Abstract
Triphenyltin (TPhT) is used worldwide in pesticide formulas for agriculture. Toxic effects of this compound to aquatic life have been reported; however, the biochemical response of fish exposed to different concentrations of TPhT hydroxide (TPhTH) was investigated for the first time in this study. The lethal concentration (LC50) of TPhTH to silver catfish, Rhamdia quelen, was calculated from an acute-exposure experiment (96 h). In addition, acethylcholinesterase (AChE) activity in brain and muscle—as well as glucose, glycogen, lactate, total protein, ammonia, and free amino acids in liver and muscle—were evaluated in a chronic-exposure experiment (15-day exposure). Speciation analysis of tin (Sn) was performed in fish tissues at the end of both experiments using gas chromatography coupled to a pulsed-flame photometric detector (GC-PFPD). Concentrations of TPhT, diphenyltin, and monophenyltin (reported as Sn) were lower than limits of quantification (10σ criteria). Waterborne TPhTH concentration used through the experiment was also evaluated by GC-PFPD, and no degradation of this species was observed. The LC50 value for silver catfish juveniles was 9.73 μg L−1 (as Sn). Decreased brain and muscle AChE activities were observed in fish exposed to TPhTH in relation to unexposed fish (control). Liver glycogen and lactate levels were significantly higher in fish kept at the highest waterborne TPhTH concentration compared with the control. Liver and muscle glucose levels of fish exposed to all TPhTH concentrations were significantly lower than those of control fish. Silver catfish exposed to all TPhTH concentrations showed lower total protein values and higher total free amino acids levels in liver and muscle compared with controls. Total ammonia levels in liver and muscle were significantly higher for the highest TPhTH concentration compared with controls. In conclusion, TPhTH caused metabolic alterations in silver catfish juveniles, and the analyzed parameters can also be used as bioindicators for TPhTH contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Triphenyltin (TPhT [(C6H5)3Sn+]) has been widely used in many countries as agricultural fungicide for the control of fungus diseases and blight on several crops (Bock 1981; Schaefer et al. 1981; Wieke Tas et al. 1989). The application of TPhT brings into the environment considerable amounts of this compound, which has provoked mortality of aquatic fauna and delays the re-establishment of organisms because of its residual toxicity (Schaefer et al. 1981; Wieke Tas et al. 1989). According to Schaefer et al. (1981), treatment of a paddy rice field with 1.12 kg of triphenyltin hydroxide (TPhTH)/ha resulted in an average water concentration of 146 μg L−1 at 2 h after application. This concentration decreased to <0.8 μg L−1 after 24 days. The average concentration of TPhTH in soil was 56 ng g−1 at 2 h, and this concentration increased to 340 ng g−1 at 3 days and then steadily decreased to 17 ng g−1 at day 24 after treatment. Even low concentrations of TPhT (in the low μg L−1 range) are toxic to fish, causing reproductive and morphological alterations and, consequently, death (Tooby et al. 1975; Johnson and Finley 1980; O’Halloran et al. 1998; Zhang et al. 2008). Moreover, sex organ alteration in females gastropods, a phenomenon known as “imposex,” is also attributed to TPhT, thus leading to decline of the population of these invertebrates (Horiguchi et al. 1997). Some studies also have shown suspected endocrine-disrupting effects of TPhT in human placenta and ovaries (Appel 2004; Osada et al. 2005; Nakanishi 2008). In the environment, TPhT can be degraded by progressive losses of aryl groups from the tin (Sn) cation, forming diphenyltin (DPhT), monophenyltin (MPhT), and, finally, inorganic Sn. Among organotin compounds (OTCs), the tri-substituted species are the most toxic, whereas the nature of the anion group has little or no effect on the biocide activity, except if this anion itself is a toxic species (Hoch 2001). In this sense, the order of toxicity of phenyltins is TPhT > DPhT > MPhT, whereas inorganic Sn is generally considered nontoxic. In Brazil, a commercial formula based on triphenyltin hydroxide is regularly employed in rice crops. Its use is also legally allowed in cotton, garlic, cocoa, carrot, bean, and potato crops (Ministério da Agricultura 2012). The recommendation for use is to apply 1 L of commercial product (400 g L−1 of TPhTH)/4 ha field.
