Abstract
Lead acetate (LA) is a toxic compound that has detrimental effects on the male reproductive system, such as decreased testicular size and function, low androgen hormone concentration, and altered testicular histology. While Nigella sativa (NS) has been shown to possess a handful of therapeutic benefits, few studies have shown its effect on damage caused by lead acetate toxicity in the male reproductive system. In this study, 75 Sprague-Dawley rats were divided into 3 groups of 25 rats, which were further subdivided into 5 subgroups each. Group 1 (negative control) was given distilled water, group 2 (positive control (PC)) administered 10 mg/kg of lead acetate (LA) orally/daily, while groups 3 (T1), 4 (T2), and 5 (T3) were given LA 10 mg/kg and graded concentrations (100, 150, and 200 mg/kg) each of NS. One group each, comprising of 25 rats, was euthanized at days 30, 60, and 90 for collection of blood plasma, epididymis, and organ tissues fixed in 10 % buffered formalin at each time interval. The right caudal epididymis was homogenized and used for the determination of spermiogram. Plasma was used for the determination of testosterone (TS), follicle stimulating hormone (FSH), and luteinizing hormone (LH) and estradiol (EST) using radioimmunoassay kits. There was reduced number of spermatogenic cells and epididymal sperm reserves in the PC group in comparison to the treatment groups. The level of TS was lower (p < 0.05) in the PC group at 90 days, while FSH was lower (p < 0.05) in T3 at 30 days and LH was higher (p < 0.05) in T1 at 90 days. The concentration of EST was lower (p < 0.05) in the PC, T1, and T2 at all time points, while the T3 group had the higher EST concentration that was similar to the control group. There was a decreased level of superoxide dismutase (SOD) and total glutathione (GSH) in the PC group and an increased GSH level in the T3 group. Sperm concentration, viability, and motility were adversely affected by LA, while concurrent treatment with NS significantly (p < 0.05) improved these parameters. This study showed the detrimental effects of LA on spermatogenesis, TS levels, and antioxidant defenses; however, these adverse effects were alleviated by oral NS administration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a common environmental contaminant found in water and soil, mostly as a result of improper industrial waste disposal. Lead poisoning has been reported in some parts of developed and developing countries as environmental contaminations due to gold mining. The most common harmful effects of lead acetate (LA) are the induction of microcytic anemia and central nervous system damage. Young children and adults have presented with signs of microcytic anemia and nervous symptoms following exposure to lead (Papanikolaou et al. 2005). In the laboratory, the administration of LA has shown various deleterious effects in the physiological status function of animals. Acute toxicity study of LA (10 mg or 100 mg) caused elevation of alanine aminotransferase enzyme (ALT) in rats after 12 and 24 h, respectively (Bharali 2013). In other studies using LA, significant changes in the hematological and biochemical parameters of rats were observed (Allouche et al. 2009; Allouche et al. 2011; Dalia 2010; Ibrahim et al. 2012). The administration of LA to male rats have also been reported to affect the levels of reproductive hormones, testosterone, estrogen, and luteinizing hormone, as well as reduce sperm viability, motility, concentration, and gonadal weight and increase sperm abnormalities and degenerative and necrotic conditions in the testis (Allouche et al. 2009; El-Tohamy and El-Nattat 2010; Elgawish and Abdelrazek 2014; Makhlouf et al. 2008).
Nigella sativa (NS) is a popular folkloric medicinal plant whose seeds, leaves, and oil have been used to treat various sicknesses for centuries. The seed and oil of NS have been reported to be safe with a high safety margin following oral intake. NS have been reported to exhibit potent anti-inflammatory, analgesic, anticarcinogenic, antidiabetic, antiulcer, antimicrobial, and antiparasitic activities (Ali and Blunden 2003; Gali-Muhtasib et al. 2006). In other studies, the immunomodulatory effects of NS were attributed to production of antioxidant enzymes, glutathione, and superoxide dismutase (Salem 2005). Similarly, NS was shown to exhibit protective roles against isoproterenol, tramadol, carbon tetrachloride, cisplastin, and sodium valproate toxicities, through production of antioxidant enzymes (Awadalla 2012; Elkhateeb et al. 2015; Hala 2011; Krishnan and Muthukrishnan 2012; Murugesan et al. 2012a, b). In related studies, both administration of NS seed and oil have been found to improve sperm counts, sperm viability and motility, and testicular weights and decrease sperm abnormalities and testosterone concentration (Bashandy 2007; Hala 2011; Mahdavi et al. 2015). Similarly, increased levels of reproductive hormones were observed after administration of NS oil in male rats (Juma and Hayfaa 2011).
