Abstract
Purpose
River impoundments disrupt natural water flow patterns and sediment distribution throughout the impacted reach, which often results in a damaging effect on aquatic ecosystems. Dam removal can release sediments that may contain fugitive agricultural nutrients and organochlorine pesticide residues (OCPRs).
Methods
Sediment samples from an impoundment on the Oostanaula Creek (HUC 03,565,432) in Athens, Tennessee, were obtained, as were surface soil samples from the agricultural watershed. A subset of cores were used for simulated weathering, and all samples were extracted and analyzed for nutrients and OCPRs.
Results
The impoundment sediments tested low in P and K, but sediment pore water contained elevated concentrations of NO3, NH4, and SO4 relative to reservoir water. Endrin aldehyde and p,p’-DDD were commonly detected in sediment and soil, while aldrin, dieldrin, and p,p’-DDE occurred in a smaller number of samples. When detected, dieldrin and endrin aldehyde frequently exceeded the threshold effect concentration (TEC), but never exceeded the probable effect concentration (PEC) in the sediment samples; p,p’-DDD always exceeded TEC and exceeded PEC in 49% of the sediment samples. The concentrations of NO3 and NH4 in the weathered sediment leachates were similar to those in the reservoir water, and NH4 became the dominant cation in leachates at the conclusion of simulated weathering. Weathering decreased sediment p,p’-DDD concentrations to less than the PEC; however, the concentrations of other OCPRs were not influenced.
Conclusion
The dam sediments may have harmful effects on sediment-dwelling organisms and a long-term impact on stream reclamation following low-head dam removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There are more than 91,000 dams in the National Inventory of Dams held by the United States Army Corps of Engineers (https://nid.sec.usace.army.mil/ords/). Approximately 50.6% of these are low-head dams (height < 7.6 m), with an estimated tens of thousands of additional low-head dams not included in this inventory. Low-head dams are those constructed to allow water to flow directly over the entire length of the dam structure, are generally 3 to 4.5 m in height, and are constructed to raise water levels for industrial and municipal water supplies (Tschantz and Wright 2011). A typical low-head dam will have a shadow of 1.2 to 4 ha, most of which contains a layer of deposited sediment that has the potential to be reused as soil amendments in wetland rehabilitation projects. For various reasons, such as dredging for navigation, regular maintenance, or dam removal projects, this deposited sediment may need to be handled or removed from the floor of the impoundment.
The presence of a dam represents the current or historic need to impound water, often for drinking water reservoirs, flood control, or hydroelectric power generation, but also the occurrence of man-made influence on natural hydrology and associated ecosystems. Since the early 1900s, there have been documented 1797 dams removed across the USA, 69 of those occurred in 2020 alone (American Rivers 2021). Over 1200 dams (height over 6 m) in Tennessee create drinking water reservoirs, provide flood control, and facilitate the generation of hydroelectric power. There are thousands of low-head dams under 6 m that are not reported to the Tennessee Department of Environment and Conservation (TDEC) because they fall below the regulated height threshold or are used for agriculture or otherwise exempt use. Many of these dams no longer serve their intended function and have exceeded their life expectancy. Removal of low-head dams provides an opportunity to restore riparian wetlands for water quality improvement; however, the sediment deposits behind dams pose a challenge in terms of waste management and water quality protection in downstream reaches. The sediment has the potential to be reused as soil amendments in wetland rehabilitation projects (Lafrenz et al. 2013; Cornwell et al. 2020); however, additional information is needed to understand the full chemical characteristics of the sediment, as well as evaluate environmental risks it may pose as the physical setting of sediment changes with regard to placement in the riverine corridor.
Because of the hydrologic modification induced by dams, sediment accumulation behind the structure diminishes the available water storage capacity over time. The physical sediment management concerns and the environmental consequences associated with dam removal are numerous (Hart et al. 2002; Tullos et al. 2016). These include dam sediment deposit mobilization (Shuman 1995; Pizzuto 2002), water flow and retention time alteration (Poff and Hart 2002), shifts in nutrient (C, N, and P) budgets (Hillbricht-Ilkowska 1999; Margolis et al. 2001), and the release of sediment contaminated with organic contaminants (organochlorine pesticides, PAHs, and PCBs) and trace metals (Ashley et al. 2006; Evans and Gottgens 2007; Cantwell et al. 2014). In agricultural watersheds, fertilizer use and manure applications represent the primary sources of N and P in aquatic systems. Typically, accumulated dam sediments reduce N and P export from watersheds through N denitrification and particulate P settling (Stanley and Doyle 2002). Nutrient dynamics following dam removal and wetland re-establishment indicate that dam sediments leach ammonia and increase the annual N load to downstream reaches, while acting as a P sink (Ahearn and Dahlgren 2005). As reduced dam sediments are exposed and become oxidized though dam removal, metals may be mobilized (De Carvalho et al. 1998). As a result, available P may be immobilized by binding with mineral surfaces (e.g., iron oxides) in the water column (De Groot and Golterman 1993; Kleeberg and Heidenreich 2004). This suggests that dam sediments may be an efficient soil amendment for increased P retention in created wetlands.
Organochlorine pesticides, their residues, and degradation products (collectively termed organochlorine pesticide residues, OCPRs) are chlorinated hydrocarbons that have high toxicity, are bio-accumulative, and have long half-lives in the environment. The OCPRs include DDT (and metabolites DDE and DDD), dieldrin, aldrin, and endrin. Although the use of most OCPRs was banned in the USA in the 1970s and 1980s, these substances are still detected in sediments derived from agricultural, urban, and mixed-use (agricultural and urban) watersheds (Tate and Heiny 1996; Gilliom et al. 2006; Evans and Gottgens 2007; Phillips et al. 2010; Wang et al. 2012; Yang et al. 2012, 2015). Correspondingly, OCPRs are also detected in agricultural surface soils (Harner et al. 1999; Bidleman and Leone 2004; Wan et al. 2005). Of the OCPRs, the most frequently detected in sediments are p,p’-DDT, p,p’-DDD, p,p’-DDE, and dieldrin (Yang et al. 2012). Studies that examine OCPR and other organic contaminant redistribution following dam removal indicate that impact on downstream reaches may be minimal. Evans and Gottgens (2007) opined that dam removal would have a limited OCPR impact because sediment concentrations were less than or equal to those in downstream sediments. Davis et al. (2017) demonstrated that low-head dam removal decreased OCPR burdens in fish. Ashley et al. (2006) and Cantwell et al. (2014) found that organic contaminant concentrations (PAHs and PCBs) were not increased in downstream sediments following dam removal. However, these studies caution that each dam removal project should be assessed on a case-by-case basis when evaluating the potential for contaminated sediment redistribution.
