 0.05) increased XO activity in treated rats. Group 1 received 0 %, group 2 received 20 %, group 3 received 40 %, group 4 received 60 %, group 5 received 80 %, and group 6 received 100 %. Values represent mean ± SD, n = 5. Values with different superscript are significantly (P < 0.05) different
Activity of Testicular Lactate dehydrogenase (LDH)
Exposure of EOMABRL using the experimental rat model significantly (P < 0.05) inhibited (in a dose-dependent manner) the activity of LDH, a functional marker of astenospermia in rat testes, by 44.1, 55.9, 61.4, 69.3, and 89.7 %, respectively, compared with the corresponding control group of animals (Fig. 5).
Effect of EOMABRL on Testicular lactate dehydrogenase (LDH) (functional marker of ATP). EOMABRL significantly (P < 0.05) decreased LDH activity in treated rats. Group 1 received 0 %, group 2 received 20 %, group 3 received 40 %, group 4 received 60 %, group 5 received 80 %, and group 6 received 100 %. Values represent mean ± SD, n = 5. Values with different superscript are significantly (P < 0.05) different
Testicular Cell Damage
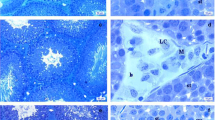
As shown in Fig. 6a (control group), the germ cells were arranged regularly, and all cell lines were present from spermatogonia to spermatid, which attached to the Sertoli cells.
Photomicrographs of eosin and hematoxylin-stained 5-μm-thick section (original magnification ×100). a Rat testis showing normal developmental stages of spermatogenesis. b EOMABRL-treated rat testis showed altered development of spermatocytes and loss of germ cells in the lumen of seminiferous tubules. Sperms or spermatids and Leydig cells appear to have undergone necrosis. Clustered cytoplasm strands are seen. c EOMABRL-treated rat testis showed elongation of seminiferous tubules. Sperms or spermatids and Leydig cells appear to have undergone necrosis. d EOMABRL-treated rat testis showed severe rupture of the basement membrane of the Leydig cells. Sperms or spermatids have undergone necrosis (necrospermia). e EOMABRL-treated rat testis showed abnormal differentiation (mitotic division) of testicular cells. In addition, severe clumping of sperms or spermatids was observed. f EOMABRL-treated rat testis showed abnormal distribution or immature formation of sperms in the seminiferous tubules. In addition, severe damage to Leydig cells was seen (L lumen, ST Spermatids, SC Spermatocyte, SG Spermatogonia, S Sperm. LG Leydig cell, CY Cytoplasm, TC Testicular cells)
In testes of animals exposed to EOMABRL (as shown in Fig. 6b–f), degenerated spermatids were clustered together and intermixed with mature spermatozoa. Sertoli cells and interstitial cells appeared to have undergone necrosis after exposure to EOMABRL. Basement membrane and intracellular spaces of most tubules were distorted and ruptured. Seminiferous tubules were elongated, and Sertoli cells were devoid of germ cells with emaciated spermatids. However, administration of EOMABRL using the rat model caused considerable damage to testicular cells at the investigated doses.
Discussion
Spermatogenesis in testis is regulated and maintained by complex hormonal control through the hypothalamus–pituitary–testicular axis and local somatic cells, such as Leydig/interstitial, Sertoli, and peritubular cells (Johnson 1997; Creasy 1999, 2001). As observed in the study, the low level of serum testosterone in EOMABRL-treated animals suggests an inhibition of its synthesis by Leydig cells because these cells are the main source of testosterone in rats (De Krester 1987). This indicates that EOMABRL was able to inhibit the (mechanism of) stimulation during the process of hormone synthesis in the Leydig cells. This corroborates with the previous work reporting that both in vivo and in vitro exposure to widely used herbicide, known as atrazine, altered endocrine function in peripubertal male rats by causing decreases in serum and intratesticular levels of testosterone (Friedmann 2002). In addition, decreased levels of LH and FSH relative to the control animals may be associated with inactivation of hypothalamus–pituitary control axis. Therefore, taken together, the normal regulation of spermatogenesis and spermiogenesis by androgen receptors in the seminiferous epithelium might perhaps be interrupted after EOMABRL exposure.
