Abstract
Many organisms appear to exhibit adaptive cost–benefit behaviors that balance foraging, safety, and pollution avoidance. However, what if the cognitive facilities needed to make decisions are compromised by industrial pollutants? Are the resulting decisions altered? Similarly, does exposure to kairomones from predators alter an organism’s ability to avoid toxicants? Furthermore, how long an exposure is necessary: A few minutes, hours, or even a lifetime? We wondered if there was an interaction between the ability to respond to a predatory event and the ability to avoid heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
We examined the aquatic pulmonate snails Physella columbiana and Lymnaea palustris, which are found in the Coeur d’Alene drainage downstream from arsenic, cadmium, lead, and zinc mines in northern Idaho State. We sought to determine the following: (1) whether short-term previous exposure to predator odor affects a snail’s ability to detect metals; (2) whether short-term previous exposure to heavy-metal pollutants affects a snail’s ability to detect fright odor; and (3) whether fright response (avoidance) decreases as a function of time. We found that a brief exposure to heavy metals impaired the snails’ ability to avoid predacious cues, and a brief exposure to predacious cues impaired their ability to avoid a stream of heavy metal-treated water. These results have important ecological ramifications because the concentrations of heavy metals we used were below levels found in the Coeur d’Alene drainage. Snails that have recently encountered evidence of predation may not be able to detect and/or move away from concentrations of heavy metals that they are normally capable of detecting. Similarly, heavy-metal exposure may impair antipredatory behaviors.
Organisms face complex environments and often must make decisions based on incomplete information (Ferrari et al. 2010). For example, based on a few chemical cues, should an organism forage and seek out potential mates or should it behave in a manner less likely to result in predation? Many organisms appear to exhibit adaptive cost–benefit behaviors (Wisenden et al. 2003; Johnston et al. 2012). However, what if the cognitive facilities needed to make decisions are compromised by industrial pollutants? Are the resulting decisions altered? And if pollutants can impair decision making, how long an exposure is necessary: a few minutes, hours, even a lifetime?
Stressors can alter the behavior of aquatic organisms (McClosky and Newman 1995; Amiard-Triquet 2009; Fraker et al. 2009). Often these behavioral changes are adaptive and ameliorate the immediate stressor whether it is abiotic, such as toxicants (Schäfers et al. 2007), or biotic, such as predators (Sih et al. 2010). A behavior employed by aquatic pulmonate snails is the ability to detect and alter their behavior based on certain kairomones (Covich et al. 1994; Jacobsen and Stabell 2004; Bernot et al. 2005) in their environment. These kairomones, or chemical cues, contain information about current environmental conditions (von Frisch 1938). They can be emitted from other snails’ mucus trails, crushed snails, or even predators themselves (Jacobsen and Stabell 2004).
In addition, pulmonate snails can detect pollutants, such as cadmium, zinc, and lead, that result from hard-rock mining near the snails’ habitat (Lefcort et al. 2000). Snails move away from areas with a higher concentration of heavy metals to areas with lower concentrations (Golding et al. 1997; Lefcort et al. 2004).
In northern Idaho, United States, heavy metals from past mining operations are patchily distributed, whereby substrates of some parts of lakes may be two orders of magnitude more polluted than areas just a few meters away (Neufeld 1987; Campbell et al. 1999; Farag et al. 1998). These lakes are in the Coeur d’Alene drainage downstream from the Environmental Protection Agency’s Bunker Hill Superfund site in the Silver Valley. The Superfund site and downstream lakes are contaminated with arsenic, cadmium, lead, and zinc (Ellis 1940; Rabe and Bauer 1977; Ridolfi Engineering 1993). The lakes have been contaminated for >125 years; therefore, at least 125 snail generations (Hunter 1975; Lefcort et al. 2010) have been exposed to the selective pressures imposed by heavy metals.
In this system we studied the ecological and behavioral effects of heavy-metal pollution on populations of P. columbiana and L. palustris aquatic pulmonate snails (Lefcort et al. 1999, 2002, 2008). Snails are a good model because they are a major component of the metal-impacted food chains in these lakes because they are in contact with bottom sediments and because they are a preferred food of fishes, such as many centrarchids and salmonids (Ellis 1940).