It is crucial to gain an accurate and complete description of the existing site conditions behind low-head dams in order to reach success measures delineated for dam removal feasibility and wetland re-establishment projects (Doyle et al. 2003). The objective of this study was to assess the existing site conditions behind a low-head dam that has exceeded its life expectancy and is no longer providing intended services due to sediment accumulations. This study focused on (1) describing the nutrient status of the dam sediments, and the types and concentrations of OCPRs present from legacy use in the agricultural watershed, and (2) examining the potential long-term effect of dam removal on sediment nutrient and OCPR concentrations using a simulated laboratory weathering technique.
2 Materials and methods
2.1 Study area and sample collection
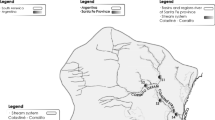
The 5.8-m high by 30-m-wide impoundment was constructed in 1968 in Valley View Hollow in Athens, Tennessee on Oostanaula Creek (OC), a tributary of the Hiwassee River of the Tennessee River Basin and the Ridge and Valley ecoregion. The reservoir was originally used as a primary drinking water source for Athens; it now serves as an emergency source. The impoundment location is 35.459 N, − 84.574 W at an elevation of 276 m, and an average monthly mean flow of 38 cfs (1.08 m3 s−1). The reservoir water surface is 4.19 m above the original creek channel; the water depth behind the impoundment varies between < 0.5 and 1.8 m because of sediment deposition. The estimated total volume of sediment (and sediment–water slurry) behind the impoundment is 21,096 m3. The reservoir receives sediment from the upper OC watershed, with an area of 55.1 km2 and a primary land use distribution of 48.2% evergreen, deciduous, or mixed forest; 39.2% pasture or hay; and 11.1% row crop. The soils in the upper OC watershed are residuum derived from Quaternary cherty clay solution residuum and Ordovician dolomitic limestone. The soils in the upper OC include Dewey (fine, kaolinitic, thermic Typic Paleudults), Etowah (fine-loamy, siliceous, semiactive, thermic Typic Paleudults), Fullerton (fine, kaolinitic, thermic Typic Paleudults), and Waynesboro series (fine, kaolinitic, thermic Typic Paleudults).
Sampling locations of sediment on the reservoir and surface soil samples in the upper OC watershed are shown in Fig. 1. Samples sites were selected to achieve a snapshot of characteristics across the spatial extent of the impoundment reservoir. Oostanaula Creek flow is impacted for a longitudinal distance of approximately 670-m upstream from the impoundment, creating a main stem reservoir as well as a small cove formed by a tributary channel from the north (Fig. 1). Sample locations were established throughout the main stem reservoir, beginning with a station at a distance of approximately 6-m upstream of the impoundment. Sample sites were established at increasing distances moving upstream through the reservoir at distances of approximately 6 m (cores 1, 2, and 3), 38 m (core 4), 76 m (cores 5, 6, and 7), 152 m (core 10), 305 m (cores 11, 12, and 13), 457 m (core 14), and 608 m (core 15) from the impoundment. A 3-station transect was used at the 6-, 76-, and 305-m stations to capture possible variability within the channel cross section. Two sample locations in the middle of the cove at approximately 140 m (core 9) and 164 m (core 8) from the impoundment were also established, creating 15 sample sites.
A motorized pontoon boat was used to access sample sites. The vessel had a relatively shallow displacement zone that minimized benthic disturbance in shallow (< 0.5 m) water and provided a stable platform to conduct sampling through the base of the boat. At each sample site, water depth was measured and one sample core was collected. Large debris (e.g., logs, sticks, rocks) was carefully cleared away from the substrate surface before sample cores were collected. A core sample comprised of a continuous vertical core of the substrate and water column at the sample location at the time of collection. The continuous core was collected in such a manner as to maintain the vertical profile of the substrate down to refusal depth, which was assumed to be the boundary layer of the sediment deposit at the top of the natural channel bottom.
Continuous cores were collected using a direct push probe (Geoprobe, Salina, KS, USA) with dual-tube tooling and 3.8-cm diameter acetate liner tubes in 122-cm length sections. The tooling was advanced into the substrate manually with a post-hole driver and with uniform effort until refusal was met. Once a tube section was fully driven, the sleeve containing the sample was extracted and capped at both ends with waterproof adhesive tape. The tooling remained driven in the substrate, keeping anything from falling into the excavated hole and minimizing the risk of cross-contamination between sample sections. Refusal in combination with an observation of tight clay in the base of the tube upon extraction was used to determine the termination depth of the sample core. All core sections were labeled and kept in a vertical position protected from direct sunlight until transported to the laboratory within 24 h.
The intact sediment cores were stored in the laboratory under refrigeration at 4 °C until dissected. For dissection, the cores were placed in a N2 glovebox and sectioned into 7.5-cm lengths using a Milwaukee M12 Plastic Pipe Shear. Sediment samples were then extruded from the sectioned cores into glass jars. The pH and redox potential of each water-saturated core subsample were then determined using a calibrated (pH 4 and 7) combination pH electrode (Fisherbrand Accumet) and Pt combination redox electrode (Fisherbrand Accumet) which was periodically tested for accuracy using a + 200 mV oxidation–reduction potential standard solution (Ricca Chemical Co., Arlington, TX, USA). The glass jars were then sealed, removed from the N2 glovebox, and stored under refrigeration at 4 °C. Watershed soil samples were collected following the procedure detailed by Huangfu et al. (2019). Briefly, the surface of 0 to 2 cm was collected and placed into paper bags. The sample locations represented forested areas, pasture, and row crop areas. The soil samples were air-dried, mixed, and disaggregated using a corundum mortar and pestle. The soil was then wet-sieved through a 53-μm sieve, and the < 53 μm suspension was freeze-dried and stored until needed.