The primary biological function of xanthine oxidoreductase (XOR) in mammals is purine degradation wherein the enzyme catalyzes the rate-limiting step in the oxidation of xanthine and hypoxanthine to uric acid (Stryer 2002). High levels of XO, a cellular redox enzyme, would be associated with reperfusion tissue injury of the testes (Cross 1987; Southorn and Powis 1988). It contributes to oxidative damage of cells through the generation of cytotoxic oxygen metabolites (H2O2; O2− and OH−) (Cross 1987). As observed in the study, significant high activity of XO in animals exposed to EOMABRL may perhaps be linked to increased purine degradation caused by low production of testicular energy (ATP) during an ischemic conditions. This observation suggests that EOMABRL exposure to animals can inhibit testicular ATP generation thereby causing ischemia and reperfusion injury to rat testes. This observation conforms to the work performed by De Lamirande and Gagnon (1992) reporting that ROS produced by XO, or by H2O2, interfered with energy production metabolism in human spermatozoa leading to decreased ATP levels.
LDH is an oxidoreductase (XOR) enzyme that catalyses the interconversion of pyruvate and lactate. Cells release LDH into the bloodstream after tissue damage. The level of cellular ATP during anaerobic conditions had been widely assessed using LDH activity because it is a fairly stable enzyme. As observed in the study, exposure to EOMABRL significantly (P < 0.05) depleted LDH activity. We hypothesised that inhibition of LDH (a key enzyme of glycolytic pathway) caused by EOMABRL in the absence of oxygen would slow down the metabolic pathway responsible for (ATP) energy production. This finding supports the previous discovery that patients with abnormal spermatogenesis showed low levels of ATP (Gale et al. 1981; Walker and Barri 1999; Parlakpinar et al. 2006). Furthermore, under aerobic conditions, pyruvate is converted to acetyl CoA catalyzed by pyruvate dehydrogenase, which may be oxidised in the tricarboxylic acid (TCA) cycle to yield ATP. It may also be used for synthesis of fatty acids, cholesterol, and ketone bodies. As observed in the study, it is suggested that heavy metals in EOMABRL would disrupt ATP production by inhibiting pyruvate dehydrogenase when competing with the phosphate group, thereby uncoupling oxidative phosphorylation. As such, this inhibits energy-linked decrease of NAD+, mitochondrial respiration, and ATP synthesis of the testes. This observation corroborates the work performed by (Klaassen and Watkins 2003), who reported that arsenic (As) and its compounds disrupts ATP production when exposed to animals by way of water drinking. This is also consistent with several studies that reported that cells exposed to As showed a considerable depletion in ATP and glycogen levels in liver and other tissues such as heart and kidney (Gresser 1981).
Furthermore, a study showed that testicular toxicity in rats is a good predictor in human subjects (Rosner et al. 2010). This investigation hypothesised that mixed-metal exposure can cause a considerable reproductive disorder when exposed in combination rather than exposed singly. Previous research showed that Cd (>0.003 mg/L) caused severe damage to spermatogenic epithelium in an animal model (WHO 1992, 1996, 2008). It has also been reported that Cd at concentrations greater than this permissible limits had direct injury on testes by damaging germ cells and Sertoli cells and consequently reducing the quality of semen. Cd2+ metabolite in the blood of occupationally exposed workers was implicated in prostate cancer (Brys et al. 1997; Waalkes and Rehms 1994). Further reports also showed that exposure to a moderate Cd concentration (0.001 mg/L) significantly decreased human semen quality and deterioration of sperm motility with no evidence of impairment in male reproductive endocrine function (Dawson et al. 1998). In the study, subchronic exposure to EOMABRL at all doses (2, 4, 6, 8, and 10 mg/g) significantly depleted male reproductive endocrine utility (such as LH, FSH, and testosterone) in a non-dose-dependent manner. The decreased response may be due to the fact that Cd was detected (0.006 mg/L) in the leachate, which together with other metals additively inactivated pituitary gonadotrophin secretion, which was greater relative to WHO limits (0.003 mg/L) (WHO 2008).