In studies of fish exposed to TPhTH, the bioconcentration and elimination factors (Wieke Tas et al. 1989) and toxicity due to acute and chronic exposure (Jarvinen et al. 1988) have been evaluated. Acetylcholinesterase (AChE) has been used by different investigators as a possible biomarker for carbamate, organophosphate, and pesticide toxicity in fish (Sancho et al. 1997; Fernández-Vega et al. 2002; Miron et al. 2005; Glusczak et al. 2006, 2007; Moraes et al. 2007; Modesto and Martinez 2010). This enzyme, present in the cholinergic synapses and motor end plates, is responsible for degrading acetylcholine at the synaptic level and is extremely important for many physiological functions of fish, such as prey location, predator evasion, orientation to food, and reproductive behavior (Saglio and Trijasse 1998; Bretaud et al. 2000; Dutta and Arends 2003; Miron et al. 2005). Possible changes in AChE activity due to exposure of fish to pollutants affect physiological functions, resulting in weakness and even death (Saglio and Trijasse 1998).
Silver catfish, Rhamdia quelen (Heptapteridae), is a native freshwater fish from southern Brazil of great economic importance. Previous studies have documented the physiological and biochemical responses of this species when exposed to herbicides (Miron et al. 2005; Crestani et al. 2006, 2007; Glusczak et al. 2007; Becker et al. 2009).
In the present work, a commercial TPhTH pesticide (Mertin 400; Syngenta, Brazil) was used. The purposes of the study were to (1) determine the lethal concentration of TPhTH to silver catfish; (2) identify phenyltin compounds in water and tissues of silver catfish after exposure to TPhTH; (3) verify AChE enzyme activity in fish exposed to this compound; and (4) determine effects of TPhTH on some metabolic parameters of this species.
Material and Methods
Instrumentation
A Varian 3800 gas chromatograph (Palo Alto, CA, USA) equipped with a pulsed flame photometric detector (PFPD), a Varian 1093 injector (held at 350 °C), and a CP 8400 autosampler (Varian) were used throughout the study. The separation of OTCs was performed using a capillary column (30 m × 0.32 mm i.d.) coated with polydimethylsiloxane (0.25-μm film thickness; Varian). Helium (White Martins, SP, Brazil, 99.9999% purity) was used as carrier gas at a flow rate of 2 mL min−1. The following temperature program was used to separate the organotin species: initial oven temperature was set to 50 °C and increased to 175 °C (10 °C min−1), then to 300 °C at 25 °C min−1, and finally held at this temperature for 2 min. The PFPD operating temperature was 350 °C with an air/hydrogen flame. The gas flow rates were 25.0, 20.0, and 22.0 mL min−1 for air 1, air 2, and H2, respectively.
Reagents and Standards
Ultrapure water was obtained from a Milli-Q system (18 MΩ cm; Millipore, Bedford, USA). Sodium tetraethylborate (Sigma-Aldrich, Seelze, Germany) solution for Sn species ethylation was prepared by dissolution of NaBEt4 in water to provide a 2 % (m/v) solution. Acetate buffer [1.0 mol L−1 (pH 4.9)] was prepared by mixing appropriate amounts of acetic acid (Merck, Darmstad, Germany) and sodium acetate (Merck) in water. Isooctane was purchased from Mallinckrodt (Phillipsburg, USA). Monophenyltin, DPhT, TPhT, and dibutyltin (DBT) chloride were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Stock standard solutions of individual organotin compounds [2500 mg kg−1 (as Sn)] were prepared by dissolving appropriate amounts of the respective compounds in methanol (Mallinckrodt) and stored at −20 °C. Working standard solutions were prepared weekly by appropriate dilution of an aliquot of the stock solutions in methanol. A commercial product containing 400 g L−1 of TPhTH (Mertin 400) was used to prepare the experimental solutions.
Fish Acclimation
Silver catfish juveniles were bought from a local fish culture and transported to the Fish Physiology Laboratory at the Universidade Federal de Santa Maria where the fish were placed in continuously aerated 250-L tanks for at least 1 week before experiments. During this acclimation period, the water parameters were as follows: dissolved oxygen (7.1 ± 0.7 mg L−1 O2), temperature (20.5 ± 1.0 °C), pH (7.2 ± 0.2), total ammonia nitrogen (TAN; 0.023 ± 0.008 mg L−1 N), unionized ammonia (0.00015 mg L−1), water hardness (23.5 ± 2.1 mg L−1 CaCO3), and alkalinity (32.1 ± 1.7 mg L−1 CaCO3). Dissolved oxygen and temperature were measured with a YSI oxygen meter. The pH was verified with a DMPH-2 pH meter. Nesslerization was used for TAN determination according to the method described in Eaton et al. (2005), and unionized ammonia levels were calculated according to Colt (2002). Water hardness was determined by the ethylene diamine tetraacetic acid titrimetric method and alkalinity according to Boyd and Tucker (1992). During the acclimation period, the photoperiod was 12 h of light to 12 h of darkness. Fish were fed once a day with commercial feed for juveniles [Supra (42 % CP)] until apparent satiety. Feeding was discontinued 24 h before the beginning of exposure to the lethal concentration (LC50) test.