These lakes contain predacious fishes, such as sunfish (Lepomis sp.). At any given time, some of the fish may be actively feeding and hence are dangerous, whereas others may not be feeding. One cue that snails use to discern hunting from quiescent predators is to focus their response not on the fish but on kairomones released from broken snail cells (von Frisch 1938; Atema and Stenzler 1977; Dickey and McCarthy 2007). When snails detect this cue, they decrease their movements and pull into their shells (Lefcort et al. 1999).
Previously (Lefcort et al. 1999, 2004) we showed that aquatic pulmonate snails avoid flowing water containing either broken snail cells or heavy metals. When placed in the base of a Y-shaped glass container and given a choice between a trickle of control water or an experimental solution, snails moved into the control stream.
However, we also found that snails with high tissue levels of heavy metals exhibit decreased antipredatory behavior (Lefcort et al. 2000), which may suggest impairment of detection abilities. These results are similar to those of Clements (1999), who found that mayflies (Rhithrogena hageni) exposed to heavy metals were more susceptible to stonefly (Claassenia sabulosa) predation.
We wondered if there was an interaction between the ability to respond to a predatory event and the ability to avoid heavy metals. If, in the field, a snail detects one negative stimulus event and responds appropriately, what happens later? In other words, how does this possibly stressful event alter behavior in the near future? If the snail subsequently comes across metal-rich sediments, does it avoid them? Similarly, does initial contact with metal-rich water alter future responses to predator cues?
To test the effect of previous exposure with one stressor on a snail’s ability to detect a second stressor, we set up Y-tube test arenas in which snails were exposed to either heavy-metal solutions or an extract of crushed snails. The heavy-metal solution was only presented briefly so that the actual tissue levels of metals would not increase. We predicted that pre-exposure to one stressor would impair their response to a subsequent stressor.
We sought to determine the following: (1) whether short-term previous exposure to predator odor affects a snail’s ability to detect metals; (2) whether short-term previous exposure to heavy-metal pollutants affects a snail’s ability to detect fright odor; and (3) whether fright response (avoidance) decreases as a function of time.
Methods
Rearing Snails
During May and June 2010, we conducted experiments on snails from Bayit Pond, Spokane, County, Washington State. Bayit Pond is a reference pond (128 m2) free of heavy metals. It was constructed in 2003 and stocked with snails from Coeur d’Alene Basin lakes. To prevent learning, snails were used in only a single replicate of a single treatment of a single experiment. Sample sizes are listed in Table 1.
Preparation of Metal Solutions
We prepared heavy-metal soil stock solution by stirring 1 L vigorously rinsed soil from a river bank within the Bunker Hill Superfund site (Lefcort et al. 1999), into a container with 40 L deionized water. After letting the solution settle for 48 h, we poured off the supernatant and filtered it. This resulted in a concentration in μg/L arsenic (>0.001), cadmium (2.1), lead (105.6), and zinc (6500.0; Anatek Labs, Spokane, WA, using inductively coupled plasma mass spectrometry and graphite furnace atomic absorption). Dilutions were then made with artificial Pond water (deionized water + KCl, MgSO4 and CaHPO4), hereafter referred to as “pond water.” For all experiments, pond water was freshly prepared on the day of testing.
Preparation of Snail Extract
We produced snail extract by first slowly cooling a snail (0.4–0.6 g) to 3 °C in a dish of pond water. The animal was then crushed and macerated with 50 mL of DI water. One drop of this extract was used in 2 L test solution. To avoid introducing novel chemical cues, no anesthesia other than cooling was used (approved by Gonzaga Institutional Animal Care and Use Committee).
Flow tank
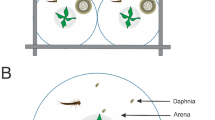
One side of the flow tank contained fresh pond water, whereas the other side contained pond water treated with an experimental solution (Lefcort et al. 2004). Together, the Y-maze flow tank trickled out the solution at the rate of ~50 mL/min (Fig. 1). We gave each individual snail 20 min to cross a black line toward either arm of the flow tank. If the snail did not successfully cross a line, it was considered a “no decision.” Hence, three outcomes were possible: control arm, experimental arm, and no decision. A total of 6.2 % (±0.6 % SD) of snails were scored as no decision.
Between snail replicates, all dishes were soaked in hot water, rinsed with deionized water, and then wiped with a paper towel to remove any chemical cues in the mucus trails left from the previous snail. The treatment arms of the Y-maze were switched (flipped) between replicates to avoid a possible left/right bias.
Experiment 1: Effect of Novelty
Snails may not be reacting to a stimulus but simply showing an interest or avoidance of a novel chemical in their environment. To control for the effect of novelty, both P. columbiana and L. palustris species underwent treatments of pre-exposure to pine needle odor. Although Ponderosa Pine (Pinus Ponderosa) are native to the area, there are no pine trees within 200 m of Bayit Pond. Therefore, pine needles are novel but not exotic.
A single fresh pine needle was gently crushed with a mortar and pestle. The pine needle was placed in 0.5 L pond water for 10 min to soak. Each snail was put in a dish containing the pine needle solution for 5 min followed by 5 min in a dish of fresh pond water. After 5 min in the pond water, the snail was placed in the Y-maze flow tank and given a choice between pine-scented water and crushed-snail extract. The results from this experiment were compared with the following experiment (Experiment 2A), which used unscented pond water as a control. This allowed us to determine if the snails were truly responding to crushed snail odor or if their response to crushed snail odor was just a response to it being a novel substance.
Experiment 2A: Avoidance of Fright Odor
We tested the avoidance behavior of both P. columbiana and L. palustris snails to fright odor emitted from crushed conspecifics and crushed heterospecifics. One of the heterospecifics was Helisoma anceps, which is an aquatic planorbid snail taxonomically distant from pulmonate snails. Each snail was first put in a dish containing pond water. After 5 min in pond water, the snail was moved to another dish containing pond water and left for 5 min (Experiment 2B required snails to be put in a second dish to be “rinsed;” therefore, all experiments used a second dish). Then the snail was placed in the Y-maze flow tank and tested.
Experiment 2B: Avoidance of Fright Odor After Preconditioning With Heavy Metals at Various Concentrations
We also tested the avoidance behavior of L. palustris to crushed conspecifics after being exposed to heavy metals. The individual snails first spent 5 min in a dish containing a mixture of multiple metals at five different concentrations. Concentrations ranged from 0.8 μg/L cadmium, 2600.0 μg/L zinc, and 42.0 μg/L lead to 0.0002 μg/L cadmium, 1.3 μg/L zinc, and 0.02 μg/L lead (Table 1). These metal levels are both above and below those found at Coeur d’Alene drainage field sites. This was followed by 5 min in a dish of pond water to “rinse” the snails. The snails were then put in the Y-maze flow tank and tested to determine their response to crushed conspecifics. One side of the flow tank contained pond water, whereas the other side contained crushed conspecifics.
Experiment 3A: Avoidance of Heavy Metals
We tested the avoidance behavior of both species of snails to heavy-metal pollutants. Each snail was put in a dish containing the pond water followed by 5 min in a second dish of pond water (control were placed in two dishes as in Experiment 3). The snail was then placed in the Y-maze flow tank and tested.
Experiment 3B: Time-Dependent Effect of Fright Odor on Avoidance of Heavy Metals
In addition, we tested the avoidance behavior of both species of snails to heavy-metal pollutants after being first exposed to crushed conspecifics. The individual snails were first put into a dish containing crushed conspecifics for 5, 15, 30, or 60 min. This was followed by 5 min in a dish of fresh pond water. The snails were then put in the Y-maze flow tank and tested to determine their response to metals. One side of the flow tank contained fresh pond water, whereas the other side contained a mixture of heavy metal whose major components were 0.08 μg/L cadmium, 260.0 μg/L zinc, and 4.2 μg/L lead.