The concentration of Pb (0.015 mg/L) detected in the leachate of the present investigation was far greater as compared with WHO permissible limits (0.01 mg/L). Previous studies showed that exposure to low inorganic Pb at 0.004 mg/L dose in blood impaired male reproductive function by decreasing sperm count, volume, and density or changing sperm motility and morphology, but no relevant effects were detected on the endocrine profile and cellular ATP (Chowdhury et al. 1986; Matthies et al. 1989; Apostoll et al. 1998; Bonde et al. 2002; Alexander et al. 1996; Telišman et al. 2000). However, exposure to EOMABRL (mixed-metals) at the investigated doses caused profoundly low serum testosterone and depleted cellular ATP levels in a non-dose-dependent manner. This effect may be linked to the increased dose of the Pb, which perhaps had compromised hypothalamic–pituitary axis (Assenato et al. 1987; Bryant 2004; Wirth and Mijal 2010). The toxic effect may also be linked to the individual, additive, synergistic, or antagonistic interactions of the metals with testicular biomolecules.
The prime major sources of Ni exposure to mammals had been traced to drinking water and contaminated foods (Aleksandra and Urszula 2008). As observed in this study, the level of Ni (0.05 mg/L) detected in EOMABRL exceeded the least observable effective concentration (LOEC) compared with WHO limits (0.02 mg/L). This suggests that Ni could pollute wells and stream water when situated close to battery recycling sites. Similarly, Fe and Ni concentrations (2.667 and 7.842 mg/L, respectively) in rats exposed to the leachate were greater than the WHO limits (0.3 0.4 mg/L, respectively). Rats exposed to the Fe and Ni contained in EOMABRL beyond WHO permissible limits showed a decreased response of LDH activity in a dose-dependent manner. This finding is in agreement with a previous report, which stated that workers exposed to metals such as Fe and Ni had lower sperm concentration, sperm motility, and decreased response of LDH activity (Li et al. 2001; Kumar et al. 2005). Mn and Fe in EOMABRL may also be linked to alteration of testosterone, FSH, and LH secretion (Xu et al. 1993; Meeker et al. 2009), and ROS production, decreased glutathione, and compromised antioxidant levels, which resulted in lipid peroxidation and oxidative damage of sperm DNA molecules (Akintunde et al. 2013; Chia et al. 1992; Telišman et al. 2000; Zeng et al. 2004; Apostoli et al. 2007).
Reports have also shown that male rats that were intraperitoneally injected with Cr ranging between 1 and 2 mg/L showed alterations in endocrine hormones with enlarged intracellular spaces, tissue loosening, and dramatic loss of gametes in the lumen of the seminiferous tubules. The similar effect of Cr in leachate-treated rats may be due to its large concentration (0.068 mg/L) relative to WHO permissible limits (0.05 mg/L). Generally, the level of heavy metals in EOMABRL was greater than those in STREAM, WL-A, WL-B, and CDW. Its high levels may be due to the fact that soil can easily form ligands with metals or because it has high capacity to retain heavy metals compared with inorganic solvents.
