Abstract
The concentrations of butyltins (BTs) in sediment from Peninsular Malaysia along the Strait of Malacca and their spatial distribution are discussed. The concentrations of BTs were high in the southern part of Peninsular Malaysia where there is a lot of ship traffic, because trade is prosperous. The concentrations of monobutyltin (MBT), dibutyltin (DBT), and tributyltin (TBT) in sediment from the coastal waters of Peninsular Malaysia were in the range 4.1–242 μg/kg dry weight (dw), 1.1–186 μg/kg dw, and 0.7–228 μg/kg dw, respectively. A higher percentage of TBT was observed in the area where TBT concentrations were high. The concentrations of monophenyltin (MPT), diphenyltin (DPT), and triphenyltin (TPT) were in the range <0.1–121 μg/kg dw, 0.4–27 μg/kg dw, and 0.1–34 μg/kg dw in sediment from Peninsular Malaysia, respectively. MPT was the dominant phenyltin species. MBT, DBT, and TBT in green mussel (Perna viridis) samples were detected in the range 41–102 μg/kg, 3–5 μg/kg, and 8–32 μg/kg, respectively. A tolerable average residue level (TARL) was estimated at 20.4 μg/kg from a tolerable daily intake (TDI) of 0.25 μg TBTO/kg body weight/day. The maximum value of TBT detected in green mussel samples was the value near the TARL. TPTs were not detected in green mussel samples. The concentrations of Diuron and Irgarol 1051 in sediment from Peninsular Malaysia were in the range <0.1–5 μg/kg dw and <0.1–14 μg/kg dw, respectively. High concentrations of these compounds were observed in locations where the concentrations of TBT were high. Sea Nine 211, Dichlofluanid, and Pyrithiones were not detected in sediment. The concentrations of antifouling biocides in Melaka and the Strait of Johor were investigated in detail. BTs were found in similar concentrations among all sampling sites from Melaka, indicating that BT contamination spread off the coast. However, Sea Nine 211, Diuron, and Irgarol 1051 in the sediment from Melaka were high at the mouth of the river. BT concentrations at the Strait of Johor were higher than those in Peninsular Malaysia and Melaka and were high at the narrowest locations with poor flushing of water. The concentrations of antifouling biocides were compared among Malaysia, Thailand, and Vietnam. A higher concentration and wide variations of TBT and TPT in sediment from Malaysia were observed among these countries. The Irgarol 1051 concentrations in sediment from Malaysia were higher than those in Thailand and Vietnam.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Organotin (OT) compounds have been utilized as an active biocide causing deleterious effects such as endocrine disruption in nontarget marine organisms (Bryan and Gibbs 1991; Laughlin and Linden 1985), and further environmental studies have indicated OT contamination of the marine environment on a worldwide scale (Clark et al. 1988). In the 1980s, the use of tributyltin (TBT) was regulated in some developed countries such as England, France and the United States, but TBT has still been detected in the aquatic environment of these countries at a contamination level that causes adverse effects on the aquatic environment. In developing countries, there have been no controls on the usage of TBT. In October 2001, the International Maritime Organization (IMO) adopted the Internal Convention on the Control of Harmful Antifouling Systems (AFS Convention), which prohibited the use of OTs as active ingredients in antifouling systems for ships. Following the international restrictions on the use of OT-based antifoulants, paint manufacturers have developed many alternative products.
In the last 20 years, the economies of some Southeast Asian countries have been growing rapidly. Environmental pollution is closely tied to economic growth, because human and industrial activities increase and trading flourishes when there is an increase of economic activity. In fact, the coastal areas of Thailand, the Philippines, and Vietnam are contaminated by chemical compounds such as persistant organic pollutants (POPs) and antifouling biocides (Kan-atireklap et al. 1997a, b, 1998; Lee et al. 1997; Monirith et al. 2003; Prudente et al. 1999). Malaysia is the fastest growing country in Southeast Asia., because Malaysia has an abundance of mineral resources and beautiful natural areas. The tourism industry and trade businesses are thriving, and the Strait of Malacca and the Strait of Johor in Malaysia have become the world’s busiest waterways. Recent surveys show that the coastal waters of Malaysia are polluted by BTs (Hashimoto et al. 1998; Sudaryant et al. 2002, 2004; Tong et al. 1996).
