Abstract
Acid rain is an environmental problem of increasing concern in China. In this study, a laboratory leaching column experiment with acid forest soil was set up to investigate the responses of soil and soil solution chemistry to simulated acid rain (SAR). Five pH levels of SAR were set: 2.5, 3.0, 3.5, 4.0, and 4.5 (as a control, CK). The results showed that soil acidification would occur when the pH of SAR was ≤3.5. The concentrations of NO3 −and Ca2+ in the soil increased significantly when the pH of SAR fell 3.5. The concentration of SO4 2− in the soil increased significantly when the pH of SAR was <4.0. The effects of SAR on soil solution chemistry became increasingly apparent as the experiment proceeded (except for Na+ and dissolved organic carbon (DOC)). The net exports of NO3 −, SO4 2−, Mg2+, and Ca2+ increased about 42–86 % under pH 2.5 treatment as compared to CK. The Ca2+ was sensitive to SAR, and the soil could release Ca2+ through mineral weathering to mitigate soil acidification. The concentration of exchangeable Al3+ in the soil increased with increasing the acidity of SAR. The releases of soluble Al and Fe were SAR pH dependent, and their net exports under pH 2.5 treