Abstract
Constructed wetland (CW) is a promising technique for removal of pollutants from wastewater and agricultural runoff. The performance of a CW to remove pollutants, however, hinges on the use of suitable substrate materials. This study examined the physicochemical properties and phosphorus (P) sorption capacities of nine different CW substrate materials using both batch experiments and the Freundlich as well as the Langmuir isotherm. The nine substrate materials used in this study were turf, topsoil, gravel, midsized sand (MSS), blast furnace slag (BFS), coal burn slag (CBS), blast furnace artificial slag (BFAS), coal burn artificial slag (CBAS), and midsized artificial sand (MSAS). Experimental data showed that sorption of P increased with initial solution P concentrations for all nine substrate materials. The maximum P sorption capacity of the substrate materials estimated by Langmuir isotherm was in the following order: turf (4243 mg/kg substrate) > BFAS (2116 mg/kg substrate) > BFS (1598 mg/kg substrate) > CBS (1449 mg/kg substrate) > top soil (1396 mg/kg substrate) > CBAS (1194 mg/kg substrate) > MSAS (519 mg/kg substrate) > gravel (494 mg/kg substrate) > MSS (403 mg/kg substrate). The specific gravity of eight substrate materials (except gravel) had very significant negative correlations with the P sorption, whereas the particle diameter of D60 and uniformity coefficient (K60) had positive correlations with the P sorption. The cation exchange capacity, organic matter, available ferrous, and exchangeable aluminum of the eight substrate materials also had very significant positive correlations with the P sorption, while the pH of the substrate materials showed a very significant negative correlation with the P sorption. Our study further suggests that turf and CBAS are the two relatively ideal substrate materials suitable for removal of P from a CW system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication of lakes and streams, resulting from surface loading and groundwater discharge of excess nutrients such as phosphorus (P) and nitrogen, is an increasing environmental concern worldwide. Constructed wetlands (CWs) have been used to remove such excess nutrients from domestic and industrial wastewaters and agricultural runoffs for decades (Sakadevan and Bavor 1998). A CW is an artificial marsh or swamp, created for treatments of anthropogenic contaminants from domestic wastewater, storm water runoff, or sewage and as a habitat for wildlife. CW is becoming a widely accepted remedial technique due to its low cost, suitable for small to medium-sized contaminated sites (Kadlec and Knight 1996; Mann and Bavor 1993; Zhu et al. 1997; Sakadevan and Bavor 1998; Drizo et al. 1999; Vymazal 1999; Del Bubba et al. 2003; Masbough et al. 2005; Xu et al. 2006).
Many studies have been directed at investigating the remedial efficiency of excess nutrients from CW systems with a variety of substrates, including pumice, quartzite, light expanded clay aggregates, lightweight aggregates, wollastonite, shale, sand, maerl, zeolite, bauxite, synthetic hydrotalcite, blast furnace slag, and fly ash (Mann and Bavor 1993; Johansson 1997; Sakadevan and Bavor 1998; Brooks et al. 2000; Gray et al. 2000; Arias et al. 2001 2003; Del Bubba et al. 2003; Kuzawa et al. 2006). Results from these studies showed that although the removal of P in a CW system occurred through substrate sorption, chemical precipitation, microbial action, and plant and algal uptake, substrate sorption plays the most important role in removal of P. The overall performance of a CW is, therefore, primarily dependent on the selection of suitable substrates.
Zhu et al. (1997) reported that a substrate’s sorption capacity of P is determined by its physicochemical properties. A substrate rich in Fe, Al, and Ca ions can easily form a phosphatic precipitant, which is very useful in removal of P from sewage. In addition, the pH and adsorptive surface of a substrate play an important role in P sorption. Substrates with fine particle materials usually have a large specific surface area and high P sorption potential. However, these substrates normally have a low hydraulic conductivity and thus can lead to overland flow and incomplete contact of substrates with sewage in CW systems (Kadlec and Knight 1996; Drizo et al. 1999). Therefore, an ideal substrate should possess both a high P sorption capacity and a suitable percolation rate.
