Abstract
Among all organotin compounds , triphenyltin hydroxide (TPhTH) is widely used as fungicide and moluscicide in Brazil. However, the effects of TPhTH on the biochemical parameters of non-target organisms, such as fish, are little known. The aim of the present study is to assess the possible toxic effects of different concentrations of waterborne TPhTH on silver catfish belonging to species Rhamdia quelen. The fish were exposed to two different concentrations of TPhTH (1.08 and 1.70 µg/L as Sn) for 15 days and then compared to the control group (triplicate, n = 3). The antioxidant profile (catalase (CAT) and the glutathione S-transferase (GST)) and the oxidative stress parameters (TBARS—thiobarbituric acid-reactive substances and protein carbonyl (PC)) were set after the exposure to TPhTH. The TBARS level and the PC content increased in several organs of the Rhamdia quelen (brain, liver, muscle and gills) under the two concentrations of TPhTH in comparison to the control group. The CAT activity in the liver and gills has enhanced in all tested TPhTH concentrations. The GST activity increased in the brain, liver and muscle tissues under all the TPhTH concentrations. The significant changes in the biomarkers indicated that the investigated pesticide could have harmful effect on fish, in the field. However, these biomarkers were measured after the fish received doses lower than the recommended for use in agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triphenyltin hydroxide (TPhTH), also known as fentin hydroxide, is an organotin compound (OTC) used as agricultural fungicide and molluscicide (Grisolia, 2002). In Brazil, this compound is only used in cotton and bean cultures in the form of hydroxide, chloride or acetate to control fungi (Wensem et al. 1991). According to Ostrakhovitch (2015), triphenyltins are highly toxic to humans and animals and can be degraded into diphenyltin (DPhT) and into monophenyltin (MPhT). Such degradation occurs in the environment through the loss of aril groups from the molecule structure. The order of phenyltin toxicity is TPhTH > DPhT > MPhT, whereas the inorganic tin (Sn) is often non-toxic (Antes et al. 2013).
Although organotin pesticides were banned from many crops, considerable amounts of them have been contaminating the soil and the water. The TPhTH is one of the most common organotin pesticides (Gui et al. 2016), but the contamination resulting from its use has encouraged several studies about aquatic organisms. Since the 1980s, some of these studies have been reporting damages caused by these organisms in Nucella lapillus (Gibbs and Bryan 1986), Thais clavigera (Leung et al. 2006) and Xenopus tropicalis (Yuan et al. 2011). Fish are suitable pollution indicators in the environment (exposure biomarkers) and the most feasible organisms for pollution monitoring in aquatic systems (Rüdel et al. 2007).
It is well-reported that toxic agents, such as pesticides, may trigger the development of reactive oxygen species (ROS) and generate oxidative stress. The antioxidant defense system of fish detoxifies their organism and eliminates reactive oxygen species (ROS). This system is formed by antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione-S-transferase (GST), and by non-enzimatic defense systems such as glutathione (GSH), vitamin E and ascorbic acid (Trenzado et al. 2006; Clasen et al. 2012). The oxidative stress in fish results from environmental pollution.
Some studies have shown the deleterious effects organotin compounds have on animals and humans due to their widespread use (Dimitriou et al. 2003; Whalen et al. 2003; Leung et al. 2006; Rüdel et al. 2007). However, none of these studies have assessed the oxidative stress caused by these compounds in freshwater fish.
Therefore, the main aim of the present study is to proceed the investigation conducted by Antes et al. (2013) about the effects of TPhTH on Rhamdia quelen. The oxidative stress parameters response to the exposure to different concentrations of waterborne TPhTH was assessed in different tissues of the herein studied species, which is a native freshwater teleost fish of great economic importance in Southern Brazil (Baldisserotto 2004). The present study is the first record in the literature about the effects of the exposure to different concentrations of waterborne TPhTH on fish.
Materials and methods
Rhamdia quelenspecimens (weigh, 107.27 ± 1.21 g; length, 22.21 ± 1.15 cm)were purchased in a commercial teleost fish farm (Rio Grande do Sul State, Brazil). The silver catfish were placed in tanks (250 L) under continuous aeration for 12 days, under natural photoperiod (12 h light/12 h dark), before the experiment. The water parameters throughout the acclimation period were: dissolved oxygen (7.1 ± 0.7 mg/L), temperature (20.5 ± 1.0 °C), pH (7.2 ± 0.2 units), total ammonia nitrogen (0.023 ± 0.008 mg/L), non-ionized ammonia (0.015 µg/L), water hardness (23.5 ± 2.1 mg CaCO3/L) and alkalinity (32.1 ± 1.7 mg CaCO3/L). There were no significant variations between the water quality parameters during the experimental period and the acclimation data.