Previous studies on the effect of NS on mature male gonadal pathology and reproductive enzyme alterations due to LA toxicity are limited; however, there are a few studies on the effect of LA on prenatal and postnatal development of the rat testes and testosterone production (Dorostghoal et al. 2011; Sharma and Garu 2011). In line with that, this study was designed to assess the level of gonadal injury, sperm production, reproductive hormonal levels, and antioxidant status of male rats exposed to LA toxicity for a period of 90 days, with sample collection at 30, 60, and 90 days. Rats were administered LA concurrently with NS at 100, 150, and 200 mg throughout the course of the experiment.
Materials and methods
Preparation of N. sativa suspension and lead acetate solution
Black seeds (N. sativa) were purchased from a local market in Serdang, Selangor, Malaysia, and certified at the National Herbarium. The seeds were identified and authenticated by Dr. Shamsul Khamis, Coordinator of Biodiversity Unit, Senior Science Officer and Botanist at the Institute of Bioscience, Universiti Putra Malaysia. The voucher specimens of the seeds are kept in the Physiology Laboratory, Faculty of Veterinary Medicine, Universiti Putra Malaysia. The seeds were cleaned and ground with a laboratory electric grinder (National Blender 8011S, Model HGB2WTS3, U.S.A.) for 10 min, and the powder was kept in sealed plastic bags. A water suspension of 10 g/L N. sativa was prepared and used for administration in this study. Lead acetate was purchased commercially from Oxford Lab. Co., India (CAS 6080–56-4) and dissolved in distilled water at a concentration of 10 mg/kg body weight.
Ethical statement
All experimental procedures were performed under clean condition at the Animal Experimental Unit, Faculty of Veterinary Medicine, Universiti Putra Malaysia. Animals were humanely handled and euthanized at stipulated dates (30, 60, and 90 days) during the experimental period, using a CO2 asphyxiation method after the anesthesia procedure with ketamine at 75–100 mg/kg body weight and xylazine 10 mg/kg body weight (Paul 2009).
Animal management and grouping
A total of 75 male Sprague-Dawley rats aged 8–10 weeks and 250–300 g were used for this study. The animals were acquired from the Animal Resource Center, Faculty of Veterinary Medicine, Universiti Putra Malaysia. The rats were kept for at least 15 days for acclimatization before commencement of the experiment. The rats were kept in a suitable temperature between 21 and 25 °C, relative humidity around 40–60 %, and dark/light cycles of 12 h a day (Meyer et al. 1982). The animals were housed in plastic cage with a diameter of 50 × 35 × 15 cm and wood shaving was used as litter material. The rats were fed with commercial rat pellets and clean water was provided ad libitum.
After the period of acclimatization, the rats were divided into five groups of 15 animals each: control (n = 15), given distilled water orally; positive control (n = 15), given lead acetate (10 mg/kg) orally; treatment 1 (n = 15), given NS (100 mg/kg) and LA (10 mg/kg), orally; treatment 2 (n = 15), given NS (150 mg/kg) and LA (10 mg/kg), orally; and treatment 3 (n = 15), given NS (200 mg/kg) and LA (10 mg/kg), orally, daily as reported by earlier researchers (Murugesan et al. 2012a, b; Jahromy et al. 2014; Abbasnezhad et al. 2015; Mustafa and Hussein 2015). At 30, 60, and 90 days, 5 rats were euthanized from each group for collection of plasma and tissue samples.