Sorption of P in different soils and matrixes has been studied in the past three decades by batch experimental measurements and by Langmuir and Freundlich isotherm estimations (Mead, 1981; Bhuiyan and Sedberry 1995; Pant et al. 2001; Cui et al. 2002; Xu et al. 2006). Pant et al. (2001) studied the sorption capacity of P by three root bed materials, namely, Lockport dolomite, Queenston shale, and Fonthill sand, in a subsurface flow CW. Those authors found that Fonthill sand is the best material for removal of P from wastewater and that the Langmuir sorption isotherm seems to be more suitable for predicting P sorption in wetland systems since this isotherm allows the calculation of maximum sorption and binding energy constant. Del Bubba et al. (2003) studied the relationships between the maximum P sorption capacity and the physicochemical properties of sands. Using the Langmuir isotherm, those authors found that among the physicochemical properties of sands, the Ca and Mg content, grain size, porosity, bulk density, and hydraulic conductivity are significantly correlated with the maximum P sorption capacity in sandy soil. More recently, Xu et al. (2006) studied the sorption capacity of P by different substrates used in CW systems through batch experiments and Langmuir predictions. Those authors reported that furnace slag has the highest P sorption capacity, followed by fly ash. The sorption capacity of P is influenced by both the physicochemical properties of the substrates and the amount of organic matter added.
However, little effort has been devoted to employing artificial soil made of coal burn slag (CBS) or blast furnace slag (BFS) mixed with topsoil and turf as the filling material for removal of P in vertical-flow CW systems. As these two kinds of artificial soil have high sorption capabilities, microbe activities, hydraulic conductivities, and oxygen diffusion rates, they could be relatively ideal substrates for effective removal of P in vertical-flow CW systems without causing overland flow. The objectives of this study were (1) to estimate the sorption capacities of P by nine different substrate materials, namely, gravel, midsized sand (MSS), CBS, BFS, top soil, turf, midsized artificial sand (MSAS), coal burn artificial slag (CBAS), and blast furnace artificial slag (BFAS) using batch experiments and the Langmuir as well as the Freundlich isotherm; (2) to estimate the efficiency of P removal by these substrates using the batch experiments; and (3) determine the relationships of the substrate physicochemical properties to their P sorption capacities.
Materials and Methods
Experiment on Sorption and Removal of P
Nine substrate materials, namely, gravel, MSS, CBS, BFS, loamy topsoil, turf, MSS, CBAS, and BFAS, were selected for this study. Theses substrate materials were obtained from local areas near the campus of South China Agricultural University, Guangzhou City. It should emphasized here that turf is the organic humus forming from grasses. Unlike peat, humus in turf is only partially decomposed. The physicochemical properties of these substrate materials are listed in Tables 1 and 2. MSAS, CBAS, and BFAS are artificial substrate materials, which were made by mixing MSS, BCS, and BFS with certain ratios of topsoil and turf, respectively (China Patent ZL01235879.7 2002).
The bulk density of substrate materials was measured using a cutting ring (volume, 100 cm3; inner diameter, 5 cm) (Wang 1989), the specific gravity was measured by pycnometer method (Pall and Mohsenin 1980), the cation exchange capacity (CEC) was measured by ammonium acetate method (Peech et al. 1947), and the pH value was measured in a 1:2.5 soil:water slurry using a glass electrode (Bruce and Rayment 1982). Organic matter was measured by dichromate titration oxidation and volumetric procedure (Walkley 1947). Exchangeable Ca and Mg were measured using an atomic absorption spectrophotometric method after extracting the soil sample with neutral ammonium acetate. Exchangeable Al was measured using an atomic absorption spectrophotometric method after extracting the soil sample with potassium chloride. Active Fe and Mn were measured using atomic absorption spectrophotometry, after extracting soil samples with DTPA (diethlenetriaminepentaacetic acid ), TEA (triethanolamine), and CaCl2. The total contents of Ca, Mg, Al, Fe, and Mn in soils were measured using atomic absorption spectrophotometry, after fusion with sodium carbonate.