Furthermore, contaminations by alternative biocides are also of concern, because many ocean liners enter the coastal areas of Malaysia. However, to date there have been no reports of pollution by alternative biocides.
In this study, the concentrations of OTs and alternative biocides are reported in sediment and green mussels from Malaysia and the distribution of antifouling biocides in Malaysia is also discussed.
Materials and Methods
Sampling Description
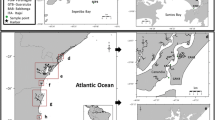
Malaysia is located in the center of Southeast Asia and consists of two geographical regions: Peninsular Malaysia and Malaysian Borneo divided by the South China Sea. Mining has declined, but the electronic industry has flourished, and as a result, Malaysia has become famous in Southeast Asia as an exporter of electronic parts. In order to confirm the widespread contaminations, sediment and biological samples were taken in Peninsular Malaysia along the Strait of Malacca from September 7 to 15, 2006 (Fig. 1). Descriptions of the sampling sites are given in Table 1. The surface sediment samples (0–5 cm) were taken using a core sampler in 13 sites. Sediment samples were not sieved. The dry weight of the sediment samples was calculated, with the difference between the weight of the sediment after having been dried at 110°C for 2 h and the weight of the sediment at the beginning. Water content and ignition loss (organic substances content) are shown in Table 1.
The green mussel (Perna viridis) samples were taken at 10 sites. The shell lengths of the green mussels were in the range 61–110 mm. Three mussel samples in each site were homogenated before analysis. Sediment samples were also taken in the coastal area of Melaka and the Strait of Johor from September 28 to 30, 2005 (Fig.1). All samples were brought back to Japan in a cold box and stored in a freezer at –20°C until chemical analysis.
Chemical Analysis
Organotin Compounds
The method used for the determination of OTs in sediment and biological samples was based on that of Midorikawa et al. (2004), with some modification. Five grams of sediment and the homogenate soft tissues of the mussels were put in a centrifuge tube and 100 μl of mixed acetone solution containing 1 μg/ml each of monobutyltin trichloride (MBTCl)-d9, dibutyltin dichloride (DBTCl)-d18, tributyltin monochloride (TBTCl)-d27, monophenyltin trichloride (MPTCl)-d5, diphenyltin dichloride (DPTCl)-d10, and triphenyltin monochloride (TPTCl)-d15 were added to the centrifuge tube as a surrogate standard. The mixture was shaken with 15 ml of 1 M HCl-methanol/ethyl acetate (1/1) for 10 min. After centrifugation for 10 min, the residue was again extracted with 10 ml of 1 M HCl-methanol/ethyl acetate (1/1). After centrifugation, the combined supernatants and 30 ml of saturated NaCl solution were transferred to a separatory funnel. The analytes were extracted twice with 15 ml of ethyl acetate/hexane (3/2) solution and the organic layer was combined. Fifty milliliters of hexane was mixed into the organic layer and left to stand for 20 min. After removal of the aqueous layer, the organic layer was dried with anhydrous Na2SO4 and concentrated up to trace level. The analytes were diluted with 5 ml of ethanol, 5 ml of acetic acid–sodium acetate buffer (pH 5.0), and 10 ml of distilled water and subsequently ethylated by shaking with 1 ml of 5% NaBEt4 for 30 min. The solution containing ethylated OTs was saponificated with 10 ml of 1 M KOH–ethanol solution by shaking for 1 h. Forty milliliters of distilled water and 20 ml of hexane were added to the solution and the mixture was shaken for 10 min. The ethylated OT residue in the aqueous layer was extracted again by shaking for 10 min with 20 ml of hexane. The combined organic layers were dried with anhydrous Na2SO4. After being concentrated up to 1 ml, the solution was cleaned by a florisil Sep-Pak column (Waters Association Co. Ltd.) The analytes were eluted with 10 ml of 5% diethyl ether/hexane. All eluting solvent was collected in a bottom flask. The solution was concentrated up to 0.5 ml after the addition of tetrabutyltin (TeBT)-d36 and tetraphenyltin (TePT)-d20 as an internal standard. The final solution was concentrated up to 0.5 ml.