Statistical Tests
Individual snails were only tested in a single replicate and only used in a single experiment. This was done to prevent any effects of learning or decreased sensitivity over time. We analyzed the data using Chi-square test with alpha set to 0.05.
Results
Experiment 1: Effect of Novelty
For the novelty control using pine needles, we found that pretreatment with pine needles had no significant effect on the fright response of either species of snails (Table 1; Fig. 2). Snails moved away from crushed snail odor and toward pine odor in a similar manner as they moved toward plain pond water in Experiment 2A.
Experiment 2A: Avoidance of Fright Odor
We found that P. columbiana and L. palustris snails moved away from the arm of the Y-maze that contained a solution of crushed-snail extract (Table 1; Fig. 3). This occurred in response to both conspecifics and heterospecifics; however, Lymnaea did not avoid planorbid extract.
Experiment 2B: Avoidance of Fright Odor After Preconditioning With Heavy Metals at Various Concentrations
We found that after exposures to metals, both P. columbiana and L. palustris exhibited a decreased ability to detect fright odor (Figs. 4a, b). If the snails were not pre-exposed to metals (Experiment 2A), they were able to detect and avoid crushed conspecifics by moving away from this stream of solution. However, if the snails were pre-exposed to varying concentrations of metals, they had a weaker response unless the metal solution was dilute.
a A test of the effect of previous exposure to heavy metal solutions on the fright response of P. columbiana (Experiment 2B). Levels ≥0.008 μg/L cadmium, 0.42 μg/L lead, and 26.0 μg/L zinc resulted in the snails not significantly (p < 0.05) avoiding the crushed snail extract at a rate of >50 %. Only zinc is illustrated, but snails were actually exposed to a multimetal solution from a polluted habitat. b A test of the effect of previous exposure to heavy-metal solutions on the fright response of L. palustris (Experiment 2B). Levels ≥0.0008 μg/L cadmium, 0.042 μg/L lead, and 2.6 μg/L zinc resulted in the snails not significantly (p < 0.05) avoiding the crushed snail extract at a rate of >50 %. Only zinc is illustrated, but snails were actually exposed to a multimetal solution from a polluted habitat
P. columbiana returned to a normal fright response at metal concentrations of 0.0008 μg/L cadmium, 0.042 μg/L lead, and 2.6 μg/L zinc. L. palustris returned to a normal fright response at metal concentrations of 0.0004 μg/L cadmium, 0.021 μg/L lead, and 1.3 μg/L zinc. For reference, water metal levels in the lakes of the Coeur d’Alene Basin are from 2 to 4 orders of magnitude greater (Farag et al. 1998; Gearheart et al. 1999).
Experiment 3A: Avoidance of Heavy Metals
We found that P. columbiana and L. palustris snails moved away from the arm of the Y-maze that contained a solution of heavy metals (Table 1). Again, the concentration of heavy metals was lower than that found at our field sites.
Experiment 3B: Time-Dependent Effect of Fright Odor on Avoidance of Heavy Metals
If the snails were not pre-exposed to fright odor (Experiment 3A), they were able to detect and avoid heavy metals by moving away from this stream of solution. However, if the snails were pre-exposed to fright odor, they had a weaker response (Fig. 5).
A test of the effect of previous exposure to crushed-snail extract on the avoidance response of L. palustris to a stream of metal-laden water (Experiment 3B). Snails unexposed and those exposed for 120 min to crushed-snail kairomones moved away from metal-laden water. Snails exposed for 5 min actually moved toward metal-laden water
After exposure to crushed conspecifics for 5-, 15-, 30-, and 60-minute increments, we found that a recent fright response interfered with avoiding water containing heavy metals (0.08 μg/L cadmium, 260.0 μg/L zinc, and 4.2 μg/L lead). However, exposure time to crushed conspecifics lengthened, L. palustris snails returned to baseline avoidance of heavy metals. We did not have enough animals to repeat this experiment with P. columbiana.