The sorption of P was measured at different time intervals between 0.5 and 48 h from batch experiments. A solution of 30 mL of K2HPO4 with five different initial P concentrations—100, 200, 300, 400, and 500 mg/L—in 0.01 M KCl solution, as a supporting electrolyte, was added to 3 g of each substrate material (in triplicate) in 50-mL Teflon-lined centrifuge tubes. Two drops of chloroform was added to each tube in order to inhibit microbial activities. The tubes were shaken on a reciprocating shaker for the desired time interval at an ambient temperature of 23°C. At the end of each time interval the samples were centrifuged at 4000 rpm for 10 min and the P in the clear supernatant solutions was filtrated and analyzed using a standard ammonium molybdate method (Sakadevan and Bavor 1998).
The removal of P from the solutions by the nine substrate materials at different time intervals was also determined through the batch experiments. Ten grams of each substrate material was added to a 50-mL centrifuge tube along with 30 mL of standard P solution (5.0 mg/L). The centrifuged tube was shaken and the supernatant in the tube was sampled at the following time intervals: 0, 0.17, 0.5, 1, 2, 4, 8, 24, and 48 h. Analogous to the sorption experiments, the supernatant was filtered prior to analysis and each treatment was in triplicate (Breen 1990).
Statistical analysis was performed with SAS version 6.12. Means and standard errors were calculated for three replicates. All data were statistically evaluated using Duncan’s multiple range test at p = 0.05.
Estimation of P Sorption and Substrate Properties
Sorption kinetics of P by substrate materials can be estimated with the Langmuir and Freundlich isotherms (Mead 1981). The Langmuir isotherm can be written
where K 1 is the maximum P sorption coefficient, K 2 the binding energy coefficient, C the P concentration in solutions (mg/L), and X the amount of P sorption in the solid phase (mg/kg). The maximum P sorption can be calculated with the slope of the curve of C/X and C (Sakadevan and Bavor 1998). The Freundlich equation is usually given as
where K and b are constants. Equation (2) can also be rearranged as
where ln K is the interception and 1/n = b is the slope.
The total porosity and particle uniformity coefficients of a substrate material can be calculated by the following equations:
where D 10 and D 60 (mm) are the diameters of particle sizes of a substrate material at which 10% and 60% of the particles pass through the sieves based on the accumulative frequency, and K 60 is the uniformity coefficient.
The saturated hydraulic conductivity is determined by Darcy’s law (Forbes 2002),
where K c is the hydraulic conductivity (cm/s), Q the total water flow rate (cm3/s), A the area of the infiltration bed (cm2), I the water head gradient, and T the time(s). The parameter values in Eq. (6) used to calculate the hydraulic conductivities of the nine substrate materials were measured from our previous study (Cui et al. 2007).
The removal of P by a substrate material was calculated using the following equation:
where C 0 is the initial P concentration of 5 mg/L and Ce is the P concentration at 48 h.
Results
Sorption of P
The amounts of P sorption by the nine substrate materials selected in this study are shown in Fig. 1. In general, the amount of P sorption increased with the initial solution P concentration for all of nine substrate materials. For instance, the amounts of P sorption by turf were 828 and 1367 mg/kg substrate, respectively, at initial P concentrations of 100 and 200 mg/L. A twofold increase in initial P concentration increased the amount of P sorption by 1.65 times. Our statistical analysis showed that differences in the amount of P sorption among all five initial P concentrations and nine substrates were significant at p = 0.05 except for MSS.
Sorption of P by BFAS also showed a very significant statistical difference except at initial P concentrations of 200 and 300 mg/L. The amounts of P sorption for the remaining seven substrates were relatively low and were statistically significant either at the upper range of initial P concentration (300–500 mg/L) or at the lower range of initial P concentration (100–200 mg/L).
Figure 1 further reveals that sorption of P by turf was the highest among all of the substrates used in this study. For example, the amounts of P sorption by turf and BFAS were about 828 and 236 mg/kg substrate, respectively, at an initial P concentration of 100 mg/L. The former was about 3.5-fold higher than the latter. Similar trends in P sorption between turf and BFAS were also observed for the rest of the four initial P concentrations. In contrast, sorption of P by MSS was the lowest at an initial P concentration level of from 100 to 300 mg/L, but higher than that by gravel at an initial P concentration level of from 400 to 500 mg/L.