A Hewlett-Packard 6890 series gas chromatography equipped with a mass spectrometry (5973 N) was used for analysis of the OTs. The separation was carried out in a capillary column coated with 5% phenyl methyl silicone (J&W Scientific Co.; 30 m length × 0.25 mm inner diameter, 0.25 μm film thickness). The column temperature was held at 60°C for the first 2 min, then increased to 130°C at 20°C/min, to 210°C at 10°C/min, to 260°C at 5°C/min and to 300°C at 10°C/min. Finally, the column temperature was held at 300°C for 2 min. The interface temperature, ion source temperature, and ion energy were 280°C, 230°C and 70 eV, respectively. Selected ion monitoring was operated under this program. The monitoring ions of 235 (233) for MBT, 261 (263) for DBT, 263 (261) for TBT, 253 (255) for MPT, 303 (301) for DPT, and 351 (349) for TPT were used to quantify the concentrations of OTs, respectively. The parenthesis shows the qualifier ions. One microliter of the sample was injected by splitless injection. The concentrations of OTs in this study are expressed as Sn4+.
Alternative Biocides
The method used for the determination of alternative compounds in sediment was based on that of Harino et al. (2005). In the centrifuge tube, 5 g of wet sediment was placed together with 10 ml of acetonitrile. The mixture was shaken for 10 min in a mechanical shaker. After removal of the supernatant, the analytes were reextracted with 10 ml acetonitrile for 10 min and the mixture was then centrifuged. The combined supernatants were concentrated by a rotary evaporator up to 5 ml and 45 ml of distilled water was added to them. The analytes were extracted three times with 10 ml of dichloromethane. The organic layer was dried by anhydrous Na2SO4. After 10 ml of methanol and 100 μl of atrazine-13C3 (1 mg/l) were added to the organic layer, the organic layer was concentrated up to 2 ml by a rotary evaporator. The analytes were determined by liquid chromatography/mass spectrometry–mass spectrometry (LC/MS-MS).
Liquid chromatography was performed on a high-performance LC (HPLC) apparatus equipped with an Agilent model 1100 series (Agilent; Yokogawa Analytical Systems, Tokyo, Japan). The separation was carried out on a narrow-bore C18 silica column (2.1 × 50 mm, 5 μm). The mobile phase was methanol–water run over a gradient (50% of methanol linear to 100% of methanol for 20 min and held for 10 min). The injection volume was 10 μl.
Electrospray mass spectrometry (ESI-MS-MS) analyses were carried out using a PE-Sciex API2000 (Sciex; Applied Biosystems Japan). Ionization of the analytes was achieved by electrospray in the positive ion mode. All the interface parameters were optimized by infusion of a standard solution (1 mg/l) of the analytes at the flow rate of 20 ml/min. The final electrospray conditions were as follows: nitrogen curtain gas, 40 l/min; ion spray voltage, 4800 V; ion source gas 1, 40 μl/min; gas 2, 70 μl/min; collision gas, 4 μl/min. LC/MS-MS acquisition was performed in the multiple reaction monitoring (MRM) mode and the precursor ion/product ion of Sea-Nine 211, Diuron, Dichlofluanid, Irgarol 1051, M1, and Pyrithiones were 282/170 (43), 233/46 (160), 333/224 (123), 254/198 (83), 214/158 (43), and 316/190 (189) respectively. The parentheses show the qualifier ion.
Results and Discussion
Evaluation of the Analytical Procedure
In order to examine the quality of the data obtained by the analytical procedure of OTs and alternative biocides, 0.1 μg of OTs were spiked to 5 g of sediments and green mussels. The recoveries of the OTs in sediment and mussels were in the range 98–115% and 95–110%, respectively, and the relative standard deviations (RSDs) of the OTs were in the range 6–10% for sediment and 5–12% for mussels, respectively (Table 2). The recoveries of the alternative biocides in the sediment were in the range 75–95%, and their RSDs were in the range of 8–15%.
