Abstract
Chlorpyrifos (CPF), an organophosphate widely applied in agriculture and aquaculture, induces oxidative stress due to free-radical generation and changes in the antioxidant defense system. The present study investigated the short-term effect of CPF exposure on the oxidative and antioxidant systems and their recovery responses in metabolically active tissues (gills, hepatopancreas [HP], and leg muscle) of freshwater crab Barytelphusa guerini. Crabs were exposed to a sublethal concentration of CPF (0.07 mg L−1) for a total of 8 days (at intervals of 1, 2, 4, and 8 days) in clean water. The following oxidative stress markers were measured: acetylcholinesterase (AChE), butylcholinesterase (BChE), and ATPase; antioxidants i.e., superoxide dismutase (SOD), catalase, and glutathione reductase (GR), lipid peroxidation (LPO), conjugating enzyme glutathione S-transferase (GST), glutathione peroxidase (GPx), and lipid content. CPF exposure led to a significant decrease in the activity of oxidative stress markers as follows: AChE (84 %), BChE (46 %), and gills Na+/K+ ATPase (62 %). At the end of the recovery period, enzyme levels were recovered except in leg muscle. Total lipids and SOD decreased; CAT and LPO levels increased; and GPx, GR, and GST showed tissue-specific activities. Maximum recovery was observed in GPx followed by GR in HP tissue of crab. Nevertheless, these responses apparently grant successful adaptation for survival in a pesticide-extreme environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Organophosphorus insecticides (OPs) are frequently used for agricultural and domestic purposes. Because OPs are relatively labile environmentally and biologically, they have replaced many organochlorine insecticides because they are more stable and less toxic to the environment (Casida and Quistad 2004). The main mechanism by which OPs influence the environment depends largely on the biochemical processes of animals and the physicochemical properties of phosphorous compounds. However, the toxic symptoms produced in animals by OPs are manifestations of the inhibition of certain enzyme systems (Livingstone 2001; Narra et al. 2013). OPs are inhibitors of a variety of enzymes, but generally they are associated with the inhibition of cholinesterase (Collange et al. 2010).
Chlorpyrifos [CPF; O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate] is an OP that is widely used in India as well as in many other parts of the world. It is a broad-spectrum pesticide used extensively for the management of domestic and agricultural pests. It is effective in controlling arthropods in soil or on foliage in >100 crop types (Tomlin 2000). Its release into the aquatic environment promotes toxic effects to nontarget aquatic species (Palma et al. 2008). CPF is a highly toxic pesticide for aquatic animals even at low concentrations (Narra et al. 2012, 2013). However, the toxicological impact of CPF exposure may be more profound in some aquatic species than in others.
In invertebrates, neurotransmission has not been as well characterized as it has been in vertebrates. Crustaceans posses both inhibitory and excitatory motoneurons, which usually employ other transmitters. When ChE is present, its primary function is as a neurotransmitter for afferent nerve fibers. ChEs are usually divided into two broad classes: acetylcholinesterases (AChEs) and butylcholinesterase (BChEs). Studies have showed ChE inhibition as a biomarker in identifying OP-exposed organisms in the field and under laboratory conditions (Sultatos 2006; Vioque-Ferńandez et al. 2007). The enzyme Na+/K+ ATPase is crucial for physiological activities, including osmotic balance, neurotransmitter release, and other cellular functions, such as cell volume regulation, calcium concentration, and membrane potential (MacGregor and Walker 1993).
Many xenobiotics, such as pesticides, cause oxidative stress leading to the generation of reactive oxygen species (ROS) and alterations in antioxidants or free oxygen-radical-scavenging enzyme systems in aquatic organisms (Livingstone 2001; Valavanidis et al. 2006). Oxidative stress occurs when the critical balance between oxidants and antioxidants is disrupted due to depletion of the antioxidant, excessive accumulation of ROS, or both, thus leading to damage (Scandalios 2005). Contaminant-stimulated ROS are associated with different pathologic processes involved in the etiology of many diseases and may be a mechanism of toxicity in aquatic organisms exposed to pollutants (Kehrer 1993).