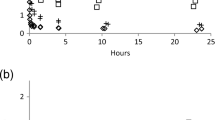
Kinetic sorption of P by all of the nine substrates at several time intervals is shown in Fig. 2. The initial P concentration used in this experiment was 5 mg/L. Two groups of substrates with distinct kinetic sorption capacities were identified. The first group of substrates, with a high P sorption capacity, included turf, CBAS, CBS, and topsoil (Fig. 2a) and the second group of substrates, with a low P sorption capacity, included gravel, MSS, MSA, BFS, and BFAS (Fig. 2b). Figure 2a shows that the sorption of P by the first group increased as time elapsed and the sorption capacity of the substrates was in the following order: turf > CBAS > CBS > topsoil. This figure further discloses that an equilibrium condition on P sorption by the substrates was not yet reached even at 48 h. Compared to the first group, the P sorption capacities of the second group were very low and approached the equilibrium condition after 24 h. At this steady condition, the sorption capacity of P by the substrates was in the following order: BFAS > BFS > MSS > MSAS > gravel.
Removal of P
Figure 3 shows the percentage removal of P from solutions by the nine substrates at five different initial P concentrations. The percentage removal of P from solutions decreased with the initial P concentration. For example, when the P concentration in the solution increased from 100 to 500 mg/L, the removal of P by turf dropped from 82% to 48.8%. A fivefold increase in initial P concentration decreased the removal of P by 33.2%.
It is also apparent that the percentage removal of P by the nine substrates at any initial P concentration level was in the following order: turf > CBAS > CBS > topsoil > BFAS > BFS > MSS > MSAS > gravel. Results indicate that the removal capability of P by gravel, which has been widely adopted in CWs, was lowest and not desirable. The removal efficiency of P in CWs could be improved and the service life of CWs could also be extended if substrates with a high P removal capability are used.
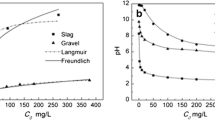
Sorption of P by the nine substrates was also estimated by the Langmuir and Freundlich isotherms. Table 3 lists the constants and regression coefficients of the Langmuir and Freundlich isotherms, which were obtained by fitting the P sorption data to the isotherms. All the correlation coefficients obtained by fitting the Langmuir isotherm to the P sorption data for all of the substrates were at a highly significant level except for the substrate CBS, which was only at a significant level. However, only six correlation coefficients obtained by fitting the Freundlich isotherm to the P sorption data for all of the substrates were at a highly significant level. Results indicate that the Langmuir isotherm was better for quantifying P sorption by the substrates used in this study. A comparison of the maximum sorption (K 1) of P estimated from the Langmuir isotherm among the nine substrates showed that turf was the highest, up to 4243 mg/kg, and MSS was the lowest, only 404 mg/kg. The order of K 1 from high to low for the remaining substrates was BFAS > BFS > CBS > topsoil > CBAS > MSAS > gravel.
Effect of Substrate Physicochemical Properties
The physical properties of eight substrates (except gravel) measured in this study include bulk density, specific gravity, total porosity, hydraulic conductivity, D 10 particle diameter, D 60 particle diameter, and K 60 uniformity coefficient (Table 1). The relationships of the above seven physical properties to P sorption associated with statistical tests are given in Table 4. These coefficients were obtained by regressing the P sorption data against each physical property.
Bulk density had a negative relationship with P sorption, as did specific gravity, hydraulic conductivity, and particle diameter of D 10. The negative relationships were highly significant between P sorption and bulk density or specific gravity but were not significant between P sorption and hydraulic conductivity or particle diameter of D 10. On the other hand, total porosity, particle diameter of D 60, and K 60 uniformity coefficient all showed positive relationships with P sorption but these were not statistically significant.
Analogous to the case of physical properties, eight substrates were chosen to measure their chemical properties, including pH, total and exchangeable (or active) Fe, Al, Ca, Mg, and Mn, CEC, and organic matter (Table 2). Correlations between the substrate chemical properties and their P sorption behaviors were estimated through linear regressions (Table 5).