The detection limits were calculated from a signal-to-noise ratio of 3.The OT’s detection limits were 0.1 μg/kg dry weight (dw) for the sediment samples and 1 μg/kg wet weight (ww) for the mussel samples. The detection limits of Sea-Nine 211, Diuron, Dichlofluanid, Irgarol 1051, M1, and Pyrithiones in the sediment samples were 0.04, 0.02, 0.1, 0.02 and 0.09 and 20 μg/kg dw, respectively.
Distribution and Concentrations of Antifouling Biocides in Peninsular Malaysia
The coastal area of Peninsular Malaysia, which is called the commercial capital, is an area in which many ships sail and are moored. It can therefore be predicted that contamination by antifouling paint in the area has occurred. Figure 2 shows the spatial distribution of BTs in sediment from Peninsular Malaysia along the Strait of Malacca. The concentrations of BTs were high in the southern part of Peninsular Malaysia. In the northern part of Peninsular Malaysia, tourism in a big industry because there are many mangrove forests and beautiful beaches, whereas in the southern part, there are fishing grounds, fish farms, and industrial ports. Severe BTs contamination was therefore found in the southern part. Sudaryanto et al. (2004) reported that elevated concentrations of BTs were found along the coast of the Strait of Malacca, particularly in locations having intensive maritime activities, such as Penang, Johor, and Johor Bahru. The concentrations of MBT, DBT, and TBT in sediment from the coastal waters of Peninsular Malaysia along the Strait of Malacca were in the range 4.1–242 μg/kg dw, 1.1–186 μg/kg dw, and 0.7–228 μg/kg dw, respectively (Table 3). The maximum concentration of TBT was observed at St. P13, where there is heavy ship traffic. The TBT concentrations in sediment from Sts. P10–11, which contains aquaculture and fishery areas, were also high. We predicted that much TBT was used in this area in the past. Furthermore, the high percentages of TBT in sediment from Sts. P10–11 and P13 show that TBT is still being used in these areas (Table 3).
Sudaryanto et al. (2004) reported BT concentrations in sediment from the coastal areas of Malaysia in 1997. The levels of BTs in sediment between 1997 and 2006 were compared using these values, because the sampling sites by Sudaryanto et al. (2004) were similar to our study areas (Fig. 3). Remarkable changes of BTs during 10 years were not observed in sediment, suggesting that the use of BTs has persisted.
The spatial distribution of PTs in Peninsular Malaysia is shown in Fig. 2. Despite the detection of PTs in most sites, a characteristic trend was not observed in the spatial distribution. The concentrations of MPT, DPT, and TPT were in the range <0.1–121 μg/kg dw, 0.4–27 μg/kg dw, and 0.1–34 μg/kg dw in sediment from the coastal waters of Peninsular Malaysia, respectively. Of total PTs, MPT was the dominant species. Kannan and Lee (1996) reported that TPT was used as a pesticide. The detections of PTs in Peninsular Malaysia might be due to its use as a pesticide and not as an antifouling biocide, because the spatial distribution of PTs is different from that of BTs.
Figure 4 shows the spatial distribution of BTs in green mussels from Peninsular Malaysia. Remarkable differences of BTs among sites were not observed. It was reported that TBTs in mussels reflected on those in water within 2 or 3 months (Short and Sharp 1989). It is presumed that BTs in water are at similar levels among the sampling sites. MBT, DBT and TBT in green mussel samples were detected in the range 41–102 μg/kg, 3–5 μg kg−1 and 8–32 μg kg−1, respectively. Of all BTs, the percentage of TBT was low, suggesting the decrease of TBT’s input. Sudaryanto et al. (2002) reported the BT concentrations in green mussels from the coastal areas in Malaysia during 1997–1998. The levels of BTs in sediment between 1997–1998 and 2006 were compared using these values (Fig. 3). During those 10 years, the TBT concentrations decreased and MBT concentrations increased, supporting the decreasing input of BTs.
