Abstract
Acid rain is a serious environmental problem in the world and is of a particular concern in southern China where most of the soils are acidic. This study investigated the dynamics of cations, phosphorus (P), and soil organic matter (SOM) in the Latosol (acidic red soil) from south China under the influences of simulated acid rain (SAR). Laboratory experiments were performed by leaching the soil columns with SAR at pH levels ranging from 2.5 to 7.0 over a 21-day experimental period. Results show that about 34, 46, 20, and 77% of the original exchangeable soil Ca+2, Mg+2, K+, and Na+, respectively, were leached out by the SAR at pH 2.5 after 21 days. Two distinct patterns of the available phosphorus (AP) concentrations were observed: one at pH≤3.5 and the other at pH ≥ 4.0. At pH≤3.5, concentrations of the AP increased from the beginning of the experiments to day 5, then decreased from day 5 to 15, and finally increased from day 15 to the end of the experiments. At pH ≥ 4.0, concentrations of the AP increased consecutively from the beginning of the experiments to day 10 and decreased from day 10 to the end of the experiments. Such a finding is useful for agricultural practices since soil P is one of the most important macronutrients for plant growth. In general, SOM content decreased with time as the Latosol was leached by the SAR at all pH levels. A maximum concentration of soil fulvic acid was found after 15 days of the experiments due to the degradation of the SOM. A multiple regression analysis showed that a very strong relationship was obtained between the soil AP and the other three parameters (i.e., pH, SOM, and sorption P).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acid rain is a collective term used to describe acids falling out of the atmosphere. A more precise term is the acid deposition, which includes both wet and dry depositions (US-EPA 2004). Wet deposition refers to acidic rain, fog, and snow. Dry deposition refers to acidic gases and particles. About 50% of the acidity in the atmosphere falls down to the earth through the dry deposition (US-EPA 2004). Acid rain is a serious environmental problem in the world. It kills fish and aquatic plants, causes deterioration of materials in lands, and is harmful to human beings. Acid rain with SO2, NOx, and NH3 may form secondary pollutants such as particles and nitrogen species that could react with organic compounds and contribute to the ozone (O3) formation (Menz and Seip 2004). In China, acid rain is mainly distributed in the areas of the Yangtze River to the south, Qinghai-Tibet Plateau to the east, and in Sichuan Basin. In recent years, a large area of acid rain with a pH ranging from 3.0 to 5.6 was found in southern China. According to the environmental protection and monitoring agencies in Guangdong Province in south China, about 50% of the rainfalls in that province are acid rains and the direct economical loss due to these acid rains is estimated to be $4.4 billion US dollars (Guo 2002). Acid rain is a particular environmental concern in southern China because most of the soils in that part of China are acidic.
Effect of acid rain on soil cation leaching loss is an increasing agricultural and environmental concern. Acid rain can mobilize cations from the soil. The H+ ion in the acidic water displaces the cations from their binding sites, reduces the cation exchange capacity, and increases the concentrations of these cations in the soil water (Brady 1984, Liu et al. 1990). The negatively charged sulphate and nitrate ions in the acid rain can act as “counterions,” which allow cations to be leached from the soil (Ivring 1983). Through a series of chemical reactions, cations are leached out and become unavailable to plants as nutrients. Acid rain can also increase the weathering of silicate minerals in soils, which leads to a loss of mineral structure and possibly reduced soil fertility (Brady 1984).
Phosphorus (P) is an essential macronutrient for plant growth. One of the major roles of P in living organisms is the transfer of energy. Sufficient P availability in the soil stimulates early plant growth and accelerates maturity. Although P is an important macronutrient, inappropriate management of soil P could pose an environmental threat to water quality. To our knowledge, little study has been devoted to investigating the availability of P in the soil due to the impact of acid rains.
Soil organic matter (SOM) is an essential component of soils that provides a carbon and energy source for soil microbes, stabilizes soil particles for preventing soil from erosion, assists plant growth by improving soil water and aeration conditions, and retains and supplies nutrients through ion exchanges. A comprehensive literature search reveals that very little attention has been given to investigate the impacts of acid rains on SOM dynamics. Calace et al. (2001) showed that the structure composition of fulvic acid from organic matter changes with increased soil acidification and a shift toward smaller molecular weight compounds was observed due to the breakup of the original molecules from SOM under acidic conditions.
Despite the fact that numerous efforts have been devoted to investigating the impact of acid rain on soil cation exchange and mineral weathering during the last two decades (Liao et al. 1997, Hodson and Landan 1999, Duan et al. 2002), our knowledge of the role of acid rain on soil property variations is still fragmented and often inconsistent. The purpose of this study was to evaluate the impact of simulated acid rain (SAR) on cations, P, and SOM dynamics in the Latosol from south China. Latosol is a red acidic soil that situates in the tropical rainforest biome where high temperature and high precipitation occur throughout the year. This soil has a loose structure and suffers from rapid erosion during heavy rainfall, which results in the leaching of nutrients and metals. Latosol is commonly distributed in south China such as Guangdong, Yunnan, and Hainan provinces.
Materials and Methods
The top 20 cm of Latosol collected from a Mango garden located in Huguang District of Zhangjiang City, Guangdong Province, China, was used for the experiments. This soil has a pH level of 4.7, an organic matter content of 21.0 g kg−1, a cation exchange capacity (CEC) of 18.8 cmol kg−1, and a base saturation of 35.6%. The initial total soil P was 0.56 g kg−1. Analytical grades of sulfuric acid (H2SO4) and nitric acid (HNO3) were purchased from Guangzhou Chemical Reagent Inc. Guangdong, China.
Rainwater in equilibrium with carbon dioxide (CO2) in air with no other species affecting pH is slightly acidic (pH > 5.6). However, rainwater is often more acidic due to the natural emissions of SO2, NOx, or organic acids. Typical pH values of acid rain resulting from anthropogenic emissions may be in a range of 3.5–5.0 (Menz and Seip 2004). In South China, however, the acid rain pH can be as low as 3.0, which primarily consists of H2SO4 and HNO3 with a ratio of 9 to 1. In order to have the SAR reflecting the natural conditions, the stock acid solution was prepared by using this ratio. The working solutions of acid rain with pH 2.5, 3.0, 3.5, 4.0, 4.5, and 5.0 were prepared in the volumetric flasks by diluting the stock solution with deionized (DI) water, whereas the working solution with pH 7.0 as a control or natural rain is prepared by adding 0.1 mL of ammonia (16.8% N) into 5000 mL of DI water.
A plastic cylinder with a 10-cm inner diameter was used to contain a 20-cm-long soil column. Then, 1000 g air-dried soil, passed through a 1-mm sieve, and mixed thoroughly, was poured into the cylinder in 2-cm increments and stirred to prevent layering. The column was tapped to settle the soil to a bulk density of 1.6 g cm−3. Prior to and after filling the column, a piece of plastic filter and two pieces of paper filters were placed at both ends of the column to prevent the leakage of the soil (Fig. 1). A total of 28 column treatments, i.e., 7 pH levels × 4 leaching time periods (at days of 5, 10, 15, and 21) with triplicates for each treatment, were carried out in this study. To reflect the natural rainfall conditions, an intermittent influent application method was employed. That is, a 250-ml influent of the SAR was slowly sprayed at a rate of 6 ml min−1 to the top of the column each day. After the column leaching experiment was terminated, the soil in the column was air-dried and analyzed for cations with an atomic adsorption spectrophotometry (ACSSSC 1983), for AP with 0.5 mol l−1 NaHCO3 extract method (NSICS 1978), for adsorption and desorption P with 0.01 mol l−1 CaCl2 method (Yan 1988), and for SOM with potassium dichromate volumetric method (NSICSA, 1978).
All of the experimental procedures and sampling analysis were performed using conventional methods (Jackson et al. 1984). Statistical analysis was performed in Excel with F-test at a significant level of α = 0.01.