Highly significant positive correlations were observed between P sorption and CEC, organic matter, active Fe, and exchangeable Al (p < 0.01), although these correlations did not exist between P sorption and exchangeable Ca and Mg, and total Mg, Al, and Fe (p > 0.05). A highly significant negative correlation was found between P sorption and pH (p < 0.01), whereas a negative correlation between P sorption and total Ca and Mn was not statistically significant (p > 0.05) (Table 5).
Discussion
Sorption characteristics of P by six natural substrates and three artificial substrates were investigated in this study. Of the six natural substrates, turf had the highest and gravel had the lowest maximum P sorption capacity, with the rest of the substrates in between in the following order: BFS > CBS > topsoil > MSS. Results imply that gravel and MSS, which are currently widely used in CW systems, were the two unfavorable substrates for P removal. P remedial efficiency would be improved if the remaining four natural substrates (i.e., excluding MSS and gravel) were adopted. However, turf and topsoil would readily cause soil clogging in vertical-flow CWs due to their swelling natures after absorbing water. Therefore, they can only be used as supplementary materials in vertical-flow CWs, not as substrates alone or as major parts of substrates. On the contrary, BFS and CBS can be used as substrates in vertical-flow CWs because they do not cause low hydraulic conductivity or medium clogging.
Three artificial substrates, namely, BFAS, CBAS, and MSAS, were made by mixing BFS, CBS, and MSS as the main materials, with the addition of certain ratios of turf and topsoil. The maximum P sorption capacities of the substrates were in the following order: BFAS > CBAS > MSAS. The maximum P sorption capacity for BFS was 1598 mg/kg, which was similar to that (1430 mg/kg) reported by Sakadevan and Bavor (1998), whereas the maximum P sorption capacity for BFAS was 2116 mg/kg. Results indicate that BFAS has more P sorption potential than BFS. According to the report by Sakadevan and Bavor (1998), the maximum P sorption by fly ash was more than 20 times higher than that of BFS and the maximum P sorption by coal ash was about 3 times higher than that of BFS. However, substrates made of fly and coal ash would be washed away by water and cause clogging of CWs due to their swelling nature. Therefore, fly and coal ash are not suitable for use as substrates in vertical-flow CWs. On the other hand, although the P sorption capacities of BFS, CBS, and their artificial soils were not the highest, they could become special substrates in vertical-flow CWs because of their low commercial prices and suitable hydraulic conductivities.
Since P fixation in CWs takes place through substrate adsorption, chemical precipitation, bacterial activities, plant and algal uptake, and interactions with organics (Kadlec and Knight 1996; Vymazal 1999), substrates play an important role in P fixation and their physicochemical properties determine their abilities for P adsorption and fixation (Zhu et al. 1997). In addition, the Fe, Al, and Ca contents, Eh, pH, and surface area of substrates also control P sorption (Vymazal et al. 1998). Materials with fine particles have the potential to increase their P sorption capabilities because of their large surface areas (Zhu et al. 1997). Our data show that sorption of P by the eight substrates (excluding gravel) had a positive correlation with the particle diameter of D 60 but a negative correlation with the particle diameter of D 10, which seems to be a contradiction to common sense. Normally, the smaller the particle diameter, the larger the surface area and thereby the higher the P sorption capacity. Although the exact reason for this phenomenon remains unknown, a possible explanation is that the overall amount of P sorption depends not only on the particle diameters but also on other physicochemical properties.