Discussion
Although toxicology experiments are often conducted on single toxicants, in reality organisms such as aquatic snails live in a complex environment. Their environment contains food, predators, parasites, often pollution, and competing conspecific and heterospecific snails. From the snail’s perspective, these are not single stressors but rather may occur sequentially or simultaneously.
Physiological and behavioral coping mechanisms may interact and preclude an optimal response. For example, migrating salmon (Oncorhynchus spp.) that are handled as they pass over dams are more susceptible to predacious Northern pikeminnow (Ptychocheilus oregonensis) for up to 1 h after handling (Mesa 1994). Similarly, Kavaliers (1988) found that a 5-min exposure to the short-tailed weasel (Mustela ermine) elicited increased pain tolerance in wild white-footed mice (Peromyscus leucopus).
Contrarily, a stressor may even have a hormetic effect (Calabrese and Baldwin 2001) and actually enhance the response to a subsequent stressor. For example, the nematode Caenorhabditis elegans exposed to heat displayed increased survival when later challenged with juglone (a chemical that generates reactive oxygen species; Cypser and Johnson 2002).
In this study, we tested P. columbiana and L. palustris aquatic pulmonate snails, which are native to northern Idaho and present in areas that contain fish predators and heavy-metal pollutants. We found that a brief exposure to heavy metals impaired their ability to avoid predacious cues, with L. palustris being more sensitive than P. columbiana to the heavy-metal pollutant concentrations.
Our results support the hypothesis that heavy metals retard the ability of P. columbiana and L. palustris to detect conspecific fright odor. This may possibly be due to alterations of ion channels (Rózsa and Salánki 1990). Thus, heavy-metal pollution commonly found in the Silver Valley of Northern Idaho could have a significant effect on the ability of native P. columbiana and L. palustris to avoid predators. This would augment the transfer of metals from lower trophic levels to higher levels, possibly including humans, because snails come into contact with polluted sediments and are a preferred food item of many game fish (Farag et al. 1998). However, fish such as common killifish (Fundulus heteroclitus), which are themselves impacted by heavy metals and other pollutants, are often less efficient predators on lower trophic levels, such as blue crabs (Callinectes sapidus) and grass shrimp (Palaemonetes pugio; Weis et al. 2001, 2011).
In addition, we confirmed our earlier finding (Lefcort et al. 2004) that both snail species avoid heavy metals. However, in this study we discovered that after pre-exposure to crushed conspecifics, L. palustris not only failed to avoid metal-treated water, they actually moved toward it. This effect was strong if given a brief 5-min exposure to crushed conspecifics, but it decreased after 15 min of exposure and returned to control levels after a lengthy 120 min. Interestingly, Fig. 5 shows a U-shaped response that appears to be hormetic (Davis and Svendsgaard 1990; Lefcort et al. 2012).
An effect of fright-odor kairomones on metal avoidance has important ecological ramifications because the concentrations of heavy metals we used are below levels found in the Coeur d’Alene drainage (Farag et al. 1998; Lefcort et al. 1999). They are also below the concentrations that we previously found to result in avoidance of heavy metals by P. columbiana and L. palustris (Lefcort et al. 2004). This suggests that snails that have recently encountered evidence of predation may not be able to detect and/or move away from concentrations of heavy metals they are usually capable of detecting. This may also explain why snails in the drainage acquire heavier metal loads than one would predict from passive aqueous absorbance (Lefcort et al. 2004; Croteau et al. 2007; Hoang et al. 2008; reviewed by Amiard-Triquet and Rainbows 2011). Concentrations of sediments and water in our previous studies (Lefcort et al. 2000, 2004) showed that snails readily avoid in the laboratory may not be avoided in the field because predator-induced kairomones are often present.