Detoxification mechanisms are enzymatic adaptations developed by animals to protect against toxic xenobiotics produced by living organisms. Anthropogenic xenobiotics, which are carried into the organism at portals (gills, gastrointestinal tract, integument. and excretory system), may not be efficiently metabolized. Hepatopancreas (HP) plays a pivotal role in metabolizing xenobiotics; a rich supply of nonspecific enzymes metabolize a broad spectrum of organic materials. Therefore, we expect that water pollution may channelize with altered antioxidant defense of freshwater field crab Barytelphusa guerini. It is important to note that information on the responses of antioxidant defenses of B. guerini under CPF intoxication is scant. Therefore, the present study was undertaken to investigate the effect of CPF on enzyme activity, such as AChE, BChE, gill Na+/K+ ATPase, lipid peroxidation (LPO), antioxidant defense system markers [superoxide dismutase (SOD), glutathione reductase (GR), glutathione S-transferase (GST), glutathione peroxidase (GPx), and catalase (CAT)], and total lipids in gill, HP, and leg muscle of freshwater crab.
Materials and Methods
Chemicals
All of the chemicals used in the present study were purchased from Sigma-Aldrich Chemical Company (Saint Louis, Missouri, USA). The test compound chlorpyrifos 20 % EC (emulsifiable concentration) was purchased from local market (National Organic Chemical Industries Limited, Bombay, India).
Animal Maintenance
Freshwater field crabs B. guerini were procured from local suppliers and brought to the laboratory in aerated large plastic tubs. Before the experiment, crabs were acclimatized to laboratory conditions and fed dry prawns for 8 days. Crabs (weighing 40 ± 5 g) were assigned to seven huge cement tanks, each of which had a 10 cm high bed of sand and a water level high enough to keep the crabs in submerged conditions. Fecal pellets and excess diet were removed and water exchanged daily to maintain water quality. A natural (12:12–h light-to-dark) photoperiod was maintained.
Physicochemical Parameters of the Medium
The average mean values of water quality during the investigation were as follows: temperature 25 ± 3 °C, pH 7.4 ± 0.4, dissolved oxygen 8.24 ± 0.22 mg L−1, total hardness 415 ± 1.2 mg L−1 as CaCO3, alkalinity 348 ± 1.6 mg L−1 as CaCO3, and chlorides 245.57 ± 1.44 mg L−1.
Pesticide Preparation and Experimental Design
The LC50 (lethal concentration required to kill half of a population of laboratory test animals) value of CPF was determined in the laboratory employing the semistatic method of Finney (1971) (using SPSS software version 12.0), and it was found to be 0.21 mg L−1. The stock solution of CPF was prepared by dissolving in acetone, and the control was performed by the addition of carrier solvent acetone. During sublethal studies, a group of 48 healthy crabs was exposed to CPF with a concentration of 0.07 mg L−1 (1/3rd of LC50) for a period of 8 days at intervals of 1, 2, 4, and 8 and another group of 48 crabs was kept in freshwater as control. In the exposure group after 8 days, 24 crabs were released into freshwater and kept in the same medium for 8 days for the recovery study. Crabs were starved 24 h before experimentation and anaesthetized on ice for 10 min; dissected tissues (gills, HP, and leg muscle) were used for further estimations. Tissues from the crabs were dissected after anesthetized by ice treatment to avoided enzymatical changes for accurate results.
ChE Assay
Tissues were homogenized in ice-cold 100 mmol L−1 phosphate buffer at pH 7.4, and the homogenates were centrifuged at 9,000×g for 30 min at 4 °C. AChE activity was measured at room temperature using the colorimetric method of Ellman et al. (1961). In a typical assay, 1,050 μL of 0.1 mol L−1 phosphate buffer, 50 μL of 0.008 mol L−1 dithiobisnitrobenzoate, 50 μL of supernatant, and 50 μL of substrate acetylcholine (ACH) 0.045 mol/L were added. The enzymatic reaction rate was measured spectrophotometrically at 412 nm against a blank. The enzyme activity was expressed as nmol substrate hydrolysed/min/mg protein.