Plasma collection for hormone and enzyme assays
Blood samples were obtained through cardiac puncture from 5 animals per group on days 30, 60, and 90, respectively (Weisbroth et al. 2013), after anesthesia with ketamine at 75–100 mg/kg body weight and xylazine 10 mg/kg body weight (Paul 2009). Blood was collected in EDTA tubes, while plasma was obtained by centrifugation of whole blood at 2500 rpm for 10 min (Hettich, Germany). Plasma samples obtained were used for the evaluation of reproductive hormones and antioxidant enzyme levels.
Collection and processing of tissue samples for histopathology
Tissue samples of the testis, epididymis, seminal vesicles, vas deferens, and prostate gland were collected in 10 % neutral buffered formalin and fixed for 48 h. Formalin-fixed tissue sections were processed through serial dehydration in ethanol, embedded in paraffin wax, sectioned at 5–6 μm, and stained with hematoxylin and eosin for histopathological examination of each animal through light microscopy. Photomicrographs of microscopic focal fields were taken using Miotic® microscope.
Sperm counts (mass motility, general motility, concentration, viability, and morphology)
The right caudal epididymis was immediately placed in 2 mL of PBS and cut into about 200 pieces using a surgical microscissor to release the spermatozoa from the epididymal tubules. Epididymal semen suspension (ESS) was immediately incubated at 37 °C for further examination. Ten microliters of ESS was added to 190 μL of formal saline, to make a dilution factor of 1:20. The total sperm concentration (TSC) was determined using a Neubauer hemocytometer as described previously by (Yokoi et al. 2003). In order to evaluate the mass motility, 5 μL of the suspension was put on a glass slide and examined under a phase-contrast microscope at a magnification of ×100. Mass motility was graded using the percentage motility of the spermatozoa. Moreover, general motility was evaluated by taking 10 μL of ESS on glass slide, covered with cover slip, and examined under phase-contrast microscope at ×400 magnification. Eosin-nigrosin stain was used to stain sperm to determine sperm viability and morphology.
Radioimmunoassay for follicle stimulating hormone, luteinizing hormone, estradiol, and testosterone measurements
Beckman Coulter Immunotech Radioimmunoassay kits were used for the plasma determination of follicle stimulating hormone, luteinizing hormone, and testosterone concentrations as previously described (Jesse et al. 2016). Briefly, 50 μL of the calibrator and plasma from different groups were added to 500 μL of tracer in an anti-LH, anti-follicle stimulating hormone (FSH), or anti-estradiol antibody-coated tubes, mixed and incubated at 18–25 °C in a shaker set at 350 rpm. Wallac Wizard Gamma Counter model 1470 was used to determine the counts per minute (cpm) of the solution. For the evaluation of testosterone concentration, 200 μL of the plasma was mixed with 2 mL of ethyl ether and vortexed vigorously for 1 min. The mixture was kept at −18 °C until a frozen aqueous phase was achieved. The aqueous phase was separately collected and in a water bath at 37 °C until evaporation. The resultant dry ether extract was redissolved in 200 μL of recovery buffer, vortexed vigorously, and mixed in tracer antibody-coated tubes. The tubes were incubated at 37 °C water bath for 1 h, and the solution was used for determination of total cpm using Wallac Wizard Gamma Counter model 1470 (Jesse et al. 2016).
Evaluation of superoxide dismutase and total glutathione concentrations
Superoxide dismutase catalyzes the dismutation of superoxide into H2O2 and molecular oxygen. This enzyme was determined using a superoxide dismutase (SOD) determination kit (Sigma, Singapore). The reaction mix was set up in a 96-well plate and incubated for 20 min at 37 °C. Absorbance was measured using a plate reader (Tecan, Austria) at 450 nm.
Total glutathione activity was measured using the glutathione assay kit (Sigma, Singapore). Glutathione standards ranging from 50 to 3.125 μM were prepared by serially diluting glutathione standard solution in 5 % 5-sulfosalicyclic acid (SSA). The reaction mix was incubated at room temperature for 5 min before the addition of NADPH solution to each well. Absorbance was measured using a plate reader (Tecan, Austria) at 412 nm.
