Abstract
The lower Melet River is a drinking water source that is surrounded by hazelnut grove, agricultural lands, resulting in the accumulation of genotoxic agents such as mining activities, various domestic and agricultural wastes. Therefore, it receives many domestic and agricultural wastes that contain the genotoxic agent. This study was aimed to assess the heavy metal concentrations in water, sediment, and bioaccumulation in the tissues of Alburnus chalcoides. Comet assay and micronucleus test were used to evaluate the genotoxic effects on the blood cells of A. chalcoides. The concentrations of heavy metals and metalloid in the water, in the sediments and in the muscle of fish were in the order of Fe > Al > Mn > As > Zn > Cu > Ni > Cr > Cd = Pb = Co, Fe > Al > Mn > Zn > Cu > Pb > Cr > As > Co > Ni > Cd and Fe > Zn > Al > Mn > Cu > Pb > As > Cr > Ni > Co > Cd, respectively. The blood cells of fish collected from the polluted location showed significantly higher DNA damage and micronucleus frequency compared to the reference location (p < 0.05). The study indicated that the DNA integrity of A. chalcoides was affected by heavy metals which originated from many anthropogenic sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
River systems are continuously polluted by anthropogenic activities, several chemical pollutants come from agricultural, industrial and domestic wastes, and other sources. These polluting agents have clastogenic and/or mutagenic properties (Waters et al. 1999). Several genotoxic contaminants such as heavy metals (Matsumoto et al. 2006) can act as genotoxicants and can cause genetic alterations on DNA of aquatic organisms (Crespo-López et al. 2009). The genotoxic effects such as strand breaks, cross-linkages, and DNA base modifications resulted in the loss of DNA integrity (Frenzilli et al. 2009).
The comet assay and the micronucleus (MN) test are two rapid and sensitive methods. They are extensively used as genotoxic biomarkers for several species (Matsumoto et al. 2006; Boettcher et al. 2010). Further, these methods are useful tools for environmental monitoring and early ecological risk assessment. Besides, these tests are early warning tools for the environment (Park and Choi 2007).
In situ toxicity assessment has been used for several aquatic organisms in river environments and this method is useful to assess water toxicity and non-point source contaminants (Kushwaha et al. 2012). Fishes are affected by water pollution by directly exposing to the physical and chemical changes in the water. Therefore, fish species are suitable genetic model to evaluate the pollution in aquatic ecosystems (Kushwaha et al. 2012). Besides, they are used in vivo, in vitro and in situ genotoxic monitoring of rivers and lakes. Fishes, such as Squalius cephalus (Sunjog et al. 2016), Oreochromis niloticus (Hemachandra and Pathiratne 2016), Channa punctatus (Javed et al. 2016), Abramis brama (Kostić et al. 2016), Alburnus alburnus (Jovanović et al. 2018), Labeo rohita (Hussain et al. 2018), have been widely used to investigate DNA damage caused by heavy metals.
The lower Melet River, is covered by agricultural and industrial fields, which is also used as the most important drinking water source of the Ordu province. Further, the geological location of the river, the formation of the rich region of mineral deposits and heavy metals from domestic wastes, hazelnut agriculture and used pesticides are the main pollution sources of the river. In the upper region of the sampling points, there is Kabadüz district where mining activities are carried out. However, there is no study about the genotoxic potential of this river. This study was carried out to detect heavy metals and metalloid concentration in water, sediment and to evaluate bioaccumulation in the muscle of A. chalcoides. Consequently, the main objective of the current study was to determine the genotoxic potential of heavy metal and metalloid arising from anthropogenic activities in the lower Melet River using the comet assay and MN test.