CEC, pH, active Fe, and exchangeable Al all had highly significant negative relationships with P sorption. Total Mg, exchangeable Ca, and Mg had positive correlations with P sorption and these correlations increased significantly with time. For example, the correlations between exchangeable Ca and P sorption among the eight substrates (except gravel) were not significant over a short time period but were significant as the sorption time increased. A similar result was also observed for total Ca. In general, P sorption was the highest in (1) basic wetlands with a large amount of calcium and (2) acidic wetlands with high concentrations of aluminum and iron (McEldowney et al. 1993) because P can be fixed by forming a precipitant with these chemicals. For instance, calcium reacted with soluble P to form hydroxyapatite uinder high-pH conditions, and hydroxyapatite can be precipitated out of solutions (Ryden et al. 1977). When municipal sewage is slightly basic, the calcium content in substrates is very important for P removal, while the Fe and Al contents in substrates are important when sewage is acidic because they can remove P by forming precipitant. Therefore, the ability of P removal by a substrate is somehow determined by the contents of exchangeable Ca and Al and active Fe, especially the contents of active Fe and exchangeable Al over a short time. The CEC of a substrate also plays an important role in P sorption but decreases as sorption time increases, thereby reducing the P removal ability.
Conclusions
Sorption of P by all nine substrates used in this study increased with the initial P concentration, and accorded with the Langmuir and Freundlich sorption isotherms. The correlation coefficients obtained by fitting the isotherms to the P sorption data for the substrates (except gravel) all reached significant or highly significant levels. The characteristics of P sorption by the substrates were better predicted by the Langmuir isotherm than by the Freundlich isotherm. The maximum sorption capacities of P by the nine substrates, estimated from the Langmuir isotherm, were in the following order: turf (4243mg/kg) > BFAS (2116mg/kg) > BFS (1598mg/kg) > CBAS (1449mg/kg) > topsoil (1369mg/kg) > CBS (1194mg/kg) > MSAS (519mg/kg) > gravel (494mg/kg) > MSS (403 mg/kg).
The removal of P from the solution was 92% for turf, 57% for CBAS, 56% for CBS, 41% for topsoil, 24% for BFAS, 21% for BFS, 19% for MSS, 12% MSAS, and 3% for gravel. For the physical properties of the substrates, bulk density and specific gravity had highly significant negative correlations with P sorption. As for the chemical properties of the substrates, CEC, organic matter content, active Fe, and exchangeable Al showed strongly significant positive correlations with P sorption, whereas pH had a highly significant negative correlation with P sorption.
It should be pointed out that our major focus in this study was the sorption capacities of the substrate materials. None of these materials is used as a sole substrate in CW treatments. In real-world applications, these substrate materials are usually mixed at certain ratios to obtain the best possible P removal rates.
References
Arias CA, Bubba MD, Brix H (2001) Phosphorus removal by sands for use as media in subsurface flow constructed reed beds. Water Res 35(3):1159–1168
Arias CA, Brix H, Johansen NH (2003) Phosphorus retention from municipal wastewater in an experimental two-stage vertical flow constructed wetland system equipped with a calcite filler. Water Sci Techno 48(5):51–58
Bhuiyan LR Jr, Sedberry JE (1995) Phosphorus sorption characteristics of selected soils of Louisiana. Commun Soil Sci Plant Anal 26(7–8):1059–1072
Breen PF (1990) A mass balance method for assessing the potential of artificial wetland for wastewater treatment. Water Res 24(6):689–697
Brooks AS, Rozenwald MN, Geohring LD, Lion LW, Steenhuis TS (2000) Phosphorus removal by wollastonite: a constructed wetland substrate. Ecol Eng 15:121–132
Bruce RC, Rayment GE (1982) Analytical methods and interpretations used by the Agricultural Chemistry Branch for soil and land use surveys. Queensland Department of Primary Industries Bulletin QB82004
China Patent No. ZL012358797 (2002) Constructed flower bed for sewage treatment