Statistical analysis
Data obtained from hormonal and antioxidant enzyme assays were summarized as mean ± S.E and analyzed with GraphPad Prism 6.0 using two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test.
Results
Body weight and TBWR
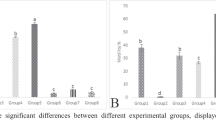
There were no significant changes in the body weights of rats in all groups throughout the experimental period. However, at day 30, the testicular body weight ratio (TBWR) was lower in the positive control (PC) and higher (p < 0.05) in the T3 group. At day 60, TBWR is significantly lower in the PC, T1, and T2, while at 90 days, it was significantly lower in the PC group in the PC in comparison to the control and treatment groups (Fig. 1).
Histopathological findings in the male reproductive system
Thirty days of sampling
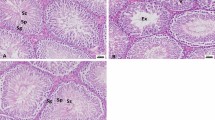
At 30 days of LA and NS administration, there was decreased sperm reserve (>10–50 %) in the tail of the epididymis of PC rats; the sperm is characterized by immature and degenerate sperm cells. A few focuses of epididymal epithelial vacuolation and lymphocytic perivascular inflammatory cells was seen. Epididymal sperm reserves showed tremendous improvements in the treatment groups T1, T2, and T3 (60–90 %). Epididymal vacuolation was also observed in the treatment groups but it was minimal (Fig. 2 (A1–A5)). In the testis, sperm production can be observed in the seminiferous tubules (ST), with an increased number of immature spermatids in the PC. The treatment groups had enhanced sperm developmental stages with many long spermatids within the seminiferous tubules (Fig. 2 (B1–B5)). There were multifocal areas of inflammatory cell infiltration in the prostate of all groups, which may be an incidental finding or toxic response to LA (Fig. 2 (C1–C5)). No significant changes were observed in the seminal vesicles of all the groups; however, mild cellular vacuolations which are indications of glandular production were seen in all groups (Fig. 2 (D1–D5)). There were epithelial vacuolations and focal to multifocal areas of leucocyte infiltration in the ductus efferentes (Fig. 2 (E1–E5)).
Photomicrograph section of the reproductive organ of NC, PC, T1, T2, and T3, respectively. A1–A5 Epididymis showing epididymal sperm reserves (black arrow) and vacuolation of epithelium (red arrow). B1–B5 Testes showing spermatogenesis stages in the seminiferous tubules (arrows). C1–C5 Prostate gland showing lymphocytic infiltration in glandular interstitium (arrows). D1–D5 Seminal vesicles showing glandular epithelium (arrows). E1–E5 Efferent duct showing luminal epithelium (arrows); H&E × 200
Sixty days of sampling
At 60 days, there was an improvement rather than deterioration of the epididymal sperm reserve in the PC group to about 50–70 %. However, there were still numerous numbers of degenerate and immature spermatozoa in the tubular lumen. The epididymal sperm reserve was optimal—70–90 % in all the treatment groups—however, a few immature cells were seen in the T1 group (Fig. 3 (A1–A5)). There was less tubule without spermatogenesis in the PC group, while a few tubules had more immature spermatids or round spermatids. Spermatogenesis was observed at optimal levels in the ST of all treatment groups (Fig. 3 (B1–B5)). The prostate gland was dilated and empty in some sections of the PC group, while focal areas of lymphocytic infiltration were seen in a few foci. The prostate gland was mostly filled with a glandular secretion in the treatment group, with a few foci of lymphocytic infiltration in all groups (Fig. 3 (C1–C5)). There were no significant lesions in the seminal vesicles (Fig. 3 (D1–D5)) and vas deferens (Fig. 3 (E1–E5)).