Results and Discussion
Soil Cation Dynamics
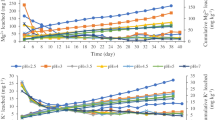
Changes in concentrations of soil exchangeable Ca+2 and Mg+2, after leaching with the SAR at seven different pH levels over a 21-day experimental period, are shown in Figure 2. Concentrations of exchangeable Ca+2 increased from 55.2 mmol kg−1 at the beginning of the experiments to 57.74–59.34 mmol kg−1 at different pH levels after 5 days and then decreased exponentially from day 5 to the end of the experiments. A statistical analysis with F-test showed that differences in concentrations of exchangeable Ca+2 and Mg+2 were significant at F0.01 = 3.14. In general, concentrations of exchangeable Ca+2 decreased with time at all pH levels (Fig. 2a). The maximum decrease in Ca+2 concentration was found at pH 2.5 after 21 days of the experiments and was about 18.8 mmol kg−1 (i.e., 55.2–36.4 = 18.8). This was equivalent to about 34% of the original soil exchangeable Ca+2 leached out by the SAR. This occurred because more Ca+2 ions were displaced by the H+ ions under such strong acidic conditions.
Similar results were obtained for Mg+2 (Fig. 2b) as compared to those of Ca+2. That is, concentrations of soil-exchangeable Mg+2 decreased with time at all pH levels. About 46% of the original exchangeable Mg+2 was leached out by the SAR at pH 2.5 after 21 days. This occurred due to the same reason as in the case of Ca+2.
Changes in concentrations of soil-exchangeable K+ and Na+, after leaching with SAR at seven different pH levels, are shown in Figure 3. Analogous to the cases of Ca+2 and Mg+2, concentrations of soil-exchangeable K+ and Na+ decreased with time at all pH levels. It is also apparent that the decrease in Na+ concentration was much steeper than that of K+. For instance, the maximum concentration decrease in Na+ was about 0.37 mmol kg−1 at pH 2.5 after 21 days (Fig. 3a) but in K+ was about 2.01 mmol kg−1 at the same pH level and time period (Fig. 3b). That is, about 20 and 77% of the original exchangeable Na+ and K+ were, respectively, leached out by the SAR. Results suggest that leaching loss of soil-exchangeable K+ by the acid rain was a series problem as K+ is a macronutrient for plant growth.
Soil P Dynamics
Changes in concentrations of soil AP with time, after leaching with SAR at different pH levels, are shown in Figure 4. The leaching experiments started with an initial soil AP concentration of 2.35 g kg−1 and no AP was detected in the effluent samples. Two distinct patterns of the AP concentrations were observed, one for the SAR at pH 2.5–3.5 (Fig. 4a) and the other for the SAR at pH 4.0–7.0 (Fig. 4b). At pH 2.5–3.5, concentrations of the AP increased from the beginning of the experiments to day 5, then decreased from day 5 to 15, and finally increased from day 15 to the end of the experiments. Although variations in AP concentrations were only within 0.4 g kg−1, an F-test showed that such variations were statistically significant at α = 0.01. The mechanisms for the changes in AP are complex and involved a variety of compounds. The increase in AP concentrations within the first 5 days did not result from desorption of soil P, which is confirmed in Figure 5a. Figure 5 shows that concentrations of the desorption P decreased within the first 5 days at all pH levels. A possible explanation of this phenomenon could be the release of the AP from the decomposition of soil organic matter (Fig. 6a) when the soil was leached with the low pH SAR.
A decrease in AP concentrations from day 5 to 15 occurred because of P reaction with soil cations. For acidic soils like Latosol, Aluminum (Al) is a dominant ion that reacts with P to form amorphous Al and iron (Fe) phosphates as well as Ca phosphates. These reactions result in very insoluble compounds of phosphate. An increase in AP concentrations after day 15 could again be due to the decomposition of SOM (Fig. 6a) although the exact reasons remain unknown.