ATPase Assay
Gills were homogenized in ice-cold 1.0 ml of 0.1 M Tris–HCl buffer (pH 7.4) using a Teflon homogenizer and then centrifuged at 1,000 rpm for 15 min at 4 °C. The supernatant was used for the estimation of Na+/K+ ATPase activity according to the method of Shiosaka et al. (1971) and expressed as μmol Pi (inorganic phosphate) liberated/mg protein.
Antioxidant Assays
SOD activity was measured according to the method of Das et al. (2000), and the activity was expressed in U (unit)/mg protein. CAT activity was measured by the method of Aebi (1974) by monitoring the decrease in absorbance of H2O2 at 240 nm and was expressed as units/mg protein. GPx activity was measured according to the method of Paglia and Velentine (1967) with cumene-hydroperoxide as the substrate and expressed as nmol of NADPH oxidized/min/mg protein. GR activity was assayed by the method of Massey and Williams (1965) and expressed in nmol of NADPH oxidized/min/mg protein. GST activity was measured according to Habig et al. (1974), and activity was expressed as the amount of enzyme catalyzing the formation of nmol thiobarbituric acid reactive substances (TBARS) formed/min/mg protein. LPO was determined by Ohkawa et al. (1979), and the level of TBARS was expressed as nmol of TBARS formed/mg protein.
Lipid Content
Total lipid content was estimated by the method of Folch et al. (1957) and was expressed as mg/g wet weight of tissue.
Statistical Analysis
The data were subjected to Statistical Package for the Social Sciences (SIPS) version 12.0 software. Duncan’s multiple range tests were used to determine the differences among treatment means at p > 0.05, and each value was expressed as the mean ± SE. All of the experiments were repeated six times (n = 6), and no significant results were observed within the control (summarized control values were taken).
Results
AChE Activity
AChE activity was inhibited in all tissues throughout the exposure period; the greatest inhibition was observed in muscle (84 %) followed by HP (75 %) and the lowest in gills (63 %) at the end of the exposure period. When the crabs were transferred into toxicant-free water, insignificant recovery (p > 0.05) was noted in gills and HP at the end of day 8, whereas muscle may require more time to recover (12 %) (Table 1).
BChE Activity
BChE activity in CPF-exposed crabs was not significantly affected (p > 0.05) on day 1, whereas significant inhibition was observed in muscle (47 %), HP (34 %), and gills (29 %) at the end of the exposure period. Insignificant recovery (p > 0.05) was noticed in gills on day 4 and that of HP and muscle on day 8 (Table 1).
Na+/K+ ATPase Activity
In gills, Na+/K+ ATPase activity was inhibited (62 %) after 8 days of exposure and recovered (p > 0.05) in a time-dependent manner at the end of recovery period (Table 1).
LPO Level
In CPF-exposed crabs, the LPO level in gills was 46 %, in muscle was 40 %, and in HP was 38 % at the end of the 8-day exposure period. Insignificant recovery (p > 0.05) was observed on day 8 in all tissues (Fig. 1a).
SOD Activity
Activity of the primary antioxidant enzyme, i.e., SOD, decreased in a time-dependent manner with a maximum in muscle tissue (66 %) followed by HP (36 %) and was lowest in gills (29 %). Complete recovery (p > 0.05) was observed on day 8 when the crabs were transferred into toxicant-free water (Fig. 1b).
CAT Activity
Activity of the second primary antioxidant enzyme, i.e., CAT, was inducted to a maximum in gills (37 %) on day 2, whereas it was highest on day 8 in HP (47 %) and muscle (68 %). When crabs were released into CPF-free water, gills insignificantly recovered (5 %) on day 4; HP recovered on day 8; and muscle (17 %) required more time to recover (Fig. 2a).