Acute Experiment
After acclimation, juveniles (12.24 ± 0.18 g and 11.07 ± 0.11 cm) were randomly distributed to 30-L tanks yielding the following seven treatments of TPhTH (three replicates each treatment, five fish per tank): 0 (control), 4.40 ± 0.61, 5.95 ± 0.33, 6.76 ± 0.72, 9.43 ± 0.52, 11.57 ± 0.64, and 13.81 ± 0.77 μg L−1 as Sn (these concentrations were determined by the analysis of water of each tank using GC-PFPD before starting the experiment). All feces and residues were removed daily by suction; consequently, ~20 % of the water in the tanks was replaced by water with previously adjusted TPhTH concentrations. Mortality was registered at 12, 24, 48, 72, and 96 h after exposure to TPhTH, and juveniles were removed when immobile and respiratory movements ceased. The LC50 value at 72- and 96-h exposure to TPhTH was calculated by the method of probits (Finney 1971).
Chronic Experiment
Silver catfish (107.27 ± 1.21 g 22.21 ± 1.15 cm) were randomly separated in 30-L tanks and exposed for 15 days to the following TPhTH concentrations [in μg L−1 (as Sn)]: 0 (control), 1.08 ± 0.27, and 1.70 ± 0.33 (three replicates each treatment; five fish per tank). Greater concentrations of TPhTH were not included because it was verified that they lead to death of the fish after a few hours. A lesser concentration of 1.08 μg L−1 TPhTH was not tested because of the limits of quantification of phenyltins in water are ~0.5 μg L−1. These concentrations were determined by the analysis of water of each tank using GC-PFPD before starting the experiments.
Fish were fed to satiety once a day during the experimental period. Uneaten food and feces were siphoned daily, and at least 10 % of the water in the tank was replaced with water with previously adjusted waterborne TPhTH concentrations. Water parameters were monitored according to the methods described previously every 3 days, and the mean values were as follows: dissolved oxygen (7.3 ± 0.5 mg L−1 O2), temperature (22.7 ± 1.0 °C), pH (7.2 ± 0.2), TAN (4.8 ± 0.3 mg L−1 N), unionized ammonia (0.037 mg L−1), water hardness (29.9 ± 3.1 mg L−1 CaCO3), and alkalinity (35.6 ± 1.7 mg L−1 CaCO3). In addition, every 3 days, the water was totally replaced and water samples collected to control TPhTH concentrations.
After the experimental period, all fish were killed by spinal cord section. The whole brain, liver, kidney, gill, and muscle were carefully removed to determine TPhTH concentrations using GC-PFPD. Moreover, samples of brain, liver, and muscle were obtained to determine AChE activity and metabolic parameters. The Ethics and Care Committee for Laboratory Animals of Federal University of Santa Maria (UFSM) approved the study protocol (2007-24).
Sn Speciation by GC-PFPD
The sample preparation procedure used for fish was adapted from Van et al. (2008). Briefly, ~400 mg of lyophilized fish tissues (brain, liver, kidney, gill, and muscle) were weighed into a glass vial, and 1 g of NaCl and 5 mL of methanol/ethyl acetate 1:1 were added. The mixture was mechanically stirred for 1 h (in the dark). After that, 5 mL of 0.1 mol L−1 HCl in methanol/ethyl acetate (1:1) were added and the mixture shaken for 1 h. Then samples were centrifuged at 1,000×g for 5 min, and 4 mL of were transferred to another glass vial; the pH was adjusted to 4.9 with sodium acetate/acetic acid buffer for further derivatization. After 1 mL of isooctane and 1 mL of 2 % (m/v) NaBEt4 solution were added, the ethylation reaction was performed by manually shaking the mixture for 10 min. The samples were then centrifuged at 1,000×g for 5 min, and the organic phase was transferred to a GC vial for analysis by GC-PFPD. For the analysis of water samples, 10 mL of samples were transferred to a glass vial and the pH adjusted to 4.9 by the addition of 2 mL of sodium acetate/acetic acid buffer solution. The ethylation was performed as described previously for fish tissue samples.
The commercial product Mertin 400, a suspension containing 400 g L−1 TPhTH applied to rice fields, was analysed by GC–ICP-MS using a procedure described in a previous work (Antes et al. 2012) to evaluate the purity of this product. The concentration of solutions used in all experiments was calculated while taking into account the determined concentration of TPhTH, and all values are expressed as Sn.
Quantification was performed by the standard addition calibration method using DBT as the internal standard and adding 5.0 μg kg−1 (as Sn) after the extraction procedure. Sample solutions were stored at −20 °C in the dark before GC-PFPD determination, and 2 μL were injected for analysis.