Cui LH, Luo SM, Zhu XZ, Liu YH (2002) Treatment of septic tank effluent using vertical-flow constructed wetlands. Wetland and remediation. II. In: Proceedings of the Second International Conference on Wetlands and Remediation. Battelle Press, online, pp 225–234
Cui LH, Zhu XZ, Luo SM (2007) The relationship between constructed wetland substrate phosphorus adsorption character and its physico-chemical properties. China Environ Sci 27(2):250–254
Del Bubba M, Arias CA, Brix H (2003) Phosphorus adsorption maximum of sands for use as media in subsurface flow constructed reed beds as measured by the Langmuir isotherm. Water Res 37:3390–3400
Drizo A, Frost CA, Grace J, Smith KA (1999) Physico-chemical screening of phosphate-removing substrates for use in constructed wetland systems. Water Res 33(17):3595–3602
Forbes MG (2002) Phosphorus retention and fractionation in masonry sand light weight expanded shale used as substrate in a subsurface flow wetland. PhD dissertation. University of North Texas, Denton
Gray S, Kinross J, Read P, Marland A (2000) The nutrient assimilative capacity of maerl as a substrate in constructed wetland systems for waste treatment. Water Res 34(8):2183–2190
Johansson L (1997) The use of LECA (light expanded clay aggregrates) for the removal of phosphorus from wastewater. Water Sci Technol 35(5):87–93
Kadlec RH, Knight RL (1996) Treatment wetlands. Lewis, CRC Press, Boca Raton, FL, pp 70–73
Kuzawa K, Jung Y-J, Kiso K, Yamada T, Nagai M, Lee T-G (2006) Phosphorus removal and recovery with a synthetic hydrotalcites as an adsorbent. Chemosphere 62:45–52
Mann RA, Bavor HJ (1993) Phosphorus removal in constructed wetlands using gravel and industrial waste substrata. Water Sci Technol 27:107–113
Masbough A, Frankowskic K, Hallb JK, Duffd SJB (2005) The effectiveness of constructed wetland for treatment of woodwaste leachate. Ecol Eng 25:552–566
McEldowney S, Hardman DJ, Waite S (1993) Pollution: ecology and biotreatment. Longman Science and Technology, London
Mead JA (1981) A comparison of the Langmuir, Freundlich and Temkin equations to describe phosphate adsorption properties of soils. Aust J Soil Res 19:333–342
Pall R, Mohsenin N (1980) A soil air pycnometer for determination of porosity and particle density. Trans ASAE 23:735–741, 745
Pant HK, Reedy KR, Lemon E (2001) Phosphorus retention capacity of root bed media of sub-surface flow constructed wetlands. Ecol Eng 17:345–355
Peech M, Alexander LT, Dean LA, Reed JF (1947) Soil survey laboratory methods 1996. Soil Survey Investigations Report No. 42. USDA, Washington, DC
Ryden JC, Mclaughlin JR, Syers JK (1977) Mechanisms of phosphate sorption by soils and hydrous ferric oxides gel. J Soil Sci 28:72–92
Sakadevan K, Bavor HJ (1998) Phosphate adsorption characteristics of soils, slag and zeolite to be used as substrates in constructed wetland systems. Water Res 32(2):393–399
Vymazal J (1999) Removal of phosphorus in constructed wetlands with horizontal sub-surface flow in the Czech Republic. In: Vymazal J (ed) Nutrient cycling and retention in natural and constructed wetlands. Backhuys, Leiden, The Netherlands, pp 1–17, 73–83
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils—effect of variations indigestion conditions and of inorganic soil constituents. Soil Sci 63:251–264
Wang RZ (1989) Soil particle density, bulk density and porosity determination; soil particle analyses; soil moisture determination. In: Li UK (eds) Routine methods of agro-chemistry and soil. Agro-chemistry Committee of Chinese Society of Soil Science Science Press, Beijing, pp. 15–66
Zhu T, Jenssen PD, Machlum T, Krogstad T (1997) Phosphorus sorption and chemical characteristics of lightweight aggregates (LWA): potential filter media in treatment wetlands. Water Sci Technol 35(5):103–108
Xu DF, Xu JM, Wu JJ, Muhammad A (2006) Studies of phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere 63:344–352
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 40571074), the Natural Science Foundation of Guangdong Province (No. 04020599), the Key Project of Guangdong Province (No. 2003C32912), and the Key Project of Dongguan City of Science and Technology (No. 2006168402).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, L., Zhu, X., Ma, M. et al. Phosphorus Sorption Capacities and Physicochemical Properties of Nine Substrate Materials for Constructed Wetland. Arch Environ Contam Toxicol 55, 210–217 (2008). https://doi.org/10.1007/s00244-007-9109-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-007-9109-y