GPx Activity
GPx activity in muscle (41 %) and gills (31 %) was enhanced throughout the exposure period, whereas it was decreased in HP (39 %) compared with the control. Depuration results showed that gills and HP were insignificantly (p > 0.05) recovered to the control level at the end of day 8, and muscle (8 %) required more time to recover (Fig. 2b).
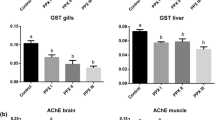
GST Activity
Activity of GST was significantly enhanced to the maximum in muscle (70 %) followed by gills (45 %) with a decreased trend observed in HP (60 %) at the end of exposure period. Gills recovered on day 4 and HP on day 8, whereas muscle (16 %) required more time for recovery (Fig. 3a).
GR
GR activities were decreased in gills (44 %) and HP (37 %), whereas there was an increase in muscle (60 %) at the end of exposure period. At the end of 8-day recovery period insignificant recovery (p > 0.05) was observed in gills and HP and muscle may require more time (15 %) (Fig. 3b).
Total Lipid
Total lipid content was decreased in tissues of crab but highest in HP (69 %) followed by muscle (59 %) and gills (55 %). When crabs were released into toxicant-free water, insignificant recovery (p > 0.05) was observed in gills and muscle, whereas HP had not recovered by the end of day 8 (Fig. 4).
Discussion
AChE activity measurement has been used for monitoring the neurotoxicity of OP pesticides (Mileson et al. 1998; Jokanović 2001). Jokanović (2001) reported that AChE is responsible for the hydrolysis of the neurotransmitter acetylcholine, which stimulates a postsynaptic response in the nervous system and the neuromuscular junction: Inactivation of the enzymes disrupts the action of the nervous and neuromotor systems. The enzymes cause dose- and time-dependent inhibition, and the rate of inhibition differs depending on species and age (Pan and Dutta 1998). In some species, AChE sensitivity in muscle tissue mirrors that in brain tissue, whereas in others species, muscle tissue is much more insensitive (Wheelock et al. 2005; Modesto and Martinez 2010). Gagnaire et al. (2008) reported that the variability of AChE and BChE enzyme characteristics has also been found between different invertebrate species and different tissues of the same organism in a time-dependent manner. In Procambarus clarkia, muscle had greater AChE and BChE activities than other tissues when exposed to the OP insecticide fenitrothion (Escartin and Porte 1996). In the present study, gill, HP and muscle AChE and BChE activities decreased compared with those of control crabs. Chang et al. (2006) reported decreased AChE activity in gills, haemolymph, and HP of Macrobrachium rosenbergii exposed to the OP pesticide trichlorfon. Decreased AChE activity was observed in the brain and ventral ganglion of blue crab Callinectes sapidus (Habig et al. 1986), HP of brown shrimp (Chambers et al. 1979), and muscle tissue of shrimp Palaemon serratus (Galgani and Bocquene 1990). When crabs were transferred into CPF-free water, AChE and BChE activities recovered to a large extent. Recovery of ChE activities in fish exposed to OPs appears to be a function largely of the degree of the initial inhibition because enzyme recovery requires de novo synthesis of the enzyme. This suggests that CPF might have caused an accumulation of ACh at the neuromuscular junction (Klaassen 2001).
Gills, the principal organ for respiration in crabs, are directly exposed to the surrounding environment and maintain acid–base, osmotic, and ionic balance of body fluid (Lucu 1990). Na+/K+ ATPase has a role in osmoregulation by providing energy for active transport of Na+ K+ and maintains the Na+/K+ ATPase gradient across cell membranes due to free energy resulting from the hydrolysis of intracellular adenosine triphosphate (ATP) (Scheiner-Bobis 2002). In the present study, inhibition in Na+/K+ ATPase suggests that the rate of ion transport was decreased in gill tissue. Na+/K+ ATPase and AChE are membrane-bound enzymes, and their activities depend on the phospholipid content of the membrane. At the end of the depuration period, Na+/K+ ATPase was found to be near that of the control. Any change in the lipid component of the membrane will directly affect the activities of these enzymes (Sahoo et al. 1999). In the present investigation, decreased total lipid content observed in gills, HP, and muscle is associated with Na+/K+ ATPase and ChE inhibition.