AChE Assay
AChE (E.C. 3.1.1.7) activity was measured as described by Ellman et al. (1961) and modified by Miron et al. (2005). Samples of brain and muscle (30 mg) were weighed and homogenized in a Potter–Elvejhem glass/Teflon homogenizer with 150 mM of NaCl. The homogenates were centrifuged for 15 min at 3,000 g at 5 °C, and the supernatant was used as the enzyme source. Aliquots (50 and 100 mL) of supernatant (brain and muscle, respectively) were incubated at 25 °C for 2 min with 100 mM of phosphate buffer (pH 7.5) and 1 mM DTNB (5-5′-dithio-bis (2-nitrobenzoic acid)) as chromogen. After 2 min, the reaction was initiated by the addition of acetylthiocholine (0.08 M) as substrate for the reaction mixture. The final volume was 2.0 mL. Absorbance was measured by spectrophotometry (Femto Scan spectrophotometer, Sao Paulo, Brazil) at 412 nm during 2 min. Enzyme activity was expressed as μmol min−1 mg of protein−1.
Determination of Metabolic Parameters
The liver and muscle were carefully removed, placed on ice, frozen in liquid nitrogen, and then stored at −20 °C for 1 week until analysis. Liver and muscle glycogen were estimated according to Bidinotto et al. (1998) after KOH and ethanol addition for precipitation of glycogen. For lactate, glucose, and ammonia determination, tissue samples were homogenized by adding 10 % trichloroacetic acid (1:20 dilution) using a motor-driven Teflon pestle and centrifuged at 1,000×g for 10 min for protein flocculation. The completely deproteinated supernatant was used for lactate determination using the method described by Harrower and Brown (1972). Glucose was measured according to Park and Johnson (1949), and ammonia was measured according to Verdouw et al. (1978). For amino acid quantification, tissues (liver and muscle) were twice mechanically disrupted by adding 2 mL of 20 mM phosphate buffer (pH 7.5), and the homogenates were centrifuged at 1,000×g for 10 min. The supernatant extracts were used for colorimetric amino acid determination according to Spies (1957). All biochemical analyses were measured spectrophotometrically and in duplicate. Protein was determined according to Bradford (1976) by the Coomassie blue method using bovine serum albumin as the standard. Absorbance of samples was measured at 595 nm.
Statistical Analysis
All data are expressed as mean ± SEM. Homogeneity of variances among treatments was tested with Levene test. Data presented homogeneous variances, so comparisons among different groups were made by one-way analysis of variance (ANOVA) and Tukey tests. Analysis was performed using the Statistica software version 7.0, and the minimum significance level was set at P < 0.05.
Results
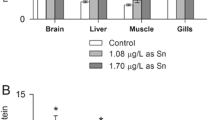
The limit of quantification (LOQ), calculated by following the signal at intercept and 10 times the SD regression of the calibration curve, for MPhT, DPhT, and TPhT in fish tissues by GC-PFPD were 8.1, 10.5, and 9.0 ng g−1 (as Sn), respectively. Concentrations of phenyltin compounds in the tissues were lower than LOQ. The LOQs were 0.54, 0.75, and 0.57 μg L−1 (as Sn) for MPhT, DPhT, and TPhT in water, respectively. According to the results obtained for Sn speciation analysis in water collected on the tanks during the experiment, only TPhT was detected.
The LC50 values for juvenile silver catfish were 12.81 [confidence interval (CI) 11.77–13.94 μg L−1] for 72 h and 9.73 μg L−1 (CI 7.94–12.10 μg L−1) for 96 h. Mortality did not reach 50 %, even at the highest concentration tested (≤48 h), so LC50 values at these times were not calculated. There was no mortality of fish maintained at 0, 4.40, and 5.95 μg L−1 TPhTH in the LC50 experiment; however, the increase of waterborne TPhTH increased mortality (Fig. 1).
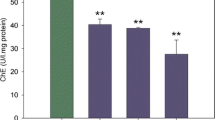
In the chronic experiment, in which nonlethal concentrations were used, brain and muscle AChE activity decreased significantly in fish exposed to TPhTH compared with control fish (Fig. 2). Liver glycogen and lactate levels were significantly greater in fish exposed to the highest concentration of TPhTH compared with the control; however, glucose levels in liver decreased in fish kept at both TPhTH concentrations. Muscle glucose levels were significantly lower in fish kept at both TPhTH concentrations than in unexposed fish. Lactate and glycogen levels in muscle were not affected significantly by TPhTH exposure (Fig. 3).