2.2 Sediment extractions
Impoundment water at selected locations was obtained from cores prior to dissection. The water samples were filtered through 0.45-μm nylon syringe filters and refrigerated until analysis. The moisture content of the core samples was determined in duplicate by placing a ~ 1-g subsample into an aluminum tin. The tins were placed in an oven set to 105 °C and allowed to dry for 16 h. Once a constant oven-dry mass was obtained, the wet and oven-dry masses were used to calculate the moisture content. To each 20 g, water-saturated sediment sample (oven-dry basis) was added a sufficient amount of distilled-deionized (DDI) water to obtain a 1:1 solid-to-solution ratio. The suspensions were placed on a platform shaker for 1 h at ambient temperature (20–22 °C); then, the solid and solution phases were separated by vacuum filtration through Whatman 42 filter paper, then through a 0.45-μm nylon syringe filter. These extractions were performed in duplicate. The impoundment sediment–water extracts were refrigerated until analysis.
The Mehlich 3 extraction procedure is commonly used to assess the nutrient status of soil (Mehlich 1984). Specifically, it is employed to predict crop response to fertilizer applications. For the Mehlich 3 extraction procedure, water-saturated impoundment sediment samples (2.5 g on an oven-dry mass basis and threefold replication) were extracted in 25 mL of the Mehlich 3-extractant (0.2 M CH3COOH–0.25 M NH4NO3–0.015 M NH4F–0.013 M HNO3–0.001 M EDTA). Samples were shaken for 5 min and then filtered through Whatman 42 filters. The extracts were filtered further through 0.45-μm nylon syringe filters, and stored under refrigeration for analysis. The sediments were also subjected to nitric acid extraction for an estimation of the total elemental content and to compare to the metal content of watershed soils (Huangfu et al. 2019). Nitric acid digestion of the sediments was performed using the procedure of Chang et al. (1984). Sediment samples (3.5 g and in triplicate) were placed in 40-mL Pyrex digestion tubes with 21 mL of 4 M HNO3. The tubes were fitted with glass funnels, placed in a digestion block, and refluxed at 70 °C for 16 h. After cooling, the suspension volume was brought to 35 mL with DDI water, mixed, filtered sequentially through Whatman 42 filter paper and a 0.45-μm nylon membrane filter, and refrigerated for analysis.
2.3 Simulated weathering
The simulated weathering of impoundment sediment samples was performed according to the procedure of Essington (1991) and Abbott et al. (2001) to examine the potential long-term effects of sediment redistribution of chemical characteristics. Sediment cores 4, 7, 12, and 13 (Fig. 1) were dissected into 15-cm sections. The sediment in each section was extruded into a 22.9 cm × 7.6 cm × 14 cm polypropylene container (weathering cell). To each weathering cell was added a sufficient volume of DDI water to obtain a 1:1 solid-to-solution ratio. After mixing, the cells were allowed to sit undisturbed for 1 h and the pH and redox potential were determined. The leachate was then extracted by vacuum filtration through Whatman 42 filter paper and retained for chemical analysis. The solids were returned to their respective cells and allowed to air dry to a constant mass (approximately 7 days of drying time). Solid samples were taken for OCPR analysis and the saturation-extraction-drying cycle was repeated using a volume of DDI water required to obtain a 1:1 solid-to-solution ratio. This 7-day cycle was repeated 8 times; OCPR analysis of the sediments was performed during even-numbered weathering cycles.
2.4 Inorganic analyses
Impoundment sediment and sediment–water extracts were analyzed for anions and cations with a Dionex ICS-1100 Ion Chromatograph using a Dionex AS11-HC column for anions (Cl, NO2, NO3, and SO4) and a CG12A column for cations (Na, NH4, K, Mg, and Ca). Phosphate in the pond and sediment pore waters was determined with a Skalar San + + System. Sediment total carbon (TC) and total nitrogen (TN) were determined using a dry combustion technique with a Thermo Electron CN Analyzer. Pond water total carbon was measured with a Shimadzu TOC-VCPH Total Organic Carbon Analyzer. The HNO3 digestions were analyzed for Al, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, Se, Sr, V, and Zn with a Spectro CIROS inductively coupled argon plasma-atomic emission spectrometer (ICP-AES) using commercially available ICP-AES standards. The Mehlich 3 extracts were similarly analyzed for Ca, K, Mg, Mn, P, S, Cu, and Zn.
2.5 Organochlorine analyses
The method for extracting and analyzing OCPRs was modified from EPA methods 3540 and 8081 (USEPA 1996 and 2007). Duplicate 3-g subsamples of < 2 mm, air dry sediment, were placing in a glass extraction vessel (SIMAX, 100 mL) with 3 g of Na2SO4. A 5 ml volume of acetone was added, and the vessels were sealed, placed on a wrist-action shaker for 1 h, and then sonified for 20 min. A 10 mL aliquot of hexane was then added, the vessels sealed, and the shaking and sonification treatment was repeated. The suspensions were left to stand for 16 h at ambient temperature. The clear acetone + hexane mixture was removed from the sediment and placed into a glass centrifuge tube (50 mL, Corex Oak Ridge type). To the remaining sediment, 5 mL of hexane was added and the shaking and sonification treatment was repeated. The mixture was allowed to set for 2 h; then, the clear hexane was removed and combined with the hexane + acetone extract. The combined solvent extract mixture was centrifuged for 3 min to remove suspended sediment. The 13–15 mL of the clear solvent extract was removed and placed into a 40-mL glass flat-bottom centrifuge vial. To this, 1 g of granular Cu was added. The Cu suspension was shaken for 30 min to remove sulfur. The Cu additions were repeated in 1-g increments until the granules did not turn black. The sulfur-free solvent extract was separated from the granular Cu and placed in a 20-mL glass vial and the solvent evaporated under a constant stream of N2. After evaporating, the sample was re-dissolved in 2 mL of hexane, then filtered through a 0.45-μm nylon syringe filter into a 1.5-mL GC–MS auto-sampler vial.