Fish were fed with commercial feed for juveniles (Supra, 42% crude protein), once a day, during the acclimation and experimental periods. The commercial product used in the present investigation—Mertin 400® (Syngenta, Brazil)—contained 400 g/L of TPhTH. The TPhTH in the water was monitored every 3 days during the experiment. It was quantified through gas chromatography-inductively coupled plasma mass spectrometry (GC-ICP-MS), according to the method previously described by Antes et al. (2013). The concentration of solutions used in the experiment was calculated according to the pre-set concentrations of TPhTH—all values were expressed as Sn.
The Rhamdia quelen specimens were randomly separated in tanks (60 L)-five per tank, in triplicate (n = 3)—at the end of the acclimation period. Next, they were exposed to 0.0 (control, TPhTH-free), 1.08 and 1.70 μg/L Sn, respectively, for 15 days. The tested concentrations were set to each tank, before the experiment had started. Uneaten food and feces were siphoned on a daily basis. At least 30% of the water in the tanks was replaced by water containing previously adjusted concentrations of waterborne TPhTH in order to keep the desired concentration (1.08 and 1.70 μg/L Sn) during the experiment. According to Antes et al. (2013), the TPhTH LC50 value for silver catfish juveniles was 9.73 µg/L(as Sn). It was verified that greater concentrations of TPhTH take fish to death after a few hours. Thus, the TPhTH concentrations 1.08 and 1.70 µg/L were used in the present study, although the concentration often used in rice crops is 0.48 mg/L.
After the exposure period, the fish were anesthetized and euthanized through spinal cord section. The organs (brain, liver, muscle and gills) were carefully removed and stored at −80 °C until the time for biochemical analyses. Lipid peroxidation was set through the TBARS levels, according to the method by Buege and Aust (1978). The PC assay was set according to Yan et al. (1995). The antioxidant enzyme ‘CAT’ followed the method described by Nelson and Kiesow (1972), and the GST activity described by Habig et al. (1974) was adopted. The protein in the samples was found through the methodology by Bradford (1976). The details about the biochemical analyses can be found in Clasen et al. (2012). Sample normality and homogeneity were tested through the Kolmogorov-Smirnov’s and Levene tests, respectively. The statistical analysis was performed through one-way analysis of variance (ANOVA) and Tukey test as post-hoc comparison. The data were homogeneous and expressed as the mean ± standard error of mean (S.E.M.) The p value was significant at ≤0.05, in all comparisons. The statistical analysis was performed in the Statistica software, version 7.0 (n = 3, in all experimental groups). The methodology adopted in the present experiment was approved by the Ethics and Animal Welfare Committee of Federal University of Santa Maria, under protocol number 2007–24.
Results and discussion
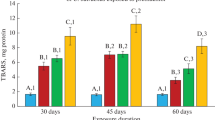
The means of limit of quantification (LOQs) in the water were 0.54, 0.75, and 0.57 µg/L Sn for MPhT, DPhT, and TPhTH, respectively. The TPhTH was the only pesticide detected in the water collected in the tanks during the experiment. It was detected through the Sn speciation analysis. All teleost fish have survived the 15-day exposure to different concentrations of TPhTH. Both the lipid peroxidation (LPO) and the protein oxidation in the present study were measured through oxidative damage biomarkers. The exposure to all tested concentrations of waterborne TPhTH has significantly increased the TBARS levels in all the assessed organs of the herein analyzed Rhamdia quelen specimens in comparison to the control group (Fig. 1a). The increased TBARS levels found in the present study clearly indicate LPO induced by TPhTH exposure. The structure of the proteins may be damaged by molecules from oxidation processes, such as LPO (as observed in the present study), or by direct ROS attacks (Ostrakhovitch 2015). This result meets those by Mitra et al. (2013), who found increased TBARS levels in the brain of rats exposed to different tributyltin doses, for 3 and 7 days. The trace metal levels can be highly modified, so they may lead to the formation of excessive free radicals; and it results in increased lipid peroxidation and protein modification.