Fish exposed to both TPhTH concentrations showed lower total protein values in liver and muscle compared with the control (Fig. 4a). Total ammonia levels in liver and muscle were significantly greater in fish kept at 1.70 and 1.08 μg L−1 TPhTH, respectively, compared with the control (Fig. 4b). In addition, total free amino acid levels in liver and muscle were significantly greater in fish maintained at both TPhTH concentrations compared with control fish (Fig. 4c).
Discussion
Because only TPhT was detected in water collected in the tanks during the experiment, the degradation of this species could not be observed under the experimental conditions. However, it is important to mention that TPhT presents relatively low stability in the environment (Antes et al. 2012), and degradation is influenced by ultraviolet radiation, pH, chemical compounds, etc. (Hoch 2001). Therefore, a real field experiment would be necessary to confirm the obtained results.
The concentrations of MPhT, DPhT, and TPhT in fish tissues were lower than the LOQ achieved using the GC-PFPD technique. However, the changes in metabolic parameters of silver catfish juveniles exposed to TPhTH confirm the negative effects caused by TPhT at concentrations <9 ng g−1 (LOQ for TPhT). Considering the instability of TPhT species in the environment (Antes et al. 2012), it is necessary to consider that alteration in metabolic parameters could also be related to the degradation products MPhT and DPhT. The use of a more sensitive analytical technique would be necessary to quantify TPhT; then a better correlation with metabolic parameters could be given.
According to our knowledge, this is the first study dedicated to investigating the metabolic responses of fish exposed to different concentrations of TPhTH. The LC50 96-h value of TPhTH to silver catfish juvenile [9.7 μg L−1 (as Sn)] indicates that this species is relatively more resistant than newly hatched larvae of fathead minnows (Pimephales promelas) [7.1 μg L−1 as TPhTH (~2.3 μg L−1 as Sn)] (Jarvinen et al. 1988). In contrast, similar values have been reported for other fishes: 8.9 μg L−1 for fingerling rainbow trout (Oncorhynchus mykiss), 19.9 μg L−1 for juvenile goldfish (Carassius auratus), 7.4 μg L−1 for juvenile bluegill (Lepomis macrochirus), and 6.4 μg L−1 for juvenile fathead minnows (28, 62 23, and 20 μg L−1 as TPhTH, respectively, as described in the literature) (Johnson and Finley 1980).
Bioconcentration and elimination of 14C-radiolabelled TPhTH was investigated by Wieke Tas et al. (1989) in larvae of two fish species: guppy (Poecilia reticulata) exposed to 6 μg L−1 TPhTH (~2 μg L−1 as Sn) for 8 days (in a semistatic system where water was renewed every day in the first 3 days, however, every other day in the remaining period) and rainbow trout exposed to 3 μg L−1 (~1 μg L−1 as Sn) for 4 days (in a static system where water was not renewed). After the exposure period, fish were maintained in tanks with clean water to verify the elimination, which was determined after 6 and 12 days in guppy and rainbow trout, respectively, by counting the radioactivity of 14C-radiolabelled TPhTH in water and fish tissue. The investigators reported that although there was no equilibrium between waterborne TPhTH and tissue levels (bioconcentration/elimination rate) after the period of exposure; although TPhTH uptake was rapid, much longer accumulation periods are required to reach this state of equilibrium (Wieke Tas et al. 1989). In addition, these investigators reported that during the elimination period, waterborne TPhTH levels remained lower than the detection limit, similar to the present study.
The AChE activity is considered a sensitive biomarker because it is inhibited in fish exposed to pesticides (Bretaud et al. 2000; Dutta and Arends 2003; Miron et al. 2005; Üner et al. 2006; Pereira-Maduenho and Martinez 2008) or heavy metals (Romani et al. 2003; Senger et al. 2006; Pretto et al. 2010) or in animals collected in rivers contaminated by pollutants (Sancho et al. 2000; de La Torre et al. 2002; Lionetto et al. 2003). The activation or inhibition of AChE can influence the process of cholinergic neurotransmission and promote undesirable effects in fish, such as loss of equilibrium and erratic swimming (Miron et al. 2005), and can affect fleeing and reproductive behavior (Saglio and Trijasse 1998; Bretaud et al. 2000). The decrease of brain AChE activity in silver catfish exposed to waterborne TPhTH was similar to results found for fishes exposed for 96 h to the glyphosate (Roundup®, Monsanto) herbicide: piava (Leporinus obtusidens) maintained at 3, 6 10, or 20 mg L−1, silver catfish maintained at 0.2 or 0.4 mg L−1 (Glusczak et al. 2006, 2007, respectively), and curimbatá (Prochilodus lineatus) maintained at 10 mg L−1 (Modesto and Martinez 2010). In addition, in the present study a decrease of muscle AChE activity in silver catfish exposed to waterborne TPhTH was observed. This response is similar to results reported by Modesto and Martinez (2010) after 24 and 96 h of exposure to glyphosate; however, it different from results reported by Glusczak et al. (2006, 2007), who did not find differences in the AChE activity of muscle. Therefore, the AChE inhibition observed in silver catfish exposed to waterborne TPhTH might lead to Ach accumulation, thus producing overstimulation of the receptors.