Cobalt is beneficial to humans because it is a part of vitamin B12, which is essential for human health and stimulates the production of red blood cells. However, larger doses >0.05 mg/L may damage reproductive health (Shahida et al. 2009). Zn within the tolerable limits (3 mg/L) plays a critical role in male reproductive function because it allows the prostate to secrete high levels of fertile semen and sex hormones (Naha and Chowdhury 2006). Cu occurs at the active site of SOD to exercise a redox function (Williams 1989). In the present findings, Co (0.049 mg/L), Zn (0.01 mg/L), and Cu (0.341 mg/L) detected in EOMABRL were considerably lower than WHO exposure limits (0.05, 3, and 2 mg/L, respectively). Low levels or deficiencies of these metals (Co, Zn, and Cu) have been implicated in the decrease of sex hormones with sequential alteration of testicular antioxidant enzymes, catalase, and superoxide dismutase (Saaranen et al. 1989; Freedman 1992; Bray et al. 1997). In addition, competitive interactions between Zn and other metallic ions (Cd, Pb, Mn) might have occurred in the testicular cells. This is because Zn has similar physicochemical properties with other ions and there is consumption of high doses of these other ions in EOMABRL. They therefore competitively bind to the active sites of the enzymes linked to spermatogenesis, thereby downregulating their activities resulting in abnormal spermatozoa (Griffiths et al. 2007; Underwood and Somers 1969; Wong et al. 2001). Generally, the biotoxic effects of whole leachate on rat testicular biomarkers examined in this study are due to their individual, additive, competitive, synergistic, or antagonistic interference with normal testicular biomolecules.
Moreover, adverse histopathological changes were observed in the gonads of treated animals, including enlarged or elongation of intracellular spaces resulting from damage to Sertoli cells as well as a dramatic loss of gametes in the lumen of the seminiferous tubule. In addition, coagulative necrosis of spermatozoa after EOMABRL exposure in experimental rats was seen. This might be caused by the disruption of the blood–testis barrier with consequent metal accumulation in the tissue (Chandra et al. 2007).
Conclusion
Humans and animals are exposed to toxicants from the environment in form of mixed metals. Exposure to EOMABRL had significant effects on reproductive functioning by depleting testicular serum hormone levels such as LH, FSH, and testosterone. XO activity, with a sequential decrease in LDH activity (a marker of cellular ATP), was also observed. Taken together, we conclude that some possible mechanisms by which EOMABRL at the tested doses (2, 4, 6, 8, and 10 mg/g) elicits toxicity in male rat testes could be through the inhibition of LDH activity and depletion of serum hormone levels with increased activity of XO. In addition, these biotoxic effects could be traced to individual, additive, competitive, synergistic, or antagonistic interactions of heavy metals, all of which eventually interrupted spermatogenic processes.
References
Aitken RJ, Ryan AL, Curry BJ, Baker MA (2004) Multiple forms of redox activity in populations of human spermatozoa. Mol Hum Reprod 9:645–661
Akintunde JK, Oboh G (2012) In vitro oxidative damage induced in livers, hearts and kidneys of rats treated with leachate from battery recycling site: Evidence for environmental contamination and tissue damage. J Clin Exp Pathol 2:129
Akintunde JK, Oboh G (2013a) Municipal auto-battery recycling-site leachate activates key enzymes linked to non-insulin dependent diabetes mellitus (NIDDM) and hypertension. J Diabetes Metab 4:235
Akintunde JK, Oboh G (2013b) Exposure to leachate from municipal battery recycling site: Implication as key inhibitor of steroidogenic enzymes and risk factor of prostate damage in rats. 28(2–3):107–115