The GC–MS analyses were performed using a Shimadzu GCMS QP2010S. A 1 μL aliquot of sample was injected at 225 °C in splitless mode. The carrier gas was He at a flow rate of 0.7 mL min−1. Separation of the OCPRs was performed with an RTx-5 ms Restek 30 m × 0.25 mm × 0.25 μm column. The oven temperature was programmed to 140 °C with a 0.5 min hold, ramping at 20 °C min−1 to 270 °C and a 5 min hold, then to 290 °C at 3 °C min−1 and a 2 min hold, and finally to 310 °C at 40 °C min−1 and a 3 min hold. The MS detector was operated in the SIM mode with a detector voltage of 0.1 kV, an ion source temperature of 260 °C, and an interface temperature of 300 °C. GSMS instrument calibration was performed using a standard mixture of 16 compounds obtained from Restek (P/N 32,022). The OCPR concentrations were determined using external calibration curves. The compounds, retention times, and target ion and fragment masses are listed in Table S1.
Selected soil and sediment samples were spiked with a surrogate standard (3,3’,4,4’,5,5’- hexachlorobiphenyl) to account for OCPR recovery. The recoveries ranged between 70 and 92%, with lower recoveries associated with higher organic carbon contents of soils and sediments. Method detection limits (MDLs) were determined for a select number of OCPRs. A hexane sample containing 4 μg L−1 aldrin and 8 μg L−1 p,p’-DDE, p,p’-DDD, and endrin aldehyde was prepared. This solution was analyzed 7 times by GC–MS using the procedure described above. The MDL values were determined by multiplying the standard deviation of the replicate analyses with the one-tailed t statistic for the 99% confidence interval. The 95% confidence interval about the MDL was derived according to the chi-square over degrees of freedom distribution. The MDLs ranged from 1.8 to 2.6 μg L−1 (1.6 to 2.3 μg kg−1) with a lower confidence limit of 1.2 μg L−1 (1.0 μg kg−1) and an upper confidence limit of 5.8 μg L−1 (5.2 μg kg−1).
3 Results and discussion
3.1 Impoundment water and sediment pore water analysis
Each core sample was represented by three components: ponded water that overlain the sediment deposit, the pore water within the sediment deposit, and the impounded sediment. The impounded and pore waters were analyzed for common inorganic constituents (Table 1). Sediment pH (6.61) was lower than that of the reservoir water (7.69). A majority of the samples were suboxic (14 > pe + pH > 9; O2 is depleted) to anoxic (pe + pH < 9; FeIII and MnV reduction occurs) (Fig. S1). Within the cores, the redox status did not vary with depth (Fig. S2). However, the redox status of core samples close to the dam, and those in the cove area, was generally poised by the FeII/FeIII couple (pe + pH ~ 9), whereas the redox status of cores at greater distances from the dam was more oxidized (near the oxic/suboxic border) (Fig. 2). The reservoir and sediment pore water chemistry were unremarkable and generally consistent with the medians and ranges of freshwater compositions (Davies and DeWiest 1966; Bowen 1979) (Table 1). The reservoir water chemistry was dominated by calcium and carbonate and controlled by calcite dissolution (Fig. S3). However, the sediment pore water was dominated by calcium and sulfate. Pore water also contained significantly higher concentrations of NH4-N, NO3-N, and SO4-S relative to the reservoir water (mean values of 5.82 vs. 2.98 mg L−1 for NH4-N; 6.56 vs. 0.91 mg L−1 for NO3-N; 84.2 vs. 0.19 mg L−1 for SO4-S), which may be associated with the degradation of organic matter in the sediment.
3.2 Inorganic impoundment sediment analysis
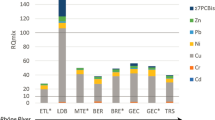
The HNO3-extractable elemental composition of the impounded sediments is similar to that of upper Oostanaula watershed soil and other soils in the region (Huangfu et al. 2019) (Table 2). Anoxic environments are commonly devoid of FeIII and MnV oxyhydroxides, which are scavengers for fugitive nutrients and other pollutants. The particularly low Fe content of the sediments relative to the surface soils indicates that FeIII oxyhydroxides have been reduced to soluble FeII in the suboxic/anoxic sediments. On average, Fe in the sediments was 1.44 g kg−1, compared to 22.2 g kg−1 in the watershed surface soils. Manganese concentrations were also significantly lower in the sediment; 1.06 g kg−1 compared to 1.55 g kg−1 in watershed soils. Correspondingly, significantly lower concentrations of elements that may associate with FeIII and MnV oxyhydroxides also displayed lower sediment concentrations relative to watershed soils, including, P, Co, and Cu.
The median soil test (Mehlich 3) levels of P and K were low (Duncan et al. 2019), indicating that the establishment of vegetation on sediments after dam removal would be difficult without fertilizer inputs. For P, 88% of the sediment samples tested low; for K, 83% tested low. Total carbon (TC) concentrations in the pond sediment ranged from below detection to 54 g kg−1 (Table 2). Total nitrogen (TN) concentrations ranged from below detection to 3.8 g kg−1 and were highly correlated to TC (r2 = 0.965, P < 0.0001; Fig. S4). On average, TN was 7.7% of TC. The higher TC (and TN) concentrations were associated with the more recently deposited sediments (Fig. S5), as concentrations decreased with increasing sediment depth.
3.3 OCPRs in the upper Oostanaula Creek watershed soils and impoundment sediments
There is limited information concerning the historical use of organochlorine pesticides in the upper Oostanaula watershed. Of the OCPRs examined (Table S1), only aldrin, dieldrin, p,p’-DDD, and endrin aldehyde were detected in watershed soils (Table 3). In general, the use of these pesticides in production agriculture in the USA and Canada ceased in the early 1970s. Both p,p’-DDD and endrin aldehyde were detected in all surface soil samples, with geometric means of 24.2 μg kg−1 and 23 9 μg kg−1. Aldrin was detected in 10 of the 12 samples, and dieldrin in 11, with geometric means of 2.4 μg kg−1 and 9.1 μg kg−1. The total concentrations of OCPRs (ΣOCPR) in the surface soils ranged from 35.6 to 151 μg kg−1, with a mean of 77.7 μg kg−1 (± 31.0 SD) and a median of 81.0 μg kg−1 (55.4–87.8 IQR). The concentrations of the detected OCPRs were not correlated with the current agronomic practices in the watershed.