The increase of liver glycogen in silver catfish exposed to 1.70 μg L−1 TPhTH (as Sn) was observed to be similar to piava specimens exposed to the herbicide quinclorac (Pretto et al. 2011). This pattern is different than that reported in other studies with toxicants, where glycogenolysis in the liver tissue, as a result of the stress response caused by this type of toxicant, has been observed (Sancho et al. 1998; Oruç and Üner 1999). An increase of lactate levels in the liver indicates metabolic disorders and a severe respiratory stress in fish tissues (Begum and Vijayaraghavan 1999), as observed in silver catfish exposed to the highest TPhTH concentration. Similar results concerning liver lactate were found by Glusczak et al. (2007) and Pretto et al. (2011) in fish exposed to herbicides. Liver and muscle glucose levels in silver catfish exposed to all TPhTH concentrations were lower than in control fish. Therefore, this response could be attributed to a metabolic disorder or to high glucose consumption by the metabolic process.
Total protein level was lower in liver and muscle of silver catfish maintained at all TPhTH concentrations than in those kept at control conditions, suggesting that the fish were using protein as an energy source. A decrease of protein in fish tissues on exposure to toxicants was previously reported (Sancho et al. 1998, 2000; Glusczak et al. 2006, 2007). The increase of total free amino acid levels in silver catfish tissues exposed to TPhTH is probably a result of the breakdown of protein for energy requirement and impaired incorporation of amino acids in protein synthesis. Moreover, the decrease of total protein levels, accompanied by the increase of total free amino acids levels, in liver and muscle suggest high protein hydrolytic activity due to increase of protease enzyme activity in both tissues. Similar results were found by David et al. (2004) in common carp (C. carpio) exposed to a sublethal concentration (1.2 μg L−1) of cypermethrin for 6, 12, 24, and 48 h. In addition, the high levels of total free amino acids can also be attributed to a decrease in the use of amino acids and its involvement in the maintenance of osmotic and acid base balance (Moorthy et al. 1984).
Ammonia is a toxic metabolite, and its excess is known to trigger the operation of detoxification or use systems, mainly through the formation of less toxic nitrogenous substances (Begum 2004). Total ammonia levels in liver and muscle of silver catfish exposed to high TPhTH concentrations (except at 1.70 μg L−1 of TPhTH in muscle) were greater than those of control group. This increase of TAN levels is probably related to the increase of protein catabolism in these tissues. Similar results were reported in liver and muscle of walking catfish (Clariasbatrachus) during carbamate exposure (Begum 2004) and those of piava exposed to Roundup (Glusczak et al. 2006).
Conclusion
The results presented in this work are important from an environmental point of view because waterborne TPhTH might change metabolic parameters of silver catfish juveniles even at low concentrations (1–2 μg L−1). The results obtained confirm that the analyzed parameters can be used as bioindicators of pesticide exposure, including TPhTH in agricultural cultures, where this compound is used to control fungus and parasites that cause diseases in plants. Despite the adverse effects observed in fish exposed to TPhTH, the concentrations of the investigated species in fish tissues were lower than the detection limits achieved by GC-PFPD. Therefore, it was not possible to establish a direct relationship of metabolic parameters and TPhT or degradation product concentrations.