Akintunde JK, Oboh G, Akindahunsi AA (2013) Testicular membrane lipid damage by complex mixture of leachate from municipal battery recycling site as indication of idiophatic male infertility in rat. J Interdiscip Toxicol 6(4):192–197
Aleksandra D, Urszula B (2008) The impact of nickel on human health. J Elem 13(4):685–696
Alexander BH, Chckoway H, Van Netten C, Muller CH, Ewers TG, Kaufman JD et al (1996) Semen quality of men employed at a lead smelter. Occup Environ Med 55:364–374
Apostoli P, Telišman S, Sager PR (2007) Reproductive and developmental toxicity of metals. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT (eds) Handbook on the toxicology of metals. Academic Elsevier, Amsterdam, pp 213–249
Apostoll P, Kiss P, Porru S, Bonde JP, Vanhoorne M (1998) Male reproductive toxicity of lead in animals and humans. Occup Environ Med 55:364–374
Assenato G, Paci C, Baser ME, Molinini R, Candela RG, Altamura BM et al (1987) Sperm count suppression without endocrine dysfunction in lead-exposed men. Arch J Environ Health 42:124–127
Association of Official Analytical Chemists (2005) Official methods of analysis (15th ed). Longman, Washington
Biswas NM, Sengupta R, Chatopadyay GR, Choudhury A, Sarkar M (2001) Effects of ethanol on cadmium-induced testicular toxicity in male rats. Reprod Toxicol 15:699–704
Bonde JP, Joffe M, Apostoll P, Dale A, Kiis P, Spano M et al (2002) Sperm count and chromatin structure in men exposed to inorganic lead: Lowest adverse effect levels. Occup Environ Med 59:234–242
Bray TM, Levy MA, Noseworthy MD (1997) The role of zinc Zn in free radical mediated diseases. In: Fischer PWF, Abb´e MR, Cockell KA, Gibson RS (eds) Trace elements in man and animals—9: Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals. NRC Research Press, Ottawa, Canada, pp 333–6
Bryant SD (2004) Lead-contaminated drinking waters in the public schools of Philadelphia. J Clin Toxicol 42:287–294
Brys M, Nawrocka AD, Miekos E (1997) Zinc and cadmium analysis in human prostate neoplasms. Biol Trace Elem Resour 59:145–152
California Environmental Protection Agency (CEPA) (2012) Chemicals known to the state to cause cancer or reproductive toxicity list as of June 22, 2012. Proposition 65: The Safe Drinking Water and Toxic Enforcement Act of 1986. Sacramento, CA. http://www.oehha.ca.gov. Accessed 19 May 2014
Centres for Disease Control and Prevention (CDCP) (2009). Fourth report on human exposure to environmental chemicals. CDCP, Atlanta, GA. http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf. Accessed 17 Sept 2012
Chandra AK, Chatterjee A, Ghosh R, Sarkar M, Chaube SK (2007) Chromium induced testicular impairment in relation to adrenocortical activities in adult albino rats. Reprod Toxicol 24:388–396
Chia SE, Ong CN, Lee ST, Tsakok FH (1992) Blood concentrations of lead, cadmium, mercury, zinc and copper and human semen parameters. Arch Androl 29:177–183
Chowdhury AR (2009) Recent advances in heavy metals induced effect on male reproductive function. J Med Sci 2:37–42
Creasy DM (1999) Hormononal mechanism in male reproductive tract toxicity. In: Harvey PW, Rush KC, Cockburn A (eds) Endocrine and hormonal toxicology. Wiley, Chichester, pp 355–405
Creasy DM (2001) Pathogenesis of male reproductive toxicity. Toxicol Pathol 29:64–76
Cross CE (1987) Oxygen radicals and human disease. Annu Int Med 107:526
Dawson EB, Ritter S, Harris WA, Evans DR, Powell LC (1998) Comparison of sperm viability with seminal plasma metal levels. Biol Trace Elem Resour 64:215–219
De Krester DM (1987) A hormonal control of reproduction (2nd ed). Oxford University, Oxford, pp 76–90
De Lamirande E, Gagnon C (1992) Reactive oxygen species and human spermatozoa: Effects on the motility of intact spermatozoa and on sperm axoneme. J Androl 13:368–378