The PC values reported for Rhamdia quelen at 1.08 and 1.70 µg/L of TPhTH showed significant increase in all analyzed organs (brain, liver, muscle and gills) (Fig. 1b). The damage in the proteins may have been caused by molecules resulting from oxidation processes, such as LPO, or by direct ROS attacks to the proteins’ structure. The ROS formation in the present study was represented by changes in the enzyme activity. The LPO and protein oxidation results highlighted that TPhTH induces oxidative damage as a clear response to TPhTH poisoning. Fish often increase the TBARS and the protein carbonyl levels after they are exposed to pesticides.
The present results suggest that the oxygen radicals produced during the aforementioned process could cause structural defects in the cellular membrane because they increase the number of double bonds in the hydrocarbon chains, fact that leads to changes in membrane permeability (Langner et al. 1998). Hartl et al. (2001) have found that the consumption rates have significantly increased in groups of fish (Platichthys flesus) exposed to organotin pesticides. The results found by Langner et al. (1998) and by Hartl et al. (2001) explain the changes observed in the current study concerning the LPO membrane and protein oxidation levels. Therefore, increased lipid peroxidation and protein carbolnylation can further affect the toxic effect of ROS; thus, lipid peroxidation and protein carbolnylation allow the metal to be more catalytically active, thereby reaching higher ROS levels (Mitra et al. 2013). The adverse effect is clearly evident through the increased CAT and GST activity.
There was better CAT activity in the liver and gills of Rhamdia quelen after its exposure to the two tested concentrations in comparison to the control group (Fig. 2a). The CAT protects organisms from oxidative damage when the ROS is partially removed (Kochhann et al. 2009). The superoxide anion (O2 −) is reduced to H2O2 through SOD, whereas H2O2 is converted into water and oxygen through CAT. The changes in the CAT activity in the present study could be a good indicative of the antioxidant system activation against TPhTH-induced toxicity.
The GST activity has significantly increased in all assessed organs and in both concentrations tested in the present study in comparison to the control group (Fig. 2b). This enzyme is involved in the detoxification of many xenobiotics and plays an important role in protecting the tissue from oxidative stress. The GST induction helps handling stress conditions. It was possible concluding that GST was an important tool to detoxify from TPhTH exposure. The present results indicate that all tested Rhamdia quelen’s organs were highly affected by TPhTH. Early warnings about the toxic effects of pollutants, including oxidative stress manifestations and adaptive responses, mainly in polluted areas, can be ruled out through the adoption of the biomarker approach.
Conclusions
The oxidative stress was imposed to Rhamdia quelen due to its exposure to TPhTH. Such outcome was evidenced by the significant increase in LPO and in protein oxidation. It may explain the increased antioxidant enzyme activity. This increased activity has also indicated the short-time activation of enzymes that have resulted from the increased production of free radicals, which was caused by the TPhTH metabolism. The influence of TPhTH on the antioxidant system of Rhamdia quele allowed understanding the ecotoxicological effect of TPhTH on the environment. Such effect helps setting the water quality standards suitable to protect aquatic vertebrates. More experiments in future research should be performed in order to help better understanding the relationship between different biomarkers; however, it was clear that these pollutants represent a risk to aquatic ecosystems.