Although MPT and DPT in mussel samples were detected in the range <0.1–22 μg/kg and <0.1–5 μg/kg, respectively, TPT was not detected.
The mussels used in the present study are an important source of food. Therefore, the health risk to persons from the BT in mussels is a concern. Penninks (1993) derived a tolerable daily intake (TDI) of TBTO of 0.25 μg/kg body weight/day. This value was based on the observed effects of TBT on the immune function in rats with a safety factor of 100 to extrapolate the toxicity test from rats to humans. The tolerable average residue levels (TARLs) were calculated from TDI based on the following formula:
The TARL for seafood in Malaysia were estimated to be 40.9 μg/kg ww for an average person weighing 60 kg and consuming 146.6 g of seafood a day (Belfroid et al. 2000). The maximum value of TBT (32 μg/kg ww) detected in mussel samples was the value near the TARL, suggesting the importance of further monitoring of the TBT concentrations in mussel samples.
As alternative compounds, Sea Nine 211, Diuron, Irgarol 1051, M1, and Pyrithione were measured in sediment from Peninsular Malaysia. The concentrations of Diuron in sediment were in the range <0.1–5 μg/kg dw. The concentrations of Diuron in sediment from the coastal waters of England were in the range <12–395 μg/kg dw, as reported by Boxall et al. (2000). Thomas et al. (2000, 2002) reported that the Diuron concentrations were in the range <0.1–1.4 μg/kg dw in Southampton. Harino et al. (2005) reported that Diuron was detected in the range 0.64–1350 μg/kg dw in the Port of Osaka, Japan. The concentrations of Diuron in sediment from Peninsular Malaysia were lower than these values.
The concentrations of Irgarol 1051 in sediment from Malaysia were in the range <0.1–14 μg/kg dw. The concentrations of Irgarol 1051 have been reported in various countries; for example, the concentrations in the Hamble estuary, Owell estuary, and Southampton, England ranged from 6 to 880 μg/kg dw (Boxall et al. 2000; Gough et al. 1994; Thomas et al. 2000, 2002). The concentrations of Irgarol 1051 in sediment from Switzerland, Germany, and Japan were in the range <0.2–8 μg/kg dw, 3–220 μg/kg dw, and 7–816 μg/kg dw, respectively (Biselli et al. 2000; Harino et al. 2005; Toth et al. 1996). The concentrations of Irgarol 1051 in Malaysia were lower than those in European countries and Japan.
Higher concentrations of Diuron and Irgarol 1051 were observed in Sts. P10 and P12–13, where the concentrations of TBT were high. This means that Diuron and Irgarol 1051 are used as an antifouling paint in Malaysia. Sea Nine 211 and Dichlofluanid in sediment from most sampling sites were not detected. However, the concentrations of alternative compounds were lower than those of TBT (Table 3). Judging from these findings, the contamination by OTs in Malaysia is a more serious problem than that by alternative compounds.
Distribution of Antifouling Biocides in Melaka and the Strait of Johor
The concentrations of antifouling biocides in Melaka and the Strait of Johor, where there is great traffic density, were investigated in detail. The spatial distribution of BTs in Melaka is shown in Fig. 5. There were similar concentrations of BTs among all sampling sites from Melaka, indicating that BTs contamination spread off the coast. The concentrations of PTs were lower than those of BTs, and the spatial distribution of PTs in Melaka was different from those of BTs (Table 4). The input of PTs to sediment might be due to its use as a pesticide, as described in the previous subsection. The concentrations of MBT, DBT, and TBT in sediment from Melaka were in the range 85–270 μg/kg dw, 3.6–17 μg/kg dw, and 2.4–31 μg/kg dw, respectively, which were similar to those in Peninsular Malaysia. MBT was the dominant species in sediment from Melaka. It is well known that TBT persists for a long time in sediment (Dowson et al. 1993; Harino et al. 1998; Maguire and Tkacz 1985). However, the higher percentage of MBT among BTs in this study shows the degradation of TBT. It was reported that in most of the locations tested in Malaysia, MBT generally occurred in the highest proportion (Sudaryanto et al. 2004). The degradation of TBT in sediment from Malaysia might be faster than those in other areas because of higher temperatures. Further study is needed to clarify why the proportion of MBT is high in sediment from Malaysia.
The spatial distribution of BTs in sediment from the Strait of Johor is shown in Fig. 6. BTs concentrations in sediment were high at Sts. J7–J11 with poor flushing of water. Sudaryanto et al. (2004) reported that higher concentrations of BTs were found in the sediment samples collected in the narrowest area of the Strait of Johor. The concentrations of MBT, DBT, and TBT in the Strait of Johor were in the range 83–542 μg/kg dw, 30–232 μg/kg dw, and 41–492 μg/kg dw, respectively, which were higher than those in Peninsular Malaysia and Melaka (Table 5). MBT was the dominant compound at most sampling sites of the Strait of Johor, suggesting that the degradation rate of TBT is faster than the input rate in sediment from this area. The concentrations of MPT, DPT, and TPT were in the range 41–66 μg/kg dw, 5–29 μg/kg dw, and 0.3–34 μg/kg dw from the Strait of Johor, respectively (Table 5). The concentrations of PTs in the Strait of Johor were similar to those in the coastal waters of Peninsular Malaysia and Melaka and MPT among PTs was the dominant compound in Strait of Johor.
The relationship between the BT and PT concentrations is shown in Fig. 7. The correlation coefficient between the BTs and PTs concentrations in the Strait of Johor (R 2 = 0.570) was higher than that in Melaka (R 2 = 0.200). It was found that PTs are used not only as pesticides but also as antifouling paint on ships that are sailed and moored in the Strait of Johor.
Sea Nine 211, Diuron, and Irgarol 1051 in the sediment from Melaka were detected in the range <0.04–4.2 μg/kg dw, <0.02–4.1 μg/kg dw, and <0.02–0.21 μg/kg dw, respectively, whereas Dichlofluanid, M1, and Pyrithiones were not detected. The concentrations of Sea Nine 211, Diuron, and Irgarol 1051 were high at St. M1, which is located at the mouth of a river. In fact, the spatial distributions of alternative biocides were different from those of BTs. BT pollution in sediment was spread across a wide area because BTs have been used for a long time. However, the contamination by alternative compounds showed localized distribution, because alternative biocides have recently begun to be used.
Sea Nine 211, Diuron, and Irgarol 1051 in the sediment from the Strait of Johor were detected in the range <0.04–0.92 μg/kg dw, <0.02–9.9 μg/kg dw, and <0.02–0.90 μg/kg dw, respectively, and Dichlofluanid, M1, and Pyrithiones were not detected. The Diuron concentration was the highest among alternative biocides.
Comparison of the Concentrations of Antifouling Biocide Among Southeast Asian Countries
The concentrations of OTs were compared among Malaysia, Thailand, and Vietnam (Fig. 8). The higher concentrations and the wide variations of TBT and TPT in sediment from Malaysia and Thailand show that larger amounts of these compounds have been used in coastal waters from these countries in the past.
The level of each alternative compound in sediment was different in each country (Fig. 8). The higher concentrations were observed in Irgarol 1051 for Malaysia, Diuron for Thailand, and Sea Nine 211 for Vietnam. This reflects the fact that the patterns of application of antifouling biocides vary among regions.
Conclusions
Judging from the concentration of antifouling biocides in sediment, contamination by OTs is a more serious issue than that by alternative compounds in Southeast Asia. Even now, higher concentrations of OTs were observed at the busiest locations for ship traffic. Information about the hazards of contamination by alternative biocides is also needed because an increase in the demand for alternative compounds is estimated in the near future. Therefore, further studies are needed to continue monitoring antifouling biocides in Southeast Asian countries and to evaluate the risk of alternative compounds.