In contrast, concentrations of the AP increased continuously from the beginning of the experiments to day 10 and decreased from day 10 to the end of the experiments at pH ≥ 4.0 (Fig. 4b). It is apparent that changes in AP concentrations depended not only on soil organic matter decomposition and formation of amorphous Al and Fe phosphates but also on the SAR pH levels. Figure 4b further reveals that the peak concentrations of the AP were at day 5 at pH≤3.5 and at day 10 at pH ≥ 4.0. Such findings are useful for agricultural practices since P is one of the most important macronutrients for plant growth.
Variations in desorption and adsorption P concentrations after leaching with the SAR are shown in Figure 5. Figure 5 demonstrates that both desorption and adsorption P concentrations decreased within the first 5 days and increased from day 5 to day 10 (except for pH 4.0 in Fig. 5a). Under normal soil conditions, a decrease in soil desorption P would cause an increase in soil adsorption P. Our study showed that desorption of soil P by the SAR was not adsorbed onto the soils and may probably be released into the soil solution.
After 10 days of the experiments, mixed results were observed for desorption P (Fig. 5a). That is concentrations of the desorption P decreased from day 10 to the end of the experiments for the SAR with pH 4.0, 4.5, and 5.0, whereas concentrations of the desorption P increased from day 10 to 15 and decreased from day 15 to the end of the experiments for the SAR with pH 3.5 and 7.0. In addition, the concentrations of the desorption P decreased from day 10 to 15 and increased from day 15 to the end of the experiments for the SAR with pH 2.5 and 3.0. Results suggest that the SAR pH has a detrimental impact upon P desorption in the Latosol.
There were large concentration valleys in the adsorption P at day 15 for the SAR at pH ranging from 2.5 to 4.5 (Fig. 5b). We attributed the decrease in adsorption P to the decomposition of SOM into the fulvic acid. As shown in Figure 6b, high fulvic acid was observed at day 15. It has been reported that SOM and fulvic acid compete for the absorbed sites with P (Sibanda and Yound 1988). As a result, more adsorption P was released from the Latosol.
Soil Organic Matter Dynamics
Impacts of the SAR on SOM dynamics in the Latosol are shown in Figure 6a. In general, SOM contents decreased with time as the Latosol was leached by the SAR at all pH levels. Such a loss occurred mainly because of the leaching of the dissolved organic matter with the SAR. A statistical analysis (i.e., F-test) showed that variations in SOM contents within different pH levels were significant at α = 0.01. About 9.9, 13.0, 11.5, 6.2, 11.1, 12.2, and 7.4% of the SOM were leached out, respectively, at the pH levels of 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, and 7.0 by the end of the experiments (21 days). A plot of the SOM loss at 21 days against the pH level showed that no correlation existed between them (data not shown). Attempts to locate data in the published literature to compare with our finding were not successful. It was apparent that little attention has been given to quantify the impacts of the SAR pH levels on SOM dynamics in the past.
Impacts of the SAR on fulvic acid dynamics are shown in Figure 6b. Concentrations of soil fulvic acid increased from the beginning of the experiments to day 15 and decreased from day 15 to the end of the experiments. For example, the initial soil fulvic acid concentration was 6.0 g kg−1 but was 7.2 g kg−1 at day 15 and 6.7 g kg−1 at the end of the experiments when the Latosol was leached by the SAR at pH 2.5. The results indicated that the maximum soil fulvic acid concentration occurred after 15 days. The increase in fulvic acid concentration occurred because of the decomposition of the SOM due to the SAR. This finding is consistent with that reported by Calace et al. (2001). These authors studied the effects of the SAR on soil humic compounds in hplic arenosol and dystic cambisol in Italy. They showed that the structure composition of fulvic acid from organic matter changes with increased soil acidification and a shift toward smaller molecular weight compounds was observed due to the breakup of the original molecules under acidic conditions.
Table 1 lists the relationships between AP and three other parameters (i.e., pH, SOM, and sorption P [SP]) at days 5, 10, 15, and 21 after the Latosol was leached by the SAR. These relationships were obtained using the multiple regression analysis. Very strong relationships were observed for all of the leaching times. This result demonstrated that the impacts of the SAR on soil AP could be quantified with pH, SOM, and SP. This finding is very useful for agricultural and environmental management.