Photomicrograph section of the reproductive organ of NC, PC, T1, T2, and T3, respectively. A1–A5 Epididymis showing epididymal sperm reserves (black arrow) and vacuolation of epithelium (red arrow). B1 ‑ B5 Testes showing spermatogenesis stages in the seminiferous tubules (arrows). C1 ‑ C5 Prostate gland showing lymphocytic infiltration in glandular interstitium (arrows). D1 ‑ D5 Seminal vesicles showing glandular epithelium (arrows). E1 ‑ E5 Vas deferens showing epithelium (black arrows), and sperm cells in the lumen (red arrow); H&E × 200
Ninety days of sampling
At 90 days of sampling, there was a reduction in the number of immature sperm cells in the epididymis of the PC group. Sperm cells are sparsely distributed in the tubular lumen. In the treatment groups, epididymal sperm is densely distributed (Fig. 4 (A1–A5)). The testis of the PC had a few vacuolations within the ST, while the T1, T2, and T3 had optimal spermatogenesis without tubular vacuolations (Fig. 4 (B1–B5)). The prostate glands were comparable in all groups, distended with fluid and had an infrequent occurrence of mild lymphocytic infiltration (Fig. 4 (C1–C5)). There were no significant lesions in the seminal vesicles (Fig. 4 (D1–D5)) and the vas deferens (Fig. 4 (E1–E5)).
Photomicrograph section of the reproductive organ of NC, PC, T1, T2, and T3, respectively. A1–A5 Epididymis showing epididymal sperm reserves (black arrow). B1 ‑ B5 Testes showing spermatogenesis stages in the seminiferous tubules (arrows), slight vacuolations (red arrow). C1 ‑ C5 Prostate gland showing glandular lumen and epithelium (arrows). D1 ‑ D5 Seminal vesicles showing glandular epithelium (arrows). E1 ‑ E5 Vas deferens showing epithelium (yellow arrows); H&E × 200
Spermiogram parameters
The concentration of sperm was significantly (p < 0.05) lower in the PC and T1 at 30 and 60 days, while at 90 days, the PC, T1, and T2 all had lower sperm concentrations in comparison to the control and T3. The individual motility was significantly lower in the PC at all time points and not different between the control and T3. The general motility was lower in the PC at all time points and higher in T2 and T3. Viability was significantly (p < 0.05) higher in the T3 at 30 and 60 days of sampling and comparable between control and T3 at 90 days. The PC had a lower viability (p < 0.05) at all sampling points. The percentage of sperm abnormality was significantly higher in the PC and decreased with increasing concentration of NS at all time points (Fig. 5).
Graphs showing spermiogram parameters of rats exposed to chronic lead acetate treatment and N. sativa treatment a sperm concentration, b individual motility, c general motility, d viability, and e percentage abnormality. Bars with different superscript letters indicate statistical significance at p < 0.05
Reproductive hormonal concentration
The concentration of FSH was lower (p < 0.05) in the T1, T2, and T3 groups at 30 days of sampling, while no differences were observed at 60 and 90 days. The concentration of LH was lower in the PC and T1 at 30 days, while the PC, T1, and T2 all had a significantly lower concentration of LH at 60 and 90 days. There were no significant changes in testosterone (TS) concentration after 30 days of LA administration, while at 60 days, the PC, T1, and T2 had significantly lower concentrations, while T3 had a higher (p < 0.05) concentration. At 90 days, the PC group had a lower concentration of TS, while all the treatment groups had comparable levels that were higher than the PC. The concentration of estradiol was significantly lower in the PC and T1 at 30 and 60 days and in PC, T1, and T2 at 90 days of sampling (Fig. 6).
Antioxidant enzyme concentration
The concentration of SOD was lower (p < 0.05) in the PC group at days 30, 60, and 90 of sampling and lower in the T1 group at day 60 only. The level of total glutathione (GSH) was lower (p < 0.05) in the PC group at day 30 and higher (p < 0.05) in the T3 on days 60 and 90 of sampling (Fig. 7).