References
Antes FG, Krupp E, Flores EMM, Feldmann J, Dressler VL (2012) Speciation and degradation of triphenyltin in typical paddy fields and its uptake into rice plants. Environ Sci Technol 45:10524–10530
Appel KE (2004) Organotin compounds: toxicokinetic aspects. Drug Metab Rev 36:763–786
Becker AG, Moraes BS, Menezes CC, Loro VL, Santos DR, Reichert JM et al (2009) Pesticide contamination of water alters the metabolism of juvenile silver catfish, Rhamdia quelen. Ecotoxicol Environ Saf 72:1734–1739
Begum G (2004) Carbofuran insecticide induced biochemical alterations in liver and muscle tissues of the fish Clarias batrachus (Linnaeus) and recovery response. Aquat Toxicol 66:83–92
Begum G, Vijayaraghavan S (1999) Effect of acute exposure of the organophosphate insecticide rogor on some biochemical aspects of Clarias batrachus (Linnaeus). Environ Res 80:80–83
Bidinotto PM, Moraes G, Souza RHS (1998) Hepatic glycogen and glucose in eight tropical freshwater teleost fish: a procedure for field determinations of micro samples. Bol Téc CEPTA 10:53–60
Bock R (1981) Triphenyltin compounds and their degradation products. Res Rev 79:1–270
Boyd CE, Tucker CS (1992) Water quality and pond soil analyses for aquaculture. Alabama Agricultural Experiment Station, Auburn University, Auburn
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bretaud S, Toutant JP, Saglio P (2000) Effects of carbofuran, diuron and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus). Ecotoxicol Environ Saf 47:117–124
Colt J (2002) List of spreadsheets prepared as a complement (Available in http://www.fisheries.org/afs/hatchery.html). In: Wedemeyer GA (ed) Fish hatchery management (2nd ed). American Fish Society Publication
Crestani M, Menezes C, Glusczak L, Miron DS, Lazzari R, Duarte MF et al (2006) Effects of clomazone herbicide on hematological and some parameters of protein and carbohydrate metabolism of silver catfish, Rhamdia quelen. Ecotoxicol Environ Saf 65:48–55
Crestani M, Menezes C, Glusczak L, Miron DS, Spanevello R, Silveira A et al (2007) Effect of clomazone herbicide on biochemical and histological aspects of silver catfish Rhamdia quelen and recovery pattern. Chemosphere 67:2305–2311
David M, Mushigeri SB, Shivakumar R, Philip GH (2004) Response of Cyprinus carpio (Linn) to sublethal concentration of cypermethrin: alterations in protein metabolic profiles. Chemosphere 56:347–352
de la Torre FR, Ferrari L, Salibián A (2002) Freshwater pollution biomarker: response of brain acetylcholinesterase activity in two fish species. Comp Biochem Physiol C: Toxicol Pharmacol 131:271–280
Dutta HM, Arends DA (2003) Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish. Environ Res 91:157–162
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (2005) Standard methods for the examination of water and wastewater (21st ed). American Public Health Association
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fernández-Vega C, Sancho E, Ferrando MD, Andreu E (2002) Thiobencarb induced changes in acetylcholinesterase activity of the fish Anguilla anguilla. Pest Biochem Physiol 72:55–63
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge
Glusczak L, Miron DS, Crestani M, Fonseca MB, Pedron FA, Duarte MF et al (2006) Effect of glyphosate herbicide on acetylcholinesterase activity and metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol Environ Saf 65:237–241
Glusczak L, Miron DS, Moraes BS, Simoes RR, Schetinger MRC, Morsch VM et al (2007) Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp Biochem Physiol C: Toxicol Pharmacol 146:519–524
Harrower JR, Brown CH (1972) Blood lactic acid. A micromethod adapted to field collection of microliter samples. J Appl Physiol 32:709–711
Hoch M (2001) Organotin compounds in the environment—an overview. Appl Geochem 16:719–743
Horiguchi T, Shiraishi H, Shimizu M, Morita M (1997) Effects of triphenyltin chloride and five other organotin compounds on the development of imposex in the rock shell, Thais clavigera. Environ Pollut 95:85–91
Jarvinen AW, Tanner DK, Kline ER, Knuth ML (1988) Acute and chronic toxicity of triphenyltin hydroxide to fathead minnows (Pimephales promelas) following brief or continuous exposure. Environ Pollut 52:289–301
Johnson WW, Finley MT (1980) Handbook of acute toxicity of chemicals to fish and aquatic invertebrates. Research Publication 137. United States Fish and Wildlife Service, United States Department of the Interior, Washington, DC
Lionetto MG, Caricato R, Giordano ME, Pascariello M, Marinosci L, Schettino T (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46:324–330
Ministério da Agricultura (2012) http://agrofit.agricultura.gov.br/agrofit_cons/. Accessed 4 Jan 2012