References
Amiard-Triquet C (2009) Behavioral disturbances: the missing link between sub-organismal and supra-organismal responses to stress? Prospects based on aquatic research. Human Ecol Risk Assess 15:87–110
Amiard-Triquet C, Rainbows PS (2011) Tolerance and the trophic transfer of contaminants. In: Amiard-Triquet C, Rainbows PS, Romeo M (eds) Tolerance to Environmental Contaminants. CRC Press, Boca Raton, pp 299–332
Atema J, Stenzler D (1977) Alarm substance of the marine mud snail, Nassarius obsoletus: biological characterization and possible evolution. J Chem Ecol 3:173–187
Bernot RJ, Kennedy EE, Lamberti GA (2005) Effects of ionic liquids on the survival, movement, and feeding behavior of the freshwater snail, Physa acuta. Environ Toxicol Chem 24:1759–1760
Calabrese EJ, Baldwin LA (2001) Hormesis: U-shaped dose response and their centrality in toxicology. Trends Pharmacol Sci 22:285–291
Campbell JK, Audet D, Kern JW, Reyes M, McDonald LL (1999) Metal contamination of palustrine and lacustrine habitats in the Coeur d’Alene Basin, Idaho. Final Report, United States Fish and Wildlife Service
Clements WH (1999) Metal tolerance and predator-prey interactions in benthic macroinvertebrate stream communities. Ecol Appl 9:1073–1084
Covich AP, Crowl TA, Alexander JE, Vaughn CC (1994) Predator-avoidance responses in freshwater decapod-gastropod interactions mediated by chemical stimuli. J North Am Benthol Soc 13:283–290
Croteau MN, Luoma SN, Pellet B (2007) Determining metal assimilation efficiency in aquatic invertebrates using enriched stable metal isotope tracers. Aquat Toxicol 83:116–125
Cypser JR, Johnson TE (2002) Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci 57:B109–B114
Davis JM, Svendsgaard DJ (1990) U-shaped dose–response curves: their occurrence and implications for risk assessment. J Toxicol Environ Health 30:71–83
Dickey BF, McCarthy TM (2007) Predator and prey interactions between crayfish (Orconectes juvenilis) and snails (Physa gyrina) are affected by spatial scale and chemical cues. Invert Biol 126:57–66
Ellis MM (1940) Pollution of the Coeur d’ Alene river and adjacent waters by mine wastes. United States Bureau of Fisheries Special Science Report
Farag AM, Woodward DF, Goldstein JN, Brumbaugh W, Meyer JS (1998) Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d’Alene River Basin, Idaho. Arch Environ Contam Toxicol 34:119–127
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Fraker ME, Hu F, Cuddapah V, McCollum SA, Relyea RA, Hempel J et al (2009) Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm Behav 55:520–529
Gearheart RA, Ridolfi CA, Miller CE, Claassen V, Thrush W (1999) Restoration alternatives plan for the Coeur d’Alene Basin natural resource damage assessment. Prepared for the Natural Resources Trustees
Golding LA, Timperley MH, Evans CW (1997) Non-lethal responses of the freshwater snail potamopyrgus antipodarum to dissolved arsenic. Earth Environ Sci 47:239–254
Hoang T, Rogevich E, Rand G, Frakes R (2008) Copper uptake and depuration by juvenile and adult Florida apple snails (Pomacea paludosa). Ecotoxicology 17:605–615
Hunter RD (1975) Growth, fecundity, and bioenergetics in three populations of Lymnaea palustris in upstate New York. Ecology 56:50–63
Jacobsen HP, Stabell OB (2004) Antipredator behaviour mediated by chemical cues: the role of conspecific alarm signaling and predator labeling in the avoidance response of a marine gastropod. Oikos 104:43–50
Johnston BR, Molis M, Scrosati RA (2012) Predator chemical cues affect prey feeding activity differently in juveniles and adults. Can J Zool 90:128–132
Kavaliers M (1988) Brief exposure to a natural predator, the short-tailed weasel, induces benzodiazepine-sensitive analgesia in white-footed mice. Physiol Behav 43:187–193
Lefcort H, Thomson SM, Cowles EE, Harowicz HL, Livaudais BM, Roberts WE et al (1999) Ramification of predator avoidance: predator and heavy metal mediated competition between tadpoles and snails. Ecol Appl 9:1477–1489