European Chemicals Bureau (2010) European Chemical Substances Information System [database on the Internet]. http://ecb.jrc.ec.europa.eu/esis. Accessed 22 Mar 2012
Farombi EO, Akintunde JK, Nsute N, Adedara IA, Arojojoye O (2011) Municipal landfill leachate induces hepatotoxicity and oxidative stress in rats. J Toxicol Indust Health 28(6):532–541
Ferrari B, Radetski CM, Veber AM, Ferard JF (1999) Ecotoxicological assessment of solid wastes: a combined liquid- and solid-phase testing approach using a battery of bioassays and biomarkers. Environ Toxicol Chem 18:1195–1202
Flora SJ, Mehta A, Rao PV, Kannan GM, Bhaokar A, Dube SN (2004) Therapeutic potential of mono-isoamyl and mono-methyl esters of meso 2,3-dimercaptosuccimic acid in gallium arsenide intoxicated rats. Toxicology 195:127–146
Freedman LP (1992) Anatomy of the steroid receptor zinc finger region. Endocrinol Rev 13:129–145
Friedmann AS (2002) Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol 16:275–279
Gale EF, Cundliffe E, Reynolds PE (1981) The molecular basis of antibiotic action, 2nd edn. Wiley, New York
Gresser MJ (1981) ADP-arsenate formation by submitochondrial particles under phosphorylating conditions. J Biol Chem 256(12):5981–5983
Griffiths LM, Loeffler SH, Socha MT (2007) Effects of supplementing complexed zinc, manganese, copper and cobalt on lactation and reproductive performance of intensively grazed lactating dairy cattle on the South Island of New Zealand. Anim Feed Sci Technol 137:69–83
Houk VS (1992) The genotoxicity of industrial wastes and effluents. Mutat Resour 277:91–138
Huang K, Li J, Xu Z (2010) Characterization and recovery of cadmium from waste nickel–cadmium batteries. Waste Manag 30:2292–2298
International Agency for Research on Cancer (2012) Monographs on the evaluation of carcinogenic risks to humans. Some drinking-water disinfectants and contaminants, including arsenic 84:39. http://www.inchem.org/documents/iarc. Accessed 13 Feb 2013
Johnson L, Welsh Jr TH, Wilker CE (1997) Anatomy and physiology of male reproductive system and potential targets of toxicants. Comprehensive toxicology. Volume 10. Reproductive and endocrine toxicology. Elsevier Science International, New York, NY, pp 5–6
Klaassen C, Watkins J (2003) Casarett and Doull’s essentials of toxicology. McGraw-Hill, New York, p 512
Kumar S, Sathwara NG, Gautam AK, Agarwal K, Shah B, Kulkarni PK et al (2005) Semen quality of industrial workers occupationally exposed to chromium. J Occup Health 47:424–430
Li H, Chen Q, Li S, Yao W, Li L, Shi X et al (2001) Effect of Cr(VI) exposure on sperm quality: human and animal studies. Annu Occup Hygiene 45:505–511
Longe EO, Balogun MR (2010) Groundwater quality assessment near a municipal landfill, Lagos, Nigeria. Res J Appl Sci Eng Technol 2(1):39–44
Longe EO, Enekwechi LO (2007) Investigation on potential groundwater impacts and influence of local hydrogeology on natural attenuation of leachate at a municipal landfill. Int J Environ Sci Technol 4(1):133–140
Marouani N, Tebourbi O, Mahjoub S, Yacoubi MT, Sakly M, Benkhalifa M, Rhouma KB (2012) Effects of hexavalent chromium on reproductive functions of male adult rats. J Reprod Biol 12(2):119–133
Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D et al (2009) Multiple metals predict prolactin and thyrotropin (TSH) levels in men. Environ Resour 109:869–873
Naha N, Chowdhury AR (2006) Inorganic lead exposure in battery and paint factory: effect on human sperm structure and functional activity. J UOEH 28:157–171
Nduka JKC, Orisakwe OE, Nwangunna CK (2007) Heavy metals other than lead in flaked paints from buildings in Eastern Nigeria. Toxicol Ind Health 23:525–528
Parlakpinar H, Tasdemir S, Polat A, Bay-Karabulut A, Vardi N, Ucar M, Yanilmaz M, Kavakli A, Acet A (2006) Protective effect of chelerythrine on gentamicin-induced nephrotoxicity. Cell Biochem Funct 24:41–48