LPO is one of the main processes induced by oxidative stress (Storey 1996). Enhancement of LPO in tissues of crabs suggests the participation of free radical-induced oxidative cell injury in mediating the toxicity of CPF (Archana et al. 2011). The most affected tissue was muscle, and the least affected was HP. Increased LPO content in gills and digestive glands of freshwater mussel exposed to pesticide was reported by Kopriicu et al. (2010). The activity of antioxidant enzymes may be induced or inhibited by xenobiotic exposure intensity, duration of stress, and susceptibility of the exposed species (Thomaz et al. 2009). SOD and CAT are key enzymes in the antioxidant system that work to maintain a low, steady concentration of ROS. SOD activity of muscle, HP, and gills of B. guerini decreased with exposure time. The decrease indicates that superoxide anion radical, after transformation to H2O2, caused oxidation of the SOD cysteine, which decreased its activity (Oruç and Uner 2000). This mechanism may have probably caused the decrease in SOD activity in the present study. The increased CAT activity in the present study may have been in response to H2O2 production. The decrease in SOD activity may have resulted in an accumulation of superoxides, which in turn increased CAT because superoxide anions are known to increase CAT activity. Transfer of CPF-intoxicated crabs to freshwater caused recovery in LPO, SOD, and CAT activities. Maximum recovery was shown by HP because it is metabolically more active than gills and muscle.
The induced GR activity in muscle and its inhibition in HP and gills in the present study suggests an organ-specific response of crab to CPF. The induction may be due to the increased production of oxidized glutathione (GSSG) as well as the high activity of GPx as suggested by Zhang et al. (2004). When the crabs were released into clean water, GR activity was close to that of the control in HP, which indicates its tissue-specific response to CPF toxicity. GSTs are phase II detoxifying enzyme that promote the conjugation of decreased glutathione with a variety of reactive electrophilic compounds, thus resulting in the formation of less toxic substances that are easily excreted from the body (Chasseaud 1979). The marked increase in GST activity in muscle and gill tissue suggests active involvement of this enzyme in the detoxification of CPF as part of the phase II biotransformation of xenobiotics (Li et al. 2007). The activity of GPx would have been induced by CPF intoxication. Whether the detoxification of peroxides can be achieved by an exact mechanism involved in the inhibition of GST in HP is not clear, and further studies are required to elucidate the importance of GST in CPF-intoxicated crabs. Release of CPF-intoxicated crabs into freshwater showed less recovery in muscle compared with that in HP and gills. Inhibition of GPx in HP of CPF-exposed crabs indicates inefficiency in neutralizing the impact of peroxides (Ahmad et al. 2000). Maximum recovery in GPx activity was observed in HP followed by gills and muscle. Aquatic organisms possess a baseline status of antioxidant systems involved in a variety of detoxification reactions, which are performed by antioxidant enzymes (SOD, CAT, GPx, and GPx) to assure the maintenance of pro-oxidant/antioxidant balance, which is crucial for cellular homeostasis (Winston and Di Giulio 1991; Livingstone 2001). B. guerini has the capability to overcome stress caused by CPF after its release into toxicant-free medium (Narra et al. 2013).
Conclusion
The present results showed that CPF induces significant depression of Na+/K+ ATPase (gills) and cholinesterases as well as significant antioxidant fluctuation in gills, leg muscle, and HP of crab. From the results of the depuration study, it is concluded that crab has the capacity to recover from toxicant stress and that longer recovery periods would be necessary to recover normal metabolism after CPF intoxication. The results of the present study are not only expected to provide additional contribution to the emerging field of invertebrate oxidative stress, they will also help in understanding the physiological and biochemical basis of adaptation of B. guerini exposed to pesticides. More investigations must be performed to better understand the cellular- and tissue-specific mechanism involved in the remediation of CPF toxicity.
References
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, New York, pp 673–678
Ahmad I, Hamid T, Fatima M, Chand HS, Jain SK, Athar M et al (2000) Induction of hepatic antioxidants in fresh-water catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochem Biophys Acta 1523:37–48
Archana AS, Metkari V, Patode P (2011) Effect of methyl parathion and chlorpyrifos on certain biomarkers in various tissues of guppy fish, Poecilia reticulata. Pestic Biochem Physiol 101:132–141
Casida JE, Quistad GB (2004) Organophosphate toxicology: safety aspects of non acetylcholinesterase secondary targets. Chem Res Toxicol 17:983–998
Chambers J, Heitz J, McCorkle F, Yarbrough J (1979) Enzyme activities following chronic exposure to crude oil in a simulated ecosystem. Environ Res 20:133–139
Chang CC, Lee PP, Hsu JP, Yeh SP, Cheng W (2006) Survival and biochemical physiological and histopathological responses of the giant freshwater prawn, Macrobrachium rosenbergii, to short-term trichlorfon exposure. Aquaculture 253:653–666
Chasseaud LF (1979) The role of glutathione and glutathione-S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res 29:175–274
Collange B, Wheelock CE, Rault M, Mazzia C, Capowiez Y, Sanchez-Hernandez JC (2010) Inhibition, recovery and oxime-induced reactivation of muscle esterases following chlorpyrifos exposure in the earthworm Lumbricus terrestris. Environ Pollut 158:2266–2272
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37:201–204
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm 7:88–95
Escartin E, Porte C (1996) Acetylcholinesterase inhibition in the crayfish Procambarus clarkii exposed to fenitrothion. Ecotoxicol Environ Saf 34:160–164
Finney DJ (1971) Probit analysis, 2nd edn. Cambridge University Press, London
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gagnaire B, Geffard O, Benoit X, Margoum C, Garric J (2008) Cholinesterase activities as potential biomarkers: characterization in two freshwater snails, Potamopyrgus antipodarum (Mollusca, Hydrobiidae, Smith 1889) and Valvata piscinalis (Mollusca, Valvatidae, Muller 1774). Chemosphere 71:553–560
Galgani F, Bocquene G (1990) In vitro inhibition of acetylcholinesterase from four marine species by organophosphates and carbamates. Bull Environ Contam Toxicol 45:243–249
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Habig C, DiGiulio R, Nomeir A, Abou-Donia M (1986) Comparative toxicity, cholinergic effects, and tissue levels of S,S,S,-tri-n-butyl phosphorotrithioate (DEF) to channel catfish (Ictalurus punctatus) and blue crabs (Callinectes sapidus). Aquat Toxicol 9:193–206
Jokanović M (2001) Biotransformation of organophosphorus compounds. Toxicology 166(3):139–160
Kehrer JP (1993) Free radicals as mediator of tissue injury and disease. Crit Rev Toxicol 23(1):21–48
Klaassen C (2001) Casarett and Doull’s toxicology. McGraw-Hill, New York
Kopriicii K, Yonar SM, Seker E (2010) Effects of cypermethrin on antioxidant status, oxidative stress biomarkers, behavior, and mortality in the freshwater mussel Unio elongatulus eucirrus. Fish Sci 76:1007–1013
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Ann Rev Entomol 52:231–253
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine Pollut Bull 42:656–666
Lucu C (1990) Ionic regulatory mechanisms in crustacean gill epithelia. Comp Biochem Physiol A 97:297–306
MacGregor SE, Walker JM (1993) Inhibitors of the Na+–K+-ATPase. Comp Biochem Physiol C 105:1–9
Massey V, Williams CH (1965) On the reaction mechanism of yeast glutathione reductase. J Biol Chem 240:4470–4481
Mileson BE, Chambers JE, Chen WL, Dettbarn W et al (1998) Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci 41:8–20