Discussion
Previous studies have widely documented the deleterious effects of LA on the developing and mature male reproductive organs and fertility (Dorostghoal et al. 2011; Sharma and Garu 2011). In these studies, the administration of different doses of LA was shown to cause reduced testicular weight, sperm concentration, and motility and increased sperm abnormalities and increased testicular degenerative changes (Allouche et al. 2009; Elgawish and Abdelrazek 2014; Makhlouf et al. 2008). Although there are numerous reported literatures on the ameliorative effect of NS oil against toxic injuries to the male reproductive organs and hormonal level decreases induced by other compounds (Al-Zahrani et al. 2012; Awadalla 2012; Hala 2011), there are no reports on the effect of both its oil and seeds on LA-induced testicular injuries and hormonal deficits in male animals. The administration of NS to normal rats has been shown to boost the sperm production profiles and TS levels, indicating that the compound enhances male fertility (Juma and Hayfaa 2011; Mahdavi et al. 2015). In this study, LA induced decreased TBWR, reduced population of sperm maturation stages in the testis, and reduced tail epididymal sperm reserves indicating suppression of spermatogenesis; however, rats treated with graded doses of NS showed remarkable improvements in both spermatogenesis and epididymal sperm reserves. The deleterious effect of LA in the PC group was most obvious at 30 days of administration and least at 90 days. In related studies, NS oil has been shown to improve testicular weights, sperm concentration and motility, as well as reduce testicular degeneration and maturation arrest in rats exposed to sodium valproate, cisplastin, and heat stress (Al-Zahrani et al. 2012; Awadalla 2012; Hala 2011). It is therefore obvious that NS prevented testicular tissue damage and even enhanced sperm production at 200 mg/kg.
The reproductive hormones TS, estradiol (EST), and luteinizing hormone (LH) are important components of male sexual development and fertility. Significant decline in TS or an increase in EST and LH has been shown to adversely affect sexual maturity and fertility in male animals (Mann and Lutwak-Mann 2012). In this study, decreases in TS and FSH were observed in the PC and T3 groups on days 60 and 90, while LH was decreased in the PC and T1 groups at day at all points. These show deleterious effects of LA on the PC group and suppression of FSH production in the high-dose treatment group (NS 200 mg/kg). The decline in the level of TS in the PC group can be attributed to the lesions observed at histopathology; this ultimately resulted in low sperm maturation stages and epididymal reserves which translated into low sperm concentration and viability. The effect of LA on male hormonal production is dependent on the dose and duration of exposure; hence, reports available in the literature are not in synergy with one another. In one study, the administration of 0.05 % LA was shown to increase TS concentration in male rats after 24 weeks of daily exposure; however, the levels of FSH and LH were unaffected (Allouche et al. 2009). This is contrary to our findings and may be due to the difference in both the dose of administration and study duration. On the other hand, another study, which concurs with our findings, investigated the effect of maternal LA administration during lactation on offspring testicular parameters and function, which resulted in a reduction of TS concentration after 90–120 days (Dorostghoal et al. 2011). The administration of NS oil to normal male rats was reported to increase the levels of TS, LH, and FSH after 30–60 days, indicating a positive effect on the hormonal production (Bashandy 2007; Juma and Hayfaa 2011; Mahdavi et al. 2015). Based on these reports, we can say that the NS seed powder played a therapeutic role on the testicular tissue and Leydig cells hence maintaining TS production at normal levels throughout the experimental course. This agrees with a previous study, where NS oil at 250 mg/kg was reported to increase the level of TS in rats exposed to sodium valproate (Hala 2011). Reactive oxygen species (ROS) such as O2 − and H2O2 are responsible for oxidative and altered levels of antioxidant enzymes such as SOD, GSH, and catalase (CAT). Increased production of ROS has been associated with tissue damage and disease (Abba et al. 2015). In this study, LA caused a decrease in GSH at day 30 and SOD at all days of sampling. This shows that LA caused a decline in the oxidant defense mechanism in untreated rats. On the other hand, treatment with NS averted these effects and even led to an increased level of GSH in the T3 group at days 60 and 90. The administration of low-dose LA has been reported to induce oxidative stress in animals; such resultant effects were attributed to oxidant organ damage and dysfunction associated with LA toxicity in man and animals (Ahamed and Siddiqui 2007). The protective effects of NS oil and seed extract against oxidant damage have been reported; NS oil restored increased malondialdehyde and decreased GSH levels in rats exposed to tramadol toxicity, while 10 % extract of NS was able to restore imbalances in CAT, GSH, SOD, MDA, glutathione peroxidase, and glutathione reductase in mice exposed to carbon tetrachloride poisoning (Elkhateeb et al. 2015). These reports concur with the results of this study and support the fact that NS is a potent antioxidant compound.