References
Belfroid AC, Purperhart M, Ariese F (2000) Organotin levels in seafood. Marine Pollut Bull 40:226–232. doi:10.1016/S0025-326X(99)00241-6
Biselli S, Bester K, Huhnerfuss H, Fent K (2000) Concentrations of the antifouling compound Irgarol 1051 and of organotins in water and sediments of German North and Baltic Sea Marine. Marine Pollut Bull 40:233–244. doi:10.1016/S0025-326X(99)00177-0
Boxall ABA, Comber SD, Conrad AU, HJowcroft J, Zaman N (2000) Input monitoring and fate modeling of antifouling biocides in UK estuaries. Marine Pollut Bull 40:898–905. doi:10.1016/S0025-326X(00)00021-7
Bryan GW, Gibbs PE (1991) Impact of low concentrations of tributyltin (TBT) on marine organisms: review. In: Newman MC, McIntosh AW (eds) Metal ecotoxicology: concepts and applications, Lewis Publishers, Ann Arbor, MI, pp 323–361
Clark EA, Sterritt RM, Lester JN (1988) The fate of tributyltin in the aquatic environment. Environ Sci Technol 22:600–604. doi:10.1021/es00171a001
Dowson PH, Bubb JM, Williams TP, Lester JN (1993) Degradation of tributyltin in sediment in freshwater and estuarine marina sediments. Water Sci Technol 28:133–137
Gough MA, Fothergill G, Hendrie JD (1994) Survey of southern England coastal waters for the s-Triazine antifouling compound Irgarol 1051. Marine Pollut Bull 28:613–620. doi:10.1016/0025-326X(94)90363-8
Harino H, Fukushima M, Kawai S, Megumi K (1998) Measurement of butyltin contamination of water and sediment in Osaka Bay, Japan. Appl Organomet Chem 12:819–825. doi:10.1002/(SICI)1099-0739(199812)12:12<819::AID-AOC788>3.0.CO;2-H
Harino H, Mori Y, Yamaguchi Y, Shibata K, Senda T (2005) Monitoring of antifouling booster biocides in water and sediment from the port of Osaka, Japan. Arch Environ Contam Toxicol 48:303–310. doi:10.1007/s00244-004-0084-2
Harino H, Ohji M, Wattayakorn G, Arai T, Rungsupa S, Miyazaki N (2006a) Occurrence of antifouling biocides in sediment and green mussels from Thailand. Arch Environ Contam Toxicol 51:400–407. doi:10.1007/s00244-005-0246-x
Harino H, Midorikawa S, Arai T, Ohji M, Cu ND, Miyazaki N (2006b) Concentrations of booster biocides in sediment and clams from Vietnam. J Marine Biol Assoc UK 86:1163–1170. doi:10.1017/S0025315406014147
Hashimoto S, Watanabe M, Noda Y, Hayashi T, Kurita Y, Takasu Y, Otsuki A (1998) Concentration and distribution of butyltin compounds in a heavy tanker route in the Strait of Malacca and in Tokyo Bay. Marine Environ Res 45:169–177. doi:10.1016/S0141-1136(97)00031-7
Kan-atireklap S, Tanabe S, Sanguansin J (1997a) Contamination by butyltin compounds in sediments from Thailand. Marine Pollut Bull 34:894–899. doi:10.1016/S0025-326X(97)00053-2
Kan-atireklap S, Tanabe S, Sanguanisin J, Tabucanon MS, Hungspreugs M (1997b) Contamination by butyltin compounds and organochlorine residues in green mussel (Perna viridis, L.) from Thailand coastal waters. Environ Pollut 97:79–89. doi:10.1016/S0269-7491(97)00070-5
Kan-atireklap S, Yen NTH, Tanabe S, Subramanian AN (1998) Butyltin compounds and organochlorine residues in green mussel (Perna viridis L.) from India. Environ Toxicol Chem 67:409–424. doi:10.1080/02772249809358631
Kannan K, Lee RF (1996) Triphenyltin and its degradation products in foliage and soils from sprayed pecan orchards and in fish from adjacent ponds. Environ Toxicol Chem 15:1492–1499. doi:10.1897/1551-5028(1996)015<1492:TAIDPI>2.3.CO;2