Modesto KA, Martinez CBR (2010) Effects of Roundup Transorb on fish: hematology, antioxidant defenses and acetylcholinesterase activity. Chemosphere 81:781–787
Narra MR, Begum G, Rajender K, Rao JV (2012) Toxic impact of two organophosphate insecticides on biochemical parameters of a food fish and assessment of recovery response. Toxicol Ind Health 28(4):343–352
Narra MR, Rajender K, Rao JV, Begum G (2013) Evaluation of biochemical stress response of chlorpyrifos in tissues of edible crab Barytelphusa guerini: withdrawal of exposure improves the nutritional value of crab. Z Naturforsch 68(c):318–326
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oruç EO, Uner N (2000) Combined effects of 2,4-d and azinphosmethyl on antioxidant enzymes and lipid peroxidation in liver of Oreochromis niloticus. Comp Biochem Physiol C 127:291–296
Paglia DE, Velentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Palma P, Palma VL, Fernandez RM, Soares AM, Barbosa IR (2008) Acute toxicity of atrazine, endosulfan and chlorpyrifos to Vibrio fischeri, Thamnocephalus platyurus and Daphnia magna relative to their concentrations in surface water from the Alentejo region of Portugal. Bull Environ Contam Toxicol 81:485–489
Pan G, Dutta HM (1998) The inhibition of brain acetylcholinerase activity of juvenile largemouth bass Micropterus salmoides by sublethal concentrations of diazinon. Environ Res 79:133–137
Sahoo A, Samanta L, Das A, Patra SK, Chainy GBN (1999) Hexachlorocyclohexane-induced behavioural and neurochemical changes in rat. J Appl Toxicol 19:13–18
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38:995–1014
Scheiner-Bobis G (2002) The sodium pump, its molecular properties and mechanisms of ion transport. Eur J Biochem 269:2424–2433
Shiosaka T, Okuda H, Fungi S (1971) Mechanisms of phosphorylation of thymidine by the culture filtrate of Clostridium perfringens and rat liver extract. Biochem Biophys Acta 246:171–183
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29(12):1715–1733
Sultatos LG (2006) Interactions of organophosphorus and carbamate compounds with cholinesterases. In: Gupta RC (ed) Toxicology of organophosphorus and carbamate compounds. Elsevier Academic, Burlington, pp 209–218
Thomaz JM, Martins ND, Monteiro DA, Rantin FT, Kalinin AL (2009) Cardio-respiratory function and oxidative stress biomarkers in Nile tilapia exposed to the organophosphate insecticide trichlorfon (NEGUVON®). Ecotoxicol Environ Saf 72:1413–1424
Tomlin CDS (2000) The pesticide manual, 12th edn. The British Crop Protection Council Publication, Surrey
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
Vioque-Ferńandez A, de Alves E, Ballesteros J, Garćıa T, Gómez L, López J (2007) Dŏnana national park survey using crayfish (Procambarus clarkii) as bioindicator: esterase inhibition and pollutant levels. Toxicol Lett 168(3):260–268
Wheelock C, Eder K, Werner I, Huang H, Jones P, Brammell B et al (2005) Individual variability in esterase activity and CYP1A levels in Chinook salmon (Oncorhynchus tshawytscha) exposed to esfenvalerate and chlorpyrifos. Aquat Toxicol 74:172–192
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161
Zhang JF, Shen H, Wang XR, Wu JC, Xue YQ (2004) Effects of water-soluble fractions of diesel oil on the antioxidant defenses of goldfish, Crassius auratus. Ecotoxicol Environ Saf 58:110–116
Acknowledgments
The author thanks Umamaheswar C. Reddy for advice concerning English language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narra, M.R. Tissue-Specific Recovery of Oxidative and Antioxidant Effects of Chlorpyrifos in the Freshwater Crab, Barytelphusa guerini . Arch Environ Contam Toxicol 67, 158–166 (2014). https://doi.org/10.1007/s00244-014-0010-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0010-1