The occurrence of the four OCPRs represents their past use or the past use of their parent compounds, in the upper Oostanaula watershed. Both aldrin and dieldrin were commonly used in corn production. While aldrin use was concentrated in the Midwestern U.S., dieldrin was used in the Southern U.S. in tobacco and cotton production (Jorgenson 2001). Dieldrin is also an alteration product of aldrin. Dieldrin has been a frequently detected OCPR in North American surface soils. Szeto and Price (1991) reported the sum and aldrin and dieldrin concentrations to range from 104 to 1280 μg kg−1 in muck soils, with mean aldrin and dieldrin levels of 78 and 692 μg kg−1 (these compounds were not detected in mineral soils). Meijer et al. (2003) reported 640 μg kg−1 (± 27 μg kg−1) and 9.3 μg kg−1 (± 1.2 μg kg−1) dieldrin and aldrin in muck soils, and 29 μg kg−1 (± 3.1 μg kg−1), and 0.54 μg kg−1 (± 0.046 μg kg−1) in mineral soils. Wan et al. (2005) also found aldrin in muck soils, with a mean concentration of 30 μg kg−1, but not in mineral soils. However, dieldrin was detected in both mineral and muck soils, with a mean concentration of 450 μg kg−1 and a range of < 20 to 2310 μg kg−1. Residual dieldrin concentrations in Southern U.S. soils reportedly range from < 0.02 to 23.8 μg kg−1 (Harner et al. 1999; Bidleman and Leone 2004), with higher concentrations (0.12 to 4246 μg kg−1) observed in Midwestern U.S. soils (Aigner et al. 1998). Endrin aldehyde is an alteration product of endrin (primarily used as an insecticide in tobacco and cotton production) and was never commercially manufactured for use as a pesticide (ATSDR 2021). The occurrence of endrin aldehyde residues in soils is not well documented. The parent compound, endrin, has been detected in high organic matter (muck) in Canadian soils. Mean endrin concentrations of 45 μg kg−1 (± 0.94 μg kg−1) were detected by Meijer et al. (2003) and Wan et al. (2005) found endrin to range from < 20 to 110 μg kg−1, with a mean of 70 μg kg−1. However, endrin concentrations in mineral soils were below detection. The use of DDT was extensive in production agriculture to control insects until it was banned in the USA in 1972 (Mansouri et al. 2017). The occurrence of the metabolite p,p’-DDD may be an artifact of DDT use in the Oostanaula watershed, although DDD was formulated and applied in the USA as a pesticide (ATSDR 2019). Commonly, DDT and DDE are detected in surface soil samples with DDD. However, only p,p’-DDD was detected in the Oostanaula watershed soils. Generally, p,p’-DDD is more prevalent in high organic matter soils, ranging from below detection to 6000 μg kg−1 (Szeto and Price 1991; Meijer et al. 2003; Wan et al. 2005). Concentrations of p,p’-DDD range from < 0.1 to 12.2 μg kg−1 in Southeastern U.S. soils (Harner et al. 1999; Bidleman and Leone 2004) and up to 460 μg kg−1 in Midwestern U.S. soils (Aigner et al. 1998).
In the impoundment sediments, p,p’-DDD and endrin aldehyde were frequently detected, while aldrin and dieldrin exceeded detection levels in a smaller number of samples (Table 3). The percent detection of the OCPRs were 33% aldrin, 53% dieldrin, 94% endrin aldehyde, and 100% p,p’-DDD. The DDT derivative, p,p’-DDE was detected in 12 of the 126 sediment samples (9.5% detection), with an average concentration of 2.9 μg kg−1 in the 12 samples. p,p’-DDD is a direct degradation product of DDT, and a dehalogenation product of DDE, in anaerobic environments (Huang et al. 2001; Mansouri et al. 2017). This accounts for the prevalence of p,p’-DDD in the suboxic pond sediments. The average concentrations of the two commonly detected compounds, p,p’-DDD and endrin aldehyde, were 33.7 ± 26.2 μg kg−1 and 62.5 ± 47.5 μg kg−1. Endrin aldehyde is the dominant OCPR detected in the sediments. The frequency distribution of the four OCPRs illustrates that aldrin and dieldrin concentrations are skewed towards the lower concentration levels, while p,p’-DDD and endrin aldehyde are skewed towards higher (Fig. 3). The geometric mean concentrations of the four OCPRs is 0.39 μg kg−1 aldrin, 1.62 μg kg−1 dieldrin, 22.9 μg kg−1 p,p’-DDD, and 39.0 μg kg−1 endrin aldehyde.
The threshold effect concentration (TEC) is a sediment quality guideline, which indicates the concentration below which adverse effects on aquatic organisms are not expected to occur. Adverse effects (incidence of toxicity) on the majority of sediment-dwelling organisms are expected to occur frequently when the probable effect concentration (PEC) is exceeded for freshwater ecosystems (MacDonald et al. 2000). The TEC and PEC values for the commonly detected OCPRs are shown in Table 3. Dieldrin and endrin aldehyde concentrations frequently exceeded TECs (52% for dieldrin; 91% for endrin aldehyde) but never exceeded the PECs in the sediment samples; p,p’-DDD always exceeded TEC and exceeded PEC in 49% of the core samples. Aldrin does not exceed the severe effect level established by Persaud et al. (1993) in any of the sediment samples.
The ΣOCPR of the sediments ranged from 21.4 to 340 μg kg−1, with a mean of 117 μg kg−1 (± 77.4 SD), a geometric mean of 94.7 μg kg−1, and a median of 98.1 μg kg−1 (54.1–160 IQR). The sediment OCPR concentrations do not vary is a function of distance from the dam (Fig. 4). Furthermore, the sediment OCPR concentrations were generally not a function of sediment depth, although endrin aldehyde and ΣOCPR were found to decrease with increasing depth in a small number of cores (Figs. S6–S9). With the exception of p,p’-DDD, the concentrations of extractable OCPRs was also significantly correlated to the total carbon content of the sediments, which is consistent with the hydrophobic character of the OCPRs and with their high partitioning coefficients with organic matter (Fig. S10). Statistically, the mean concentrations of aldrin, dieldrin, and p,p’-DDD in the sediments were not different than their values in the watershed soils. This suggests that the watershed soils may be a constant source of sediment OCPRs and that the degradation rates of aldrin, dieldrin, and p,p’-DDD do not vary between the different environments. However, endrin aldehyde was enriched in the sediments relative to the surface soils, as was ΣOCPR.