Robaire B, Hales BF (2003) Advances in male mediated developmental toxicity. Kluwer/Plenum, New York
Rosner M, Dolznig H, Schipany K, Mikula M, Brandau O (2010) Human amniotic fluid stem cells as a model for functional studies of genes involved in human genetic diseases or oncogenesis. Oncotarget 2:705–712
RoyChowdhurry A, Chinoy NJ, Gautam AK, Rao RV, Parikh DJ, Shah GM et al (1986) Effects of lead on human sperm. Adv Contracept Deliv Syst 11:208–210
Saaranen M, Kantola M, Saarikoski S (1989) Human seminal plasma cadmium: comparison with fertility and smoking habits. Andrologia 21:140–145
Shahida N, Ihsnullah Z, Shah MT, Iqbal Z (2009) Effect of time intervals on levels of selected heavy metals in surface and ground water in Peshawar basin. J Chem Soc Pak 31(5):757–771
Siddique HR, Gupta SC, Dhawan A, Murthy RC, Saxena DK, Chowdhuri DK (2005) Genotoxicity of industrial solid waste leachates in Drossophila melanogaster. Environ Mol Mutagen 46:189–197
Southorn PA, Powis G (1988) Free radicals in medicine. II Involvement in human disease. Mayo Clin Proc 63:390–408
Stryer L (2002) Biochemistry, 5th edn. Freeman, New York, p 6
Telišman S, Cvitković P, Jurasović J, Pizent A, Gavella M, Ročić B (2000) Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect 108:45–53
Underwood EJ, Somers M (1969) Studies of zinc nutrition in sheep. 1. The relation of zinc to growth, testicular development, and spermatogenesis in young rams. Aust J Agric Res 20:889–897
Van Matthies J, Donat H, Schwarz I (1989) Einfluss Von Schwarmetallionen auf die mannliche fertilitat. Zentralbl Gynakol 111:155–166
Waalkes MP, Rehms S (1994) Cadmium and prostate cancer. J Toxicol Environ Health 43:251–269
Walker PDY, Barri SV (1999) Oxidant mechanisms in gentamicin nephrotoxicity. J Ren Fail 21:433
Watanabe G, Taya K, Sasamoto S (1985) Radioimmunoassay for progesterone, testosterone and estradiol-17β using125I-iodohistamine radioligands. Jpn J Anim Reprod 31:186–197
Weisshaar HD (1975) Normal ranges of alpha-HBDH, LDH, AP, and LAP as measured with substrate-optimated test charges. Med Welt 26:387–392
Williams RJP (1989) An introduction to the biochemistry of zinc. In: Mills CF (ed) Zinc in human biology. Springer-Verlag, London, pp 15–31
Winder C (1989) Reproductive and chromosomal effects of occupational exposure to lead in the male. Reprod Toxicol 3:221–233
Wirth JJ, Mijal RS (2010) Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med 56:147–167
Wong WY, Flik G, Groenen PMW (2001) The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol 15:131–136
World Health Organisation (1993) Guidelines for drinking water quality: Recommendations (2nd ed). Volume 1. WHO, Geneva, Switzerland
World Health Organisation (1996) Health criteria and other supporting information. In: Guidelines for drinking-water quality (2nd ed). WHO, Geneva, Switzerland, pp 940–949
World Health Organisation (2008) International year of fresh water. General Assembly Resolution A/RES/55/196. www.wateryear2003.org. Accessed 13 Mar 2011
World Health Organization (1992) Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, London
Xu B, Chia SE, Tsakok M, Ong CN (1993) Trace elements in blood and seminal plasma and their relationship to sperm quality. Reprod Toxicol 7:613–618
Zar JH (1984) Biostatistical analysis. Prentice-Hall, Inc., Upper Saddle River, NJ, p 620
Zeng X, Jin T, Jiang X, Kong Q, Ye T, Nordberg GF (2004) Effects on the prostate of environmental cadmium exposure—A cross-sectional population study in China. Biometals 17:559–565
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akintunde, J.K., Oboh, G. & Akindahunsi, A.A. Inhibition of Key Markers Linked With Spermatogenesis and Cellular ATP by Subchronic Exposure to Leachate in a Rat Model. Arch Environ Contam Toxicol 68, 159–168 (2015). https://doi.org/10.1007/s00244-014-0068-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0068-9