Summary and Conclusions
Laboratory experiments were performed to investigate the role of SAR upon cations, P, and organic matter dynamics in the Latosol from south China. Latosol is an acidic red soil and arises in the tropical rainforest biome where high temperature and high precipitation occur. This soil has a loose structure and suffers from rapid erosion during heavy rainfall, which results in the leaching of nutrients and metals.
Our study showed that about 34, 46, 20, and 77% of the original exchangeable soil Ca+2, Mg+2, K+, and Na+, respectively, were leached out with the SAR at pH 2.5 by the end of the experiments. Results suggest that leaching of soil-exchangeable K+ by the acid rain was a serious environmental problem as K+ is one of the most important macronutrients for plant growth. Two distinct patterns of the AP concentrations were observed, one at pH≤3.5 and the other at pH ≥ 4.0. At pH≤3.5, concentrations of the AP increased from the beginning of the experiments to day 5, then decreased from day 5 to 15, and finally increased from day 15 to the end of the experiments. Under normal soil conditions, a decrease in soil desorption P would cause an increase in soil adsorption P. However, our study showed that desorption of soil P by the SAR was not adsorbed onto the soils and is probably released into the soil solution. Very strong relationships were observed between AP and other parameters (i.e., pH, SOM, and SP) at all of the leaching times. The results demonstrated that the impacts of the SAR on soil AP could be quantified by these parameters. This finding is very useful for agricultural and environmental management. Further study is warranted to investigate the impacts of the SAR upon cations, P, and SOM dynamics in the Latosol under field conditions.
References
ACSSSC (Agricultural Committee of Soil Science Society of China) (1983) Standard analysis method of soil agricultural chemicals. Science and Technology Publisher, Beijing, pp 179–190 (in Chinese)
Brady (1984) The nature and properties of soils. 9th edition. New York, London
Calace N, Fiorentini F, Petronio BM, Pietroletti M (2001) Effects of acid rain on soil humic compounds. Talanta 54:837–846
Duan L, Hao J, Xie SD, Zhou DP (2002) Determining weathering rates of soils in China. Geoderma 110:205–225
Hodson ME, Landan SJ (1999) A long-term soil leaching column experiment investigating the effect of variable sulphate loads on soil solution and soil drainage chemistry. Environ Pollut 104:11–19
Guo YL (2002) Acid rain problem and prevention in China. J Shanxi Finance and Economics University 24:106 (in Chinese)
Ivring PM (1983) Acidic precipitation effects on crops. A review and analysis of research. J Environ Qual 12:442–453
Jackson DR, Garrett BC, Bishop TA (1984) Comparison of batch and column methods for assessing leachability of hazardous waste. Envlron Sei Technol 18:668–673
Liao B, Seip HM, Larssen T (1997) Response of two Chinese forested soils to acidic inputs: leaching experiment. Geoderma 75:53–73
Liu KH, Mansell RS, Rhue RD (1990) Cation removal during application of acid solution into air dry soil columns. Soil Sci Soc Am J S4:1747–1753u
Menz FC, Seip HM (2004) Acid rain in Europe and the United States: an update. Environ Sci Policy 7:253–265
NSICSA (Nanjing Soil Institute of China Science Academy) (1978) Soil physical and chemical analysis. Science and Technology Publisher, Shanghai. pp 105–136 (in Chinese)
Sibanda HM, Yound SD (1988) Competitive adsorption of humus acids and P on goethite, gibbsite and two tropical soils. Soil Sci 37:197–204
US-EPA (2004) Acid rain program, 2003 progress report. Clean Air Markets Division, Office of Air and Radiation, U.S. Environmental Protection Agency, EPA 430-R-04-009
Yan CS (1988) Soil fertility study method (in Chinese). Agricultural Publisher, Beijing, pp 201–202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ling, DJ., Zhang, JE., Ouyang, Y. et al. Role of Simulated Acid Rain on Cations, Phosphorus, and Organic Matter Dynamics in Latosol. Arch Environ Contam Toxicol 52, 16–21 (2007). https://doi.org/10.1007/s00244-006-0004-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-006-0004-8