Laughlin RB Jr, Linden O (1985) Fate and effects of organotin compounds. AMBIO 14:88–94
Lee DB, Prudente MS, Tanabe S, Tatsukawa R (1997) Organochlorine residues in soils and sediments from Manila and nearby provinces, Philippines. Toxicol Environ Chem 60:171–181. doi:10.1080/02772249709358462
Maguire RJ, Tkacz RJ (1985) Degradation of the tri-n-butyltin species in water and sediment from Tronto harbour. J Agric Food Chem 33:947–953. doi:10.1021/jf00065a043
Midorikawa S, Arai T, Harino H, Ohji M, Duc Cu N, Miyazaki, N (2004) Concentrations of orgnotin compounds in sediment and clams collected from coastal areas in Vietnam. Environ Pollut 131:401–408. doi:10.1016/j.envpol.2004.03.007
Monirith I, Ueno D, Takahashi S, Nakata H, Sudaryanto A, Subramarian A, Karuppiah S, Ismail A, Mushtar M, Zheng J, Richardson BJ, Prudente M, Hue ND, Tana TS, Tkalin AV, Tanabe S (2003) Asia-Pacific mussel watch: monitoring contamination of persistant organochlorine conpounds in coastal waters of Asian countries. Marine Pollut Bull 46:281–300. doi:10.1016/S0025-326X(02)00400-9
Penninks AH (1993) The evaluation of data-derived safety factors for bis (tri-n-butyltin) oxide. Food Addit Contam 10:351–361
Prudente M, Ichihashi H, Kan-atireklap S, Watanabe I, Tanabe S (1999) Butyltins, organochlorines and metal levels in green mussel, Perna viridis L. from the coastal waters of the Philippines. Fish Sci 65:441–447
Short JW, Sharp JL (1989) Tributyltin in bay mussel (Mytilus edulis) of the Pacific coast of the United States. Environ Sci Technol 23:740–743. doi:10.1021/es00064a014
Sudaryanto A, Takahashi S, Monirith I, Ismail A, Muchtar M, Zheng J, Richardson BJ, Subramanian A, Prudente M, Hue ND, Tanabe S (2002) Asia-Pacific mussel watch: monitoring of butyltin contamination in coastal waters of Asian developing countries. Environ Toxicol Chem 21:2119–2130. doi:10.1897/1551-5028(2002)021<2119:APMWMO>2.0.CO;2
Sudaryanto A, Takahashi S, Iwata H, Tanabe S, Ismail A (2004) Contamination of butyltin compounds in Malaysian marine environment. Marine Pollut Bull 130:347–358
Thomas K, Blake SJ, Waldock MJ (2000) Antifouling paint booster biocide contamination in UK marine sediment. Marine Pollut Bull 40:739–745. doi:10.1016/S0025-326X(00)00010-2
Thomas K, McHugh M, Waldock M (2002) Antifouling paint booster biocides in UK coastal waters: inputs, occurrence and environmental fate. Sci Total Environ 293:117–127. doi:10.1016/S0048-9697(01)01153-6
Tong SL, Pang FY, Phang SM, Lai HC (1996) Tributyltin distribution in the coastal environment of Peninsular Malaysia. Environ Pollut 91:209–216. doi:10.1016/0269-7491(95)00048-8
Toth S, Becker-van Slooten K, Spack L, de Alencastro LF, Tarradellas J (1996) Irgarol 1051, an antifouling compound in freshwater, sediment and biota of Lake Geneva. Bull Environ Contam Toxicol 57:426–433. doi:10.1007/s001289900208
Acknowledgment
The authors thank Professor Hideshige Takada and his group at the Tokyo University of Agriculture and Technology for assistance with the sampling in Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harino, H., Arai, T., Ohji, M. et al. Contamination Profiles of Antifouling Biocides in Selected Coastal Regions of Malaysia. Arch Environ Contam Toxicol 56, 468–478 (2009). https://doi.org/10.1007/s00244-008-9252-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-008-9252-0