Miron D, Crestani M, Schetinger RM, Morsch MV, Baldisserotto B, Tierno AM et al (2005) Effects of the herbicides clomazone, quinclorac, and metsulfuron methyl on acetylcholinesterase activity in the silver catfish (Rhamdia quelen) (Heptapteridae). Ecotoxicol Environ Saf 61:398–403
Modesto KA, Martinez CBR (2010) Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299
Moorthy KS, Kasi Srinivasa Moorthy K, Kasi Reddy B, Swami SK, Chetty CS (1984) Changes in respiration andionic content in tissues of freshwater mussel exposed tomethylparathion toxicity. Toxicol Lett 21:287–291
Moraes BS, Loro VL, Glusczak L, Pretto A, Menezes C, Marchezan E et al (2007) Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens). Chemosphere 68:1597–1601
Nakanishi T (2008) Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. J Toxicol Sci 33:269–276
O’Halloran K, Ahokas JT, Wright PFA (1998) Response of fish immune cells to in vitro organotin exposures. Aquat Toxicol 40:141–156
Oruç EO, Üner N (1999) Effects of 2,4-Diaminon some parameters of protein and carbohydrate metabolism in the serum, muscle and liver of Cyprinus carpio. Environ Pollut 105:267–272
Osada S, Nishikawa J, Nakanishi T, Tanaka K, Nishihara T (2005) Some organotin compounds enhance histone acetyltransferase activity. Toxicol Lett 155:329–335
Park JT, Johnson MJ (1949) A submicro determination of glucose. J Biol Chem 181:149–151
Pereira-Maduenho L, Martinez CBR (2008) Acute effects of diflubenzuron on the freshwater fish Prochilodus lineatus. Comp Biochem Physiol C: Toxicol Pharmacol 148:265–275
Pretto A, Loro VL, Morsch VM, Moraes BS, Menezes C, Clasen B et al (2010) Acetylcholinesterase activity, lipid peroxidation, and bioaccumulation in silver catfish (Rhamdia quelen) exposed to cadmium. Arch Environ Contam Toxicol 58:1008–1014
Pretto A, Loro VL, Menezes C, Moraes BS, Reimche GB, Zanella R et al (2011) Commercial formulation containing quinclorac and metsulfuron-methyl herbicides inhibit acetylcholinesterase and induce biochemical alterations in tissues of Leporinus obtusidens. Ecotoxicol Environ Saf 74:336–341
Romani R, Antognelli C, Baldracchini F, de Santis A, Isani G, Giovannini E et al (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 145:321–329
Saglio P, Trijasse S (1998) Behavioral responses to atrazine and diuron in goldfish. Arch Environ Contam Toxicol 35:484–491
Sancho E, Ferrando MD, Andreu E (1997) Sublethal effects of an organophosphate insecticide on the European eel, Anguilla anguilla. Ecotoxicol Environ Saf 36:57–65
Sancho E, Ferrando DM, Fernández C, Andreu E (1998) Liver energy metabolism of Anguilla anguilla after exposure to fenitrothion. Ecotoxicol Environ Saf 41:168–175
Sancho E, Cerón JJ, Ferrando MD (2000) Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla. Ecotoxicol Environ Saf 46:81–86
Schaefer CH, Miura T, Dupras Jr EF, Wilder WH (1981) Environmental impact of the fungicide triphenyltin hydroxide after application to rice fields. J Econ Entomol 74:597–600
Senger MR, Rosemberg DB, Rico EP, Arizi MB, Dias RD, Bogo MR, Bonan CD (2006) In vitro effect of zinc and cadmium on acetylcholinesterase and ectonucleotidase activities in zebrafish (Danio rerio) brain. Toxicol In Vitro 20:954–958
Spies JR (1957) Colorimetric procedures for amino acids. Methods Enzymol 3:467–477
Tooby TE, Hursey PA, Alabaster JS (1975) The acute toxicity of 102 pesticides and miscellaneous substances to fish. Chem Indust 21:523–526
Üner N, Oruç EO, Sevgiler Y, Sahin N, Durmaz H, Usta D (2006) Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21:241–245
Van DN, Bui TTX, Tesfalidet S (2008) The transformation of phenyltin species during sample preparation of biological tissues using multi-isotope spike SSID-GC-ICPMS. Anal Bioanal Chem 392:737–747
Verdouw H, Vanechteld CJA, Deckkers EMJ (1978) Ammonia determinations based on indophenol formation with sodium salicylate. Water Res 12:399–402
Wieke Tas J, Hermens LM, Van den Berg M, Seinen W (1989) Bioconcentration and elimination of triphenyltin hydroxide in fish. Mar Environ Res 28:215–218
Zhang Z, Hu J, Zhen H, Wu X, Huang C (2008) Reproductive inhibition and transgenerational toxicity of triphenyltin on medaka (Oryzias latipes) at environmentally relevant tip levels. Environ Sci Technol 42:8133–8139
Acknowledgments
The authors thank the Conselho Nacional de Pesquisa e Desenvolvimento Científico for research fellowships to B. Baldisserotto, V. L. Loro, E. M. M. Flores, and V. L. Dressler. A. G. Becker, F. G. Antes, and B. Clasen received doctoral fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antes, F.G., Becker, A.G., Parodi, T.V. et al. Toxicity of Triphenyltin Hydroxide to Fish. Arch Environ Contam Toxicol 65, 733–741 (2013). https://doi.org/10.1007/s00244-013-9944-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-013-9944-y