Lefcort H, Ammann E, Eiger SM (2000) Antipredatory behavior as an index of heavy-metal pollution? A test using snails and caddisflies. Arch Environ Contam Toxicol 38:311–316
Lefcort H, Aguon MQ, Bond KA, Chapman KR, Chaquette R, Clark J et al (2002) Indirect effects of heavy metals on parasites may cause shifts in snail species compositions. Arch Environ Contam Toxicol 43:34–41
Lefcort H, Abbott DP, Cleary DA, Howell E, Keller NC, Smith MM (2004) Aquatic snails from mining sites have evolved to detect and avoid heavy metals. Arch Environ Contam Toxicol 46:478–484
Lefcort H, Freedman Z, House S, Pendleton M (2008) Hormetic effects of heavy metals in aquatic snails: is a little bit of pollution good? Ecohealth 5:10–17
Lefcort H, Vancura J, Lider E (2010) 75 Years after mining ends stream insect diversity is still affected by heavy metals. Ecotoxicology 19:1416–1425
Lefcort H, Humphries A, Tordillos K, Vancura J, Wood S (2012) Aquatic snails detect and avoid both heavy metals and fright odor on sand substrates. In: Hamalainen EM, Jarvinen S (eds) Snails: biology, ecology and conservation. Nova Science Publishing, Happauge
McClosky JT, Newman MC (1995) Sediment preference in the Asiatic clam (Corbicula fluminea) and viviparid snail (Campeloma decisum) as a response to low-level metal and metalloid contamination. Arch Environ Contam Toxicol 28:195–202
Mesa MG (1994) Effects of multiple acute stressors on the predator avoidance ability and physiology of juvenile Chinook salmon. Trans Am Fish Soc 123:786–793
Neufeld J (1987) A summary of heavy metal contamination in the lower Coeur d’Alene river valley with particular reference to the Coeur d’Alene river wildlife management area. Idaho Department of Fish and Game publication, Coeur d’Alene, pp 1–37
Rabe FW, Bauer SB (1977) Heavy metals in lakes of the Coeur d’Alene River Valley, Idaho. Northwest Sci 51:183–197
Ridolfi Engineering and Associates Inc (1993) Confirmation of exposure of natural resources to hazardous substances in the Coeur d’Alene basin of northern Idaho. Ridolfi Engineering and Associates Inc. Publications, Seattle
Rózsa KS, Salánki J (1990) Heavy metals regulate physiological and behavioral events by modulating ion channels in neuronal membranes of molluscs. J Environ Monit Assess 14:363–375
Schäfers C, Klöppel H, Takahashi Y (2007) Zooplankton avoidance behavior following spray drift exposure to fenpyroximate. Human Ecol Risk Assess 13:527–534
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM et al (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
von Frisch K (1938) Zur psychologie des fisch-schwarmes. Naturwissenschaften 37:601–606
Weis JS, Smith G, Zhou T (2001) Effects of contaminants on behavior. Biochemical mechanisms and ecological consequences. Biosciences 51:209–217
Weis JS, Bergey L, Reichmuth J, Candelmo A (2011) Living in a contaminated estuary: behavioral changes and ecological consequences for five species. Biosciences 61:375–385
Wisenden BD, Pollock MS, Tremaine RJ, Webb JM, Wismer ME, Chivers DP (2003) Synergistic interactions between chemical alarm cues and the presence of conspecific and heterospecific fish shoals. Behav Ecol Sociobiol 54:485–490
Acknowledgments
We thank John Shea for graciously providing us with Planorbidae snails. We also thank Joey Haydock and two anonymous reviewers for critically reading and improving an earlier draft of this manuscript. We are grateful to Beth Sobba for creating Fig. 1. This study was funded by a grant by the Gonzaga Summer Research Program and The Merck Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lefcort, H., Wehner, E.A. & Cocco, P.L. Pre-Exposure to Heavy Metal Pollution and the Odor of Predation Decrease the Ability of Snails to Avoid Stressors. Arch Environ Contam Toxicol 64, 273–280 (2013). https://doi.org/10.1007/s00244-012-9821-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9821-0