Conclusion
In conclusion, this study reports the presence of gonadal spermatogenesis suppression, decreased TS, and reduced antioxidant defense in rats exposed to low-dose chronic LA administration. Similarly, it also showed the remarkable effects of graded dose NS administration in ameliorating the adverse effects of LA in the rats. From these findings, NS has shown its antioxidant potentials against LA-induced oxidant damage and it is therefore a good candidate for further exploration in other heavy metal-associated toxicities.
References
Abba Y, HassimH HH, Noordin MM (2015) Antioxidant vitamins, oxidant injuries and diseases. PJSRR 1:58–66
Abbasnezhad A, Hayatdavoudi P, Niazmand S, Mahmoudabady M (2015) The effects of hydroalcoholic extract of Nigella sativa seed on oxidative stress in hippocampus of STZ-induced diabetic rats. Avicenna J Phytomed, 1–7
Ahamed M, Siddiqui M (2007) Low level lead exposure and oxidative stress: current opinions. CCA 383:57–64
Ali B, Blunden G (2003) Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17:299–305
Allouche L, Hamadouche M, Touabti A (2009) Chronic effects of low lead levels on sperm quality, gonadotropins and testosterone in albino rats. Exp Toxicol Pathol 61:503–510
Allouche L, Hamadouche M, Touabti A, Khennouf S (2011) Effect of long-term exposure to low or moderate lead concentrations on growth, lipid profile and liver function in albino rats. Adv Biol Res 5:339–347
Al-Zahrani S, Mohany M, Kandeal S, Badr G (2012) Thymoquinone and vitamin E supplementation improve the reproductive characteristics of heat stressed male mice. J Med Plant Res 6:493–499
Awadalla EA (2012) Ameliorative effect of the crude oil of the Nigella sativa on oxidative stress induced in rat testes by cisplatin treatment. Biomedicine and Preventive Nutrition 2:265–268
Bashandy AS (2007) Effect of fixed oil of Nigella sativa on male fertility in normal and hyperlipidemic rats. Int J Pharmacol 3:27–33
Bharali M (2013) Effect of acute lead acetate exposure on liver of mice. Journal of Global Biosciences 2:121–125
Dalia M (2010) Effect of using pectin on lead toxicity. J Am Sci 6:541–554
Dorostghoal M, Dezfoolian A, Sorooshnia F (2011) Effects of maternal lead acetate exposure during lactation on postnatal development of testis in offspring Wistar rats. Iran J Basic Med Sci 14:122–131
Elgawish RAR, Abdelrazek HM (2014) Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicology Reports 1:795–801
Elkhateeb A, El Khishin I, Megahed O, Mazen F (2015) Effect of Nigella sativa Linn oil on tramadol-induced hepato-and nephrotoxicity in adult male albino rats. Toxicology Reports 2:512–519
El-Tohamy M, El-Nattat W (2010) Effect of antioxidant on lead-induced oxidative damage and reproductive dysfunction in male rabbits. J Am Sci 6:613–622
Gali-Muhtasib H, El-Najjar N, Schneider-Stock R (2006) The medicinal potential of black seed (Nigella sativa) and its components. Adv Phytomed 2:133–153
Hala M (2011) Protective effect of Nigella sativa, linseed and celery oils against testicular toxicity induced by sodium valproate in male rats. J Am Sci 7
Ibrahim NM, Eweis EA, El-Beltagi HS, Abdel-Mobdy YE (2012) Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed 2:41–46
Jahromy MH, Jalili M, Mohajer AJ, Poor FK, Dara SM (2014) Effects of Nigella sativa seed extract on perphenzine-induced muscle rigidity in male mice. World J Neurosci 2014