The detection of OCPRs in the Oostanaula Creek impoundment sediments is consistent with the findings of other studies that analyze aquatic sediments. In a survey of more than 230 streambed sediments impacted by agriculture in the USA, Gilliom et al. (2006) detected 24 out of 31 targeted OCPRs. DDT and derivatives were frequently detected (49% detection of total DDTs), with p,p’-DDD detected in 31% of the samples. Dieldrin (detected 17% of samples), endrin (1.3%), and aldrin (0.8%) were also detected. Surveys that are more recent indicate that OCPRs still persist in freshwater sediments. Carriger et al. (2006) analyzed sediment samples from Florida freshwater canals and identified DDD as one of the OCPRs with the highest potential chronic risk to arthropods. Wang et al. (2012) examined sediments from the Salton Sea area of California for 14 OCPRs. They detected OCPRs in all samples, with DDT derivatives and dieldrin among those occurring in the greatest concentrations, with DDE frequently exceeding the TEC and PEC levels. Yang et al. (2015) examined riverbed sediments in Florida and detected 10 of the 20 targeted OCPRs.
3.4 Simulated weathering
Sediment samples from cores 4 and 7 (57 m from the dam), and cores 12 and 13 (305 m from the dam) were grouped into the top (0 to 98 cm or 0 to 70 cm in depth) and bottom (98 to 194 cm and 70 to 106 cm in depth) segments and exposed to eight simulated weathering cycles. During the simulated weathering, leachate pH was generally invariant; averaging 7.1 (Fig. S11). The redox status of the leachates was suboxic during the initial weathering cycles, becoming oxidized as weathering progressed. In general, the concentrations of common salts in the sediment leachates either decreased or did not vary with weathering (Figs. 5 and 6). The salts with decreasing leachate concentrations with weathering were Ca, Mg, SO4, Cl, and NO3. After cycles 4 and 5, leachate concentrations of Mg, Cl, and NO3 achieved constant levels and did not vary with additional leaching. The average NO3-N concentration in the cycle 4 through 8 leachates was 1.5 mg L−1. The leachate concentrations of Na, K, PO4, and NH4 (in the deep sediments) generally did not vary during weathering. Calcium sulfate was the dominant soluble salt throughout the study. However, increases in NH4 concentrations in the shallow sediment leachates after cycle 2 were observed, which may be associated with the nature and quality of organic residues present. Furthermore, the concentrations of NH4 in the deep sediment leachates exceeded those of Ca near the end of the weathering study, a result of the active degradation of sediment organic matter. In these systems, NH4 was the dominant cation in the cycle 7 and 8 leachates, averaging 2 mg L−1 NH4-N in the shallow sediment samples. In the deep sediment leachates, NH4-N averaged 4.8 mg L−1 over all weathering cycles. Although both NO3-N and NH4-N are continually released during sediment weathering, their concentrations in the sediment leachates are statistically similar to those in the reservoir water. However, these concentrations exceed those documented by TDEC for ambient water quality in the upper Oostanaula, which range from 0.04 to 0.07 mg L−1 NH4-N and 0.10 to 0.43 mg L−1 NO3-N (https://tdeconline.tn.gov/dwr/, accessed Dec. 2019). Thus, the sediments may act as a continual source of N after dam removal (Ahearn and Dahlgren 2005).
Analyses were conducted to investigate the stability of OCPRs during oxidative weathering (Fig. 7). During the 8-week simulated weathering study, the sediment concentrations of dieldrin, aldrin, and endrin aldehyde did not significantly vary with depth or distance from the impoundment. The median concentrations of dieldrin, aldrin, and endrin aldehyde for all sediments and all cycles were 8.69 μg kg−1 (4.67–14.00 μg kg−1 IQR), 1.66 μg kg−1 (< 0.1–5.16 μg kg−1 IQR), and 29.0 μg kg−1 (12.3–53.2 μg kg−1 IQR). However, p,p’-DDD concentrations significantly decreased from a median of 56.5 μg kg−1 (41.9–67.1 μg kg−1 IQR) for cycles 2 and 4 (all sediments), to a median of 15.4 μg kg−1 (12.6–20.6 μg kg−1 IQR) for cycles 6 and 8 (all sediments). Although the cycles 6 and 8 p,p’-DDD concentrations still exceed the TEC, they are below the PEC (Table 3). These results indicated that there is a potential for the OCPRs to have a long-term impact on the reclamation of sediment from the low-head dam and that oxidation of the suboxic sediments may facilitate p,p’-DDD degradation (ATSDR 2019).
4 Conclusions
It is crucial to gain an accurate and complete description of the existing site conditions behind low-head dams in order to reach success measures delineated for dam removal feasibility and wetland re-establishment projects that utilize accumulated dam sediments. This study evaluated the nutrient status and the types and concentrations of OCPRs in sediments behind a low-head dam that had exceeded its life expectancy and was no longer providing the intended services due to sediment accumulation. This study also examined the potential long-term effect of dam removal on sediment nutrients and OCPR concentrations using a simulated laboratory weathering technique. Dam sediments were nutrient (P and K)-poor, but may continually release N as NH4 and NO3 during oxidative weathering, with pore water concentrations that are similar to those in the reservoir water, but greater than those in the upper Oostanaula Creek. The watershed soils and dam sediments contain OCPRs derived from legacy use in the agricultural watershed that ceased approximately 50 years prior. Relative to the sediment quality guidelines, the sediments may have harmful effects on sediment-dwelling organisms. Dieldrin exceeded the TEC in 52% of the sediment samples, while endrin aldehyde concentrations exceeded the TEC in 91% of samples. The concentrations of these OCPRs never exceeded the PECs. The p,p’-DDD concentrations always exceeded the TEC and exceeded the PEC in 49% of the core samples. With the exception of p,p’-DDD, the oxidation of the sediments during simulated weathering did not alter OCPR concentrations. Weathering reduced the concentration of p,p’-DDD to below the PEC value. Based on the simulated weathering study, there is a potential for the persistent OCPRs to have a long-term implications for the utilization of the sediment in stream reclamation and wetland rehabilitation following low-head dam removal.