References
Antes FG, Becker AG, Parodi TV, Clasen B, Lópes T, Loro VL, Baldisserotto B, Flores EMM, Dressler VL (2013) Toxicity of triphenyltin hydroxide to fish. Arch Environ Contam Toxicol 65:733–741
Baldisserotto B (2004) Silver catfish culture. World Aquac Mag 35:65–67
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1978) Microssomal lipid peroxidation. Methods Enzymol 52:302–309
Clasen B, Loro VL, Cattaneo R, Moraes B, Lópes T, Avila LA, Zanella R, Reimche GB, Baldisserotto B (2012) Effects of the commercial formulation containing fipronil on the non-target organism Cyprinus carpio: implications for rice-fish cultivation. Ecotoxicol Environ Saf 77:45–51
Dimitriou P, Castristsi-Catharios J, Miliou H (2003) Acute toxicity effects of tribultylin chloride and tryphenyltin chloride on gilthead sea bream Sparus aurata L. embryos. Ecotoxicol Environ Saf 54:30–35
Gibbs PE, Bryan GW (1986) Reproductive failure in populations of the dog-whelk, Nucella lapillus, caused by imposex induced by tributyltin from antifouling paints. J Mar Biol Assoc UK 66:767–777
Grisolia CK (2002) A comparison between mouse and fish micronucleus test using cyclophosphamide, mitomycin C and various pesticides. Mutat Res 518:145–150
Gui W, Tian C, Sun Q, Li S, Zhang W, Tang J, Zhu G (2016) Simultaneous determination of organotin pesticides by HPLC-ICP-MS and their sorption, desorption and transformation in freshwater sediments. Water Res 95:185–194
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hartl MGJ, Hutchinson S, Hawkins LE (2001) Organotin and osmoregulation: quantifying the effects of environmental concentrations of sediment-associated TBT and TPhT on the freshwater-adapted European flounder, Platichthys flesus (L.). J Exp Mar Biol Ecol 256:267–278
Kochhann D, Pavanato MA, Llesuy SF, Correa LM, Riffel APK, Loro VL, Mesko MF, Flores EMM, Dressler VL, Baldisserotto B (2009) Bioaccumulation and oxidative stress parameters in silver catfish (Rhamdia quelen) exposed to different thorium concentrations. Chemosphere 77:384–391
Langner M, Gabrielska J, Kleszczynska H, Pruchnik H (1998) Effect of phenyltin compounds on lipid bilayer organization. Appl Organometal Chemosphere 12:99–107
Leung KMY, Kwong RPY, Horiguchi T, Qiu JW, Yang R, Song M, Jiang G, Zheng GJ, Lam PKS (2006) Ecological risk assessment of endocrine disrupting organotin compounds using marine neogastropods in Hong Kong. Chemosphere 65:922–938
Mitra S, Gera R, Siddiqui WA, Khandelwal S (2013) Tribultyltin induces oxidative damage, inflammation and apoptosis via disturbance in blood – brain barrier and metal homeostasis in cerebral cortex of rat brain: An in vivo and in vitro study. Toxicol 310:39–52
Nelson DP, Kiesow LA (1972) Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solution in the UV). Anal Biochem 49:474–478
Ostrakhovitch EA (2015) Chapter 56 – Tin. In: Nordberg G, Fowler B, Nordberg M (eds) Handbook on the toxicology of metals, Vol. II – Special metals, 4th edn. Academic Press, Elsevier, p 331, 1241–1285
Rüdel H, Müller J, Steinhanses J, Schröter-Kermani C (2007) Retrospective monitoring of organotin compounds in freshwater fish from 1988 to 2003: results from the German environmental specimen bank. Chemosphere 66:1884–1894
Trenzado C, Hidalgo MC, Garcia-Gallego M, Morales AE, Furné M, Domezain J, Sanz A (2006) Antioxidant enzymes and lipid peroxidation in sturgeon Acipenser naccarii and trout Oncorhynchus mykiss; a comparative study. Aquaculture 254:758–767
Wensem JV, Akkerhuis GAJMJ, Straalen NMV (1991) Effects of the fungicide triphenyltin hydroxide on soil fauna mediated litter decomposition. Pestic Sci 32:307–316
Whalen MM, Wilson S, Gleghorn C, Loganathan BG (2003) Brief exposure to triphenyltin produces irreversible inhibition of the cytotoxic function of human natural killer cells. Environ Res 92:213–220
Yan LJ, Traber MG, Packer L (1995) Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal Biochem 228:349–351
Yuan J, Zhang X, Yu L, Sun Z, Zhu P, Wang X, Shi H (2011) Stage-specific malformations and phenotypic changes induced in embryos of amphibian (Xenopus tropicalis) by triphenyltin. Ecotoxicol Environ Saf 74:1960–1966
Acknowledgements
The authors would like to thank Conselho Nacional de Pesquisa e Desenvolvimento Científico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for supporting the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animal rights and informed consent
The current article does not contain any studies performed by any of the present authors involving human participants. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Clasen, B., Becker, A.G., Lópes, T. et al. Triphenyltin hydroxide induces changes in the oxidative stress parameters of fish. Ecotoxicology 26, 565–569 (2017). https://doi.org/10.1007/s10646-017-1780-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1780-9