Jesse FFA, Abba Y, Tijjani A, Sadiq MA, Konto M, Adamu L, Wahid AH, MohdAzmi ML, Eric LTC, Ab Rahman MF (2016) Gonado-hypophyseal lesions and reproductive hormonal changes in Brucella melitensis-infected mice and its lipopolysaccharides (LPSs). Comp Clin Path 25:31–36
Juma FT, Hayfaa M (2011) A: the effects of Nigella sativa oil administration on some physiological and histological values of reproductive aspects of rats. Iraqi J Vet Med 35:52–60
Krishnan N, Muthukrishnan S (2012) Effect of Nigella sativa seed extract on carbon tetrachloride-induced hepatotoxicity in rats. Acute Med 2:107–113
Mahdavi R, Heshmati J, Namazi N (2015) Effects of black seeds (Nigella sativa) on male infertility: a systematic review. J Herb Med 5:133–139
Makhlouf MMM, Eldien HMS, Zagloul DAM, Abu Dief EE, Abd ElHaliem NG (2008) The effect of the lead acetate on testicular structure and protective effect of vitamin E in adult albino rat. Egypt J Histol 31:406–418
Mann T, Lutwak-Mann C (2012) Male reproductive function and semen: themes and trends in physiology, biochemistry and investigative andrology. Springer Science & Business Media
Meyer O, Blom L, Sondergaard D (1982) The influence of minerals and protein on the nephrocalinosis potential for rats of semi synthesis diets. Lab Anim 16(3):271–273
Murugesan M, Ragunath M, Prabu T, Nadanasabapathi S, Sakthivel M, Manju V (2012a) Protective role of black cumin (Nigella sativa) on isoproterenol induced myocardial infarction in rats. Int J Pharmacol and Clin Sci 1:45–53
Murugesan M, Ragunath M, Prabu T, Nadanasabapathi S, Sakthivel M, Manju V (2012b) Protective role of black cumin (Nigella sativa) on isoproterenol induced myocardial infarction in rats. Int J Pharmacol Clin Sci 1:45–53
Mustafa H N, Hussein AM (2015) Does allicin combined with vitamin B-complex have superior potentials than α-tocopherol alone in ameliorating lead acetate-induced Purkinje cell alterations in rats? An immunohistochemical and ultrastructural study. Folia Morphol
Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM (2005) Lead toxicity update. A brief review. Med Sci Monit 11:RA329–RA336
Paul F (2009) Laboratory animal anesthesia. Academic press is an imprint of Elsevier. Newcastle University, pp. 208
Salem ML (2005) Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol 5:1749–1770
Sharma R, Garu U (2011) Effects of lead toxicity on developing testes in Swiss mice. Univ J Environ Res Technol 1:390–398
Weisbroth SH, Flatt RE Kraus AL (Eds). (2013) The biology of the laboratory rabbit. Academic Press
Yokoi K, Uthus EO, Nielsen FH (2003) Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 93(1–3):141–153
Acknowledgments
We want to acknowledge the Ministry of Higher Education (MOHE), Malaysia, through its Fundamental Research Grant scheme for the financial support of this research.
Author contribution
All authors contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The animal experimental protocol used in this study was approved by the Institutional Animal Care and Use Committee (IACUC) with reference number: UPM/IACUC/AUP-R047/2015, in accordance with the standard guidelines on usage and care of laboratory animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Assi, M.A., Hezmee, M.N.M., Abba, Y. et al. Assessment of therapeutic effects of Nigella sativa against chronic lead acetate-induced reproductive dysfunction in male Sprague-Dawley rats. Comp Clin Pathol 26, 87–97 (2017). https://doi.org/10.1007/s00580-016-2349-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-016-2349-3