Although this was a localized study, the results lead to a recommended protocol for assessing existing site conditions and sediment quality in a low-head dam shadow. A detailed land use inventory of the contributing watershed, identifying the key constituents of concern based on historical land use activities is required. The dam sediment deposit volume and the chemical characteristics of the potential mobile sediments would then drive the risk evaluation associated with the released or reused sediments in wetland restoration, and determine the best management practices needed to address the identified risks.
References
Abbott DE, Essington ME, Mullen MD, Ammons JT (2001) Fly ash and lime-stabilized biosolid mixtures in mine spoil reclamation: simulated weathering. J Environ Qual 30:608–616. https://doi.org/10.2134/jeq2001.302608x
Ahearn DS, Dahlgren RA (2005) Sediment and nutrient dynamics following a low-head dam removal at Murphy Creek, California. Limnol Oceanogr 50:1752–1762. https://doi.org/10.4319/lo.2005.50.6.1752
Aigner EJ, Leone AD, Falconer RL (1998) Concentrations and entantiomeric ratios of organochlorine pesticides in soils from the U.S. corn belt. Environ Sci Technol 32:1162–1168. https://doi.org/10.1021/es970750h
American Rivers (2021) American Rivers Dam Removal Database. figshare. Dataset. https://doi.org/10.6084/m9.figshare.5234068. v8. Accessed 18 June 2021
Ashley JTF, Bushaw-Newton K, Wilhelm M, Boettner A, Drames G, Velinsky DJ (2006) The effects of small dam removal on the distribution of sediment contaminants. Environ Monit Assess 114:287–312. https://doi.org/10.1007/s10661-006-4781-3
ATSDR (2021) Toxicology profile for endrin. Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services
ATSDR (2019) Toxicology profile for DDT, DDE, and DDD. Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services
Bidleman TF, Leone AD (2004) Soil-air exchange of organochlorine pesticides in the Southern United States. Environ Pollut 128:49–57. https://doi.org/10.1016/j.envpol.2003.08.034
Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, London
Cantwell MG, Perron MM, Sullivan JC, Katz DR, Burgess RM, King J (2014) Assessing organic contaminant fluxes from contaminated sediments following dam removal in an urbanized river. Environ Monit Assess 186:4841–4855. https://doi.org/10.1007/s10661-014-3742-5
Carriger JF, Rand GM, Gardinali PR, Perry WB, Tompkins MS, Fernandez AM (2006) Pesticides of potential ecological concern in sediment from South Florida canals: an ecological risk prioritization for aquatic arthopods. Soil Sediment Contam 15:21–45. https://doi.org/10.1080/15320380500363095
Chang A, Warneke J, Page A, Lund L (1984) Accumulation of heavy metals in sewage sludge-treated soils. J Environ Qual 13:87–91. https://doi.org/10.2134/jeq1984.00472425001300010016x
Cornwell JC, Owens MS, Staver LW, Stevenson JC (2020) Tidal marsh restoration at Poplar Island I: transformations of estuarine sediments into marsh soil. Wetlands 40:1673–1686. https://doi.org/10.1007/s13157-020-01294-5
Davies SN, DeWiest RCM (1966) Hydrogeology. Wiley-Interscience, NY
Davis RP, Sullivan SMP, Stefanik KC (2017) Reductions in fish-community contamination following lowhead dam removal linked more to shifts in food-web structure than sediment pollution. Environ Pollut 231:671–680. https://doi.org/10.1016/j.envpol.2017.07.096
De Carvalho PSâM, Zanardi E, Buratini SVâ, Lamparelli MCâ, Martins MC (1998) Oxidizing effect on metal remobilization and Daphnia similis toxicity from a Brazilian reservoir sediment suspension. Water Res 32:193–199. https://doi.org/10.1016/S0043-1354(97)00186-3
De Groot CJ, Golterman HL (1993) On the presence of organic phosphate in some Camargue sediments: evidence for the importance of phytate. Hydrobiologia 252:117–126. https://doi.org/10.1007/s10533-006-9049-z
Doyle MW, Harbor JM, Stanley EH (2003) Toward policies and decision-making for dam removal. Environ Manag 31:453–465. https://doi.org/10.1007/s00267-002-2819-z
Duncan, L, Savoy H, Florence R, McClure A, Raper T, Walker F, Essington M, Buschermohle M, Jagadamma S, Hawkins S, Tyler D, Allen G, Qualls D, Wilson J (2019) UT fertilizer recommendation development. University of Tennessee Extension Report W795
Essington ME (1991) Laboratory weathering of combusted oil shale. J Environ Qual 20:794–801. https://doi.org/10.2134/jeq1991.00472425002000040014x
Evans JE, Gottgens JF (2007) Contaminant stratigraphy of the Ballville Reservoir, Sandusky River, NW Ohio: implications for dam removal. J Great Lakes Res 33:182–193. https://doi.org/10.3394/0380-1330(2007)33[182:CSOTBR]2.0.CO;2
Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP, Wolock DM (2006) The quality of our nation’s waters: pesticides in the nation’s streams and groundwater, 1992–2001. U.S. Geol Surv Circ 1291
Harner T, Wideman JL, Jantunen LMM, Bidleman TF, Parkhurst WJ (1999) Residues of organochlorine pesticides in Alabama soils. Environ Pollut 106:323–332. https://doi.org/10.1016/S0269-7491(99)00110-4
Hart DD, Johnson TE, Bushaw-Newton KL, Horwitz RJ, Bednarek AT, Charles DF, Kreeger DA, Velinsky DJ (2002) Dam removal: challenges and opportunities for ecological research and river restoration. Bioscience 52:669–681. https://doi.org/10.1641/0006-3568(2002)052[0669:DRCAOF]2.0.CO;2
Hillbricht-Ilkowska A (1999) Shallow lakes in lowland river systems: role in transport and transformations of nutrients and in biological diversity. Hydrobiologia 408–409:349–358. https://doi.org/10.1023/A:1017034813729