Materials and Methods
Melet River, the biggest river of Ordu province (Turkey), forms a border between Middle and Eastern Black Sea parts. The lower Melet River is used as a water source that receives many detergents, industrial, domestic and agricultural wastes for Ordu province. It is surrounded by hazelnut groves and agricultural lands. Kabadüz district is a settlement located on the river and is located in the upper region of sampling points. Mining activities are carried out in this region and these activities constitute a resource in terms of copper, lead and zinc elements. Due to its geological location, especially, mineral deposits of copper (Cu), lead (Pb), silver (Ag), zinc (Zn), iron (Fe), gold (Au) and manganese (Mn) are noteworthy in the river basin (Gülderen and Bektaş 2011).
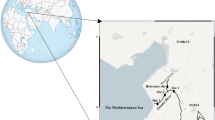
Two locations were selected to determine the genotoxic potential of the river (Fig. 1). The formation of a control group in natural river systems is an important issue for monitoring studies. Regarding this situation, Çavaş (2004) reported that the upper regions of the river are used as a reference region because they are cleaner than lower regions. In addition, the lower regions with discharge points are more polluted regions. Therefore, Location 1 was selected as a control site because anthropogenic effects were relatively low compared to Location 2. The other site was Location 2 which involved all the metal accumulation of the river. The heavy metals and metalloids which might arise from anthropogenic sources are concentrated at this site. Three different stations were selected for each locations (Location 1; 40º 54’ 26” N- 37º 56’ 07” E, 40º 54’ 20” N- 37º 56’ 08” E, 40º 54’ 12” N -37º 56’ 06” E and Location 2; 40º 58’ 58” N- 37º 56’ 00” E, 40º 58’ 29” N- 37º 56’ 10” E, 40º 58’ 11” N- 37º 56’ 23” E).
The water and sediment samples were seasonally (April, July, October, January) collected from Location 1 and Location 2 three times in 20RG-15/4/2015–2932715. The ten metals (Mn, Fe, Co, Cu, Zn, Al, Ni, Cr, Cd, Pb) and a metalloid (As) concentrations in water, sediment, and muscle of A. chalcoides were also estimated by inductively coupled plasma mass spectrometry (ICP-MS). The element concentrations were compared with permissible limits according to National Regulation (Official Gazette ) in water, according to the natural limit of earth’s crust (Turekian ve Wedepohl 1961) in sediment and permissible limits according to FAO (1983) and IAEA (2003) in the muscle of A. chalcoides.
The comet assay and micronucleus (MN) test were selected as in situ genotoxicity assays to measure genotoxic effects in erythrocyte cells of the A. chalcoides. The fish specimens that inhabiting naturally in these regions were collected from selected stations by using gill nets. A total of 40 specimens were caught from Location 1 (n = 20) and Location 2 (n = 20). Fishes were brought to the laboratory alive. Firstly, the fish were decapitated (Yazıcı and Şişman 2015) and then the blood cells of A. chalcoides individuals were collected directly from the heart of each specimen in heparinized tubes for comet assay and micronucleus test before dissection.
The comet assay was performed under alkaline conditions according to the procedure of Singh et al. (1988) with some modifications (Tice et al. 2000). The slides were precoated with a layer of %1 normal melting point (NMP) agarose. 50 µL blood and 150 µL of 0.75% low melting point (LMP) agarose were mixed and pipetted on the slide for the second layer. The slides were covered with cover glass and placed on a cooled layer. Then, the cover glass was removed and slides were placed into cold lysis buffer for 1 h. The slides were then put into cold alkaline electrophoresis buffer (10 N NaOH and 200 Mm EDTA, pH > 13) to allow DNA unwinding for 20 min at 300 mA at 4 °C. After electrophoresis, slides were placed into neutralizing buffer for 15 min. Staining was performed with EtBr per slides. The stained slides were observed under a fluorescent microscope (Leica) equipped with a TXR filter. 100 erythrocyte cells were randomly examined for each fish sample and comet images were scored using Comet IV Computer Software (Perceptive Instruments, UK). One drop blood was smeared immediately on the slides to perform the micronucleus (MN) test. When the smeared blood was dried, the slides were fixed in ethanol for 20 min and stained with 5% Giemsa solution. 2000 erythrocytes were examined per individual (Boettcher et al. 2010).
The descriptive statistics related to values of heavy metals and metalloid concentrations of water, sediment, muscle of fish, micronucleus frequency, and comet assay parameters were calculated. Spearman correlations were calculated for each element in the water-muscle and sediment-muscle. For values of micronucleus (MN) test and comet assay parameters, firstly the Kolmogorov-Smirnov test was used to determine whether the data had a normal distribution or not. Since the data were not normally distributed, evaluations were made using the Mann–Whitney U test. P-value less than 0.05 is statistically significant. All statistical tests were performed using MINITAB 16 statistical program.
Results and Discussion
The heavy metals and metalloid levels in the water, sediment, and muscle of A. chalcoides were presented for Location 1 and Location 2 in Table 1. Generally, element concentrations of water in Location 2 were higher than Location 1 (p > 0.05). The concentrations of heavy metals and metalloid in sediment followed the decreasing order: Fe > Al > Mn > Zn > Cu > Pb > Cr > As > Co > Ni > Cd, while in water, the order was Fe > Al > Mn > As > Zn > Cu > Ni > Cr > Cd = Pb = Co. While the strong correlations between water and muscle were determined in Al, Co and Zn at the Location 1, the strong correlation was found in Cr at the Location 2. Similarly, the strong correlations between sediment and muscle were determined in Ni, Mn, and Zn at the Location 1, the strong correlations were found in As, Cd, and Mn at the Location 2. Besides, especially Al, As, Fe and Cu concentrations were observed higher level than permissible limits according to National Regulation (Official Gazette RG-15/4/2015–29327) in Location 2. Cr and Mn concentrations in sediment were higher in Location 2 than Location 1 (p < 0.05). Generally, Al, As, Mn, Fe, Co and Cu concentrations in muscle of A. chalcoides inhabiting Location 2 were higher than Location 1 samples (p > 0.05). Pb and As concentrations in muscle were higher than the permissible limits according to FAO (1983) and IAEA (2003) in both Location 1 and Location 2.
The images of the observed cells during microscopic examinations for the MN test were given in Fig. 2. The results of micronucleus frequency in blood cells of fish samples were presented in Table 2. Location 2 was a more polluted location than Location 1 and the micronucleus frequency was higher in Location 2 than Location 1. In addition, this difference between Location 1 and Location 2 was statistically important (p < 0.01) (Fig. 2; Table 2). Therefore, MN frequency was determined as 3.65 ± 0.65 in Location 1 and 5.10 ± 0.94 in Location 2 (p < 0.01). Yazıcı (2012) reported that MN frequency was higher in polluted locations for Leuciscus cephalus and Capoeta capoeta inhabiting Karasu River (Turkey). Yazıcı and Şişman (2015) found similar results for Barbus plebejus inhabiting the polluted location of Karasu River (Turkey). Aquatic organisms exposed to waters contaminated by heavy metals have demonstrated DNA damage in many studies (Matsumoto et al. 2006).
The values of comet parameters were used to detect DNA damage in blood cells of A. chalcoides and the results were presented in Table 2. The DNA damage levels were shown in Fig. 3a. The DNA damage was higher in Location 2 (Fig. 3c) than in Location 1 (Fig. 3b). The blood cells of fish from polluted location showed significantly higher DNA damage in proportion to the reference location (p < 0.01). In the previous studies, three comet parameters such as tail length (TL), tail moment (TM) and tail intensity (TI) were generally used for determining DNA damage (Morin et al. 2011; Sunjog et al. 2014). In this study, six comet parameters were used to evaluate DNA damage. According to the results, comet formations in erythrocyte cells were generally increased in Location 2 compared to Location 1. When the results of the comet parameters were evaluated, tail length, tail intensity, and tail moment values were higher in Location 2 than Location 1 (Table 2). Additionally, the difference between the two station values was statistically important (p < 0.05) (Table 2). The mean tail length values were determined as 25.78 ± 0.656 µm for Location 1 and 29.38 ± 1.023 µm for Location 2, respectively. The mean values of tail length, tail moment, %DNA in the tail, tail intensity and %DNA in head parameters were determined and the values in Location 2 were higher than Location 1. These differences between the locations were statistically important (p < 0.01) (Table 2). Okuşluk (2008) conducted a study about the determination of pollution in the Mogan Lake (Turkey) using comet assay in Cyprinus carpio. The C. carpio samples were compared in Mogan Lake and commercial fish farm (control group). The tail length value of C. carpio individuals was 31.100 ± 10.390 µm for Lake Mogan and it was calculated as 22.800 ± 1.082 µm for the control group. These results were similar to the present study. In both studies, it was determined that increased concentrations of heavy metals and metalloid due to anthropogenic factors in polluted areas statistically increased (p < 0.05) genotoxic effect in fish.
The heavy metals and metalloids occur naturally in the environment. Earth’s crust has these components and anthropogenic effects cause them to mix into sediments, soils and waters (Sunjog et al. 2016). Many water sources were exposed to heavy metal and metalloid contamination from industrial, agricultural and human sources. In recent years, agricultural and industrial activities have changed the lower Melet River ecosystem. Also, pesticide residues mixed into the river. Pesticides can be divided into two groups, organic and inorganic. Inorganic pesticides are generally in a simple structure and are widely used in agriculture in pest control as they are easily soluble in water (Urkude et al. 2019). Inorganic pesticides contain different heavy metals such as iron, arsenic, copper, cadmium, lead, aluminum, and mercury, the heavy metals do not degrade readily and remain in the water and soil for a long time (Bigyan and Poonam 2014).
Additively, domestic wastes and quarry activities caused heavy metal pollution. In addition to all these factors, the most important was that the station is under the influence of the mine (Cu, Pb, and Zn) operating in Kabadüz district. Therefore, pollution in this environment can cause genetic alterations in fish species and other organisms living in this habitat. The average concentrations of Al, As, Fe, Mn, Cr and Ni in water were generally higher in Location 2 than Location 1. Also, Al, As, Fe and Cu concentrations were higher than permissible limits (Official Gazette RG-15/4/2015–29327). The high As concentration in water and muscle of fish, especially more than the permissible limit, affect organisms such as growth, ion regulation, reproduction, enzyme activities, and immune functions of fish species and the aquatic ecosystems (Kumari et al. 2016). In the present study, the high level of As was detected (Table 1) and it may be transported due to pesticide contents used in agricultural fields. Aluminum is one of the most abundant elements in the Earth’s crust. The presence of a quarry on the river leads to continuingly element entry into the water. Aluminum acts as a toxic agent on fish species in the aquatic environment (Rosseland et al. 1990). Pb is an element that is widely distributed throughout the world. Pb-based paints, storage batteries, mining of ore, application of Pb-containing pesticides and combustion of coal are other anthropogenic sources of lead (ATSDR 2019).
Matsumoto et al. (2006) determined the genotoxic effects of water samples at different pollution levels of a river by comet assay and micronucleus test using erythrocytes of Oreochromis niloticus. In the study, researchers reported that due to possible Cr compounds in river water, the comet assay values and MN frequencies increased in fish erythrocytes in the polluted area and suggested that chromium residues can be genotoxic. In the current study, the mean Cr concentration in Location 2 was also higher than Location 1 for both water and sediment of the Melet River. Cr originated from sediment can cause DNA damage in Location 2, because the mean Cr concentration was higher than the earth’s crust (Turekian and Wedepohl 1961). Scalon et al. (2010) analyzed possible DNA damage from Al, I, Cr, Cu, Zn, Pb, and Ni pollution. Al and I levels increased in the middle and lower regions of the Sinos River. The higher levels of heavy metal concentrations in polluted sites can cause DNA damage in aquatic species.
The changes in DNA damage were probably related to endogenous or seasonal effects. Many studies indicated seasonal variations in DNA damage in fish species and found that warmer seasons caused to increase DNA strand breaks (Buschini et al. 2004; de Andrade et al. 2004a, b; Osman et al. 2012). On the contrary, DNA damage decreased during warmer seasons (Wirzinger et al. 2007). The results showed that water temperature plays a role in the genotoxicity dynamic (Sunjog et al. 2014). Moreover, other chemical compounds such as pesticides, organochlorinated pesticide (OCP) residues, polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) were created genotoxic effects for fish species. The increased DNA damage was reported in fish species such as Ameiurus nebulosus (Amado et al. 2006) and Cyprinus carpio (Pandrangi et al. 1995) living in the contaminated waters with PAH and PCB. According to the present study results, it is obvious that high concentrations of heavy metals cause DNA damage in the fish. However, such natural aquatic ecosystems are influenced by heavy metals as well as other genotoxic agents such as PCBs, OCPs, and PAHs. For this reason, A. chalcoides living in the lower Melet River are thought to affect not only heavy metals but also other pollutants.
It was determined that metalloid and heavy metal concentrations in water, sediment and fish muscle of lower Melet River (Turkey) were increased in Location 2 and these element concentrations may cause genotoxic effects in at least one native fish species, A. chalcoides. The observed DNA damage in A. chalcoides may also be caused by PCBs, OCPs, and PAHs as well as heavy metal contamination found in water sediment and fish tissue. In the present study, for the first time, the MN test and comet assay were used for genotoxic monitoring for an aquatic system on the lower Melet River. The current study indicated that A. chalcoides can be used as an indicator organism for in situ monitoring in environmental contamination and genotoxicity. The obtained data were useful for monitoring studies and provided an idea for new future studies using fish blood in other aquatic locations in Turkey.
References
Amado LL, Robaldo RB, Geracitano L, Monserrat JM, Bianchini A (2006) Biomarkers of exposure and effect in the Brazilian flounder Paralichthys orbignyanus (Teleostei: Paralichthyidae) from the Patos Lagoon estuary (Southern Brazil). Mar Pollut Bull 52(2):207–213
ATSDR (2019) Toxicological profile for lead U.S. Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry, 561p
Bigyan N, Poonam T (2014) Assessment of Pesticide Use and Heavy Metal Analysis of Well Water in JhikuKhola Watershed, Kavrepalanchowk, Nepal. Int Res J Environment Sci 3(10):79–83
Boettcher M, Grund S, Keiter S, Kosmehl T, Reifferscheid G, Seitz N, Rocha PS, Hollert H, Braunbeck T (2010) Comparison of in vitro and in situ genotoxicity in the Danube River by means of the comet assay and the micronucleus test. Mutat Res Gen Tox En 700:11–17
Buschini A, Martino A, Gustavino B, Monfrinotti M, Poli P, Rossi C, Santoro M, Dörr AJM, Rizzoni M (2004) Comet assay and micronucleus test in circulating erythrocytes of Cyprinus carpio specimens exposed in situ to lake waters treated with disinfectants for potabilization. Mutat Res 557(2):119–129
Çavaş T (2004) Investigation of the genotoxic effects of industrial effluents using the micronucleus test and AgNOR analysis techniques under in-situ and laboratory conditions. PhD Thesis, Mersin University, Mersin
Crespo-López ME, Macêdo GL, Pereira SI, Arrifano GP, Picanço-Diniz DL, do Nascimento JL, Herculano AM (2009) Mercury and human genotoxicity: critical considerations and possible molecular mechanisms. Pharmacol Res 60:212–220
de Andrade VM, de Freitas TR, da Silva J (2004a) Comet assay using mullet (Mugil sp.) and sea catfish (Netuma sp.) erythrocytes for the detection of genotoxic pollutants in aquatic environment. Mutat Res 560(1):57–67
de Andrade VM, da Silva J, da Silva FR, Heuser VD, Dias JF, Yoneama ML, de Freitas TRO (2004b) Fish as bioindicators to assess the effects of pollution in two southern Brazilian rivers using the Comet assay and micronucleus test. Environ Mol Mutagen 44(5):459–468
FAO (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circulars No:764, Fish and Agriculture Organization, Roma, Italy
Frenzilli G, Nigro M, Lyons BP (2009) The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mut Res – Reviews Mut Res 681(1):80–92
Gülderen A, Bektaş MU (2011) Ordu çevre durum raporu 2011. T.C. Ordu Valiliği Çevre ve Şehircilik İl Müdürlüğü, Ordu, 209p (In Turkish)
Hemachandra CK, Pathiratne A (2016) Combination of physico-chemical analysis, Allium cepa test system and Oreochromis niloticus erythrocyte based comet assay/nuclear abnormalities tests for cyto-genotoxicity assessments of treated effluents discharged from textile industries. Ecotox Environ Safe 131:54–64
Hussain B, Sultana T, Sultana S, Masoud MS, Ahmed Z, Mahboob S (2018) Fish eco-genotoxicology: Comet and micronucleus assay in fish erythrocytes as in situ biomarker of freshwater pollution. Saudi J Biol Sci 25:393–398
IAEA (2003) World-wide intercomparison exercise for the determination of trace elements and methylmercury in fish homogenate international atomic energy agency– 407 Report No: IAEA/AL/144 IAEA/MEL/72
Javed M, Ahmad I, Usmani N, Ahmad M (2016) Bioaccumulation, oxidative stress and genotoxicity in fish (Channa punctatus) exposed to a thermal power plant effluent. Ecotox Environ Safe 127:163–169
Jovanović J, Kolarević S, Milošković A, Radojković N, Simić V, Dojčinović B, Kračun-Kolarević M, Paunović M, Kostić J, Sunjog K, Timilijić J, Djordjević J, Gačić Z, Žegura B, Vuković-Gačić B (2018) Evaluation of genotoxic potential in the Velika Morava River Basin in vitro and in situ. Sci Total Environ 621:1289–1299
Kostić J, Kolarević S, Kračun-Kolarević M, Aborgiba M, Gačić Z, Lenhardt M, Vuković-Gačić B (2016) Genotoxicity assessment of the Danube River using tissues of freshwater bream (Abramis brama). Environ Sci Pollut Res 23:20783–20795
Kumari B, Kumar V, Sinha AK, Ahsan J, Ghosh AK, Wang H, DeBoeck G (2016) Toxicology of arsenic in fish and aquatic systems. Environ Chem Lett 15(1):43–64
Kushwaha B, Pandey S, Sharma S, Srivastava R, Kumar R, Nagpure NS, Dabas A, Srivastava SK (2012) In situ assessment of genotoxic and mutagenic potential of polluted river water in Channa punctatus and Mystus vittatus. Int Aquat Res 4:16
Matsumoto ST, Mantovani MS, Malaguttii MIA, Dias AL, Fonseca IC, Marin-Morales MA (2006) Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genet Mol Biol 29(1):148–158
Morin B, Filatreau J, Vicquelin L, Barjhoux I, Guinel S, Leray-Forget J, Cachot J (2011) Detection of DNA damage inyolk-sac larvae of the Japanese Medaka, Oryzias latipes, by the comet assay. Anal Bioanal Chem 399:2235–2242
Official Gazette RG-15/4/2015–29327 Regulation on Surface Water Quality. Ministry of Forestry and Water Management, Date of Official Gazette: 30.11.2012, Number of Official Gazette: 28483, Ankara
Okuşluk Ö (2008) Determination of possible pollution in Mogan Lake by comet assay in common carp (Cyprinus carpio L.) Master Thesis Gazi University, Ankara
Osman AG, Abuel-Fadl KY, Kloas W (2012) In situ evaluation of the genotoxic potential of the river Nile: II. Detection of DNA strand-breakage and apoptosis in Oreochromis niloticus niloticus (Linnaeus, 1758) and Clarias gariepinus (Burchell,1822). Mutat Res 747(1):14–21
Pandrangi R, Petras M, Ralph S, Vrzoc M (1995) Alkaline single cell gel (Comet) assay and genotoxicity monitoring using bullheads and carp. Environ Mol Mutagen 26(4):345–356
Park SY, Choi J (2007) Cytotoxicity, genotoxicity and ecotoxicity assay using human cell and environmental species for the screening of the risk from pollutant exposure. Environ Int 33(6):817–822. https://doi.org/10.1016/j.envint.2007.03.014
Rosseland BO, Eldhuset TD, Staurnes M (1990) Environmental effects of aluminium. Environ Geochem Health 12(1–2):17–27
Scalon MCS, Rechenmacher C, Siebel AM, Kayser ML, Rodrigues MT, Maluf SW, Rodrigues MAS, Silva LB (2010) Evaluation of Sinos River water genotoxicity using the comet assay in fish. Braz J Biol 70(4):1217–1222
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175(1):184–191
Sunjog K, Kolarević S, Kračun-Kolarević M, Gačić Z, Skorić S, Ðikanović V, Lenhardt M, Vuković- Gačić B (2014) Variability in DNA damage of chub (Squalius cephalus L.) blood, gill and liver cells during the annual cycle. Environ Toxicol Pharmacol 37:967–974
Sunjog K, Kolarević S, Kračun-Kolarević M, Višnjić-Jeftić Ž, Skorić S, Gačić Z, Lenhardt M, Vasić N, Vuković- Gačić B (2016) Assessment of status of three water bodies in Serbia based on tissue metal and metalloid concentration (ICP-OES) and genotoxicity (comet assay). Environ Pollut 213:600–607
Tice RR, Agurell E, Anderson D (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35(3):206–221
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth’s Crust. Geol Soc Am Bull 72(2):175–191
Urkude R, Dhurvey V, Kochhar S (2019) Pesticide Residues in Beverages. In: Grumezescu AM, Holban AM (eds) Quality Control in the Beverage Industry. Academic Press, Amsterdam, pp 529–560
Waters MD, Stack HF, Jackson MA (1999) Genetic toxicology data in the evaluation of potential human environmental carcinogens. Mutat Res 437:21–49
Wirzinger G, Weltje L, Gercken J, Sordyl H (2007) Genotoxic damage in field-collected three-spined sticklebacks (Gasterosteus aculeatus L.): a suitable biomonitoring tool? Mutat Res 628:19–30
Yazıcı Z (2012) Cytogenetic effects of water pollution on some fish species living in Karasu River, Erzurum. Master Thesis, Atatürk University, Erzurum
Yazıcı Z, Şişman T (2015) Genotoxic effects of water pollution on Barbus plebejus living in Karasu River, Erzurum. Yunus Araştırma Bülteni 2:9–16
Acknowledgements
This study is based on part of the PhD thesis by Seda KONTAŞ at Ordu University. The study was supported by the Scientific Research Projects Commission of Ordu University (BAP; TF-1612). The corresponding author would as to thank TUBITAK-BIDEB within the scope of the 2211-E National Scholarship Program for PhD students.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kontaş, S., Bostancı, D. Genotoxic Effects of Environmental Pollutant Heavy Metals on Alburnus chalcoides (Pisces: Cyprinidae) Inhabiting Lower Melet River (Ordu, Turkey). Bull Environ Contam Toxicol 104, 763–769 (2020). https://doi.org/10.1007/s00128-020-02857-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02857-2