Huang HJ, Liu SM, Kuo CE (2001) Anaerobic biodegradation of DDT residues (DDT, DDD, and DDE) in estuarine sediment. J Environ Sci Health B36:273–288. https://doi.org/10.1081/PFC-100103569
Huangfu Y, Essington ME, Hawkins SA, Walker FR, Schwartz JS, Layton AC, Hull RA (2019) Evaluation of nitric acid extraction of elements from soils and sediments in two watersheds in east Tennessee. Commun Soil Sci Plant Anal 50:1358–1369. https://doi.org/10.1080/00103624.2019.1614611
Jorgenson JL (2001) Aldrin and dieldrin: a review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ Health Perspect 109(Supplement 1):113–139. https://doi.org/10.1289/ehp.01109s1113
Kleeberg A, Heidenreich M (2004) Release of nitrogen and phosphorus from macrophyte stands of summer dried out sediments of a eutrophic reservoir. Archiv Hydrobiologie 159:115–136. https://doi.org/10.1127/0003-9136/2004/0159-0115
Lafrenz MD, Bean RA, Uthman D (2013) Soil ripening following dam removal. Phys Geogr 34:124–135. https://doi.org/10.1080/02723646.2013.799033
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31. https://doi.org/10.1007/s002440010075
Mansouri A, Cregut M, Abbes C, Durand MJ, Landoulsi A, Thouand G (2017) The environmental issues of DDT pollution and bioremediation: a multidisciplinary review. Appl Biochem Biotechnol 181:309–339. https://doi.org/10.1007/s12010-016-2214-5
Margolis BE, Castro MS, Raesly RL (2001) The impact of beaver impoundments on the water chemistry of two Appalachian streams. Can J Fish Aquat Sci 58:2271–2283. https://doi.org/10.1139/f01-166
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Meijer SN, Shoeib M, Jantunen LMM, Jones KC, Harner T (2003) Air-soil exchange of organochlorine pesticides in agricultural soils. 1. Field Measurements Using a Novel in Situ Sampling Device Environ Sci Technol 37:1292–1299. https://doi.org/10.1021/es020540r
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management aquatic sediment quality in Ontario. Water Resources Branch, Ontario Ministry of the Environment, Toronto, 27
Phillips PJ, Nowell LH, Gilliom RJ, Nakagaki N, Murry KR, VanAlstyne C (2010) Composition, distribution, and potential toxicity if organochlorine mixtures in bed sediments of streams. Sci Total Environ 408:594–606. https://doi.org/10.1016/j.scitotenv.2009.09.052
Pizzuto J (2002) Effects of dam removal on river form and process. Bioscience 52:683–691
Poff NL, Hart DD (2002) How dams vary and why it matters for the emerging science of dam removal. BioScience 52:659–668. https://doi.org/10.1641/0006-3568(2002)052[0659:HDVAWI]2.0.CO;2
Shuman JR (1995) Environmental considerations for assessing dam removal alternatives for river restoration. Reg Rivers: River Manag 11:249–261. https://doi.org/10.1002/rrr.3450110302
Stanley EH, Doyle MW (2002) A geomorphic perspective on nutrient retention following dam removal. BioScience 52:693–701. https://doi.org/10.1641/0006-3568(2002)052[0693:AGPONR]2.0.CO;2
Szeto SY, Price PM (1991) Persistence of pesticide residues in mineral and organic soils in the Fraser Valley of British Columbia. J Agric Food Chem 39:1679–1684. https://doi.org/10.1021/jf00009a027
Tate CM, Heiny JS (1996) Organochlorine compounds in bed sediment and fish tissue in the South River Basin, USA, 1992–1993. Arch Environ Contam Toxicol 30:62–78. https://doi.org/10.1007/BF00211330
Tschantz BA, Wright KR (2011) Hidden dangers and public safety at low-head dams. J Dam Safety 9:9–17
Tullos DD, Collins MJ, Bellmore JR, Bountry JA, Connolly PJ, Shafroth PB, Wilcox AC (2016) Synthesis of common management concerns associated with dam removal. J Am Water Resource Assoc 52:1179–1206. https://doi.org/10.1111/1752-1688.12450
USEPA (1996) Method 3540c: soxhlet extraction. Available at https://www.epa.gov/sites/production/files/2015-12/documents/3540c.pdf. Accessed 18 Dec 2019
USEPA (2007) Method 8081b: organochlorine pesticides by gas chromatography. Available at https://www.epa.gov/sites/production/files/2015-12/documents/8081b.pdf. Accessed 18 Dec 2019
Wan MT, Kuo J, Pasternak J (2005) Residues of endosulfan and other organochlorine pesticides in farm areas of the Lower Fraser Valley, British Columbia, Canada. J Environ Qual 34:1186–1193. https://doi.org/10.2134/jeq2004.0361
Wang W, Delgado-Moreno L, Conkle JL, Anderson M, Amrhein C, Ye Q, Gan J (2012) Characterization of sediment contamination patterns by hydrophobic pesticides to preserve ecosystem functions of drainage lakes. J Soils Sediments 12:1407–1418. https://doi.org/10.1007/s11368-.12-0560-7
Yang L, Li X, Zhang P, Melcer ME, Wu Y, Jans U (2012) Concentrations of DDTs and dieldrin in Long Island Sound sediment. J Environ Monit 14:878–885. https://doi.org/10.1039/c2em10642f
Yang YY, Toor GS, Williams CF (2015) Pharmaceuticals and organochlorine pesticides in sediments of an urban river in Florida, USA. J Soils Sediments 15:993–1004. https://doi.org/10.1007/s11368-015-1077-7
Acknowledgements
We also thank Ms. Galina Melnichenko and Ms. Melanie Stewart of the Biosystems Engineering and Soil Sciences Department’s Soil and Water Quality Laboratory for their expertise in analyzing the soil and sediment samples.
Funding
Financial support of this research by USEPA project number A16-0345 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Jan Schwarzbauer
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Essington, M.E., Ludwig, A.L., Essington, E.A. et al. Persistence of organochlorine pesticide residues in sediments derived from an agricultural watershed in Tennessee, USA. J Soils Sediments 22, 1852–1864 (2022). https://doi.org/10.1007/s11368-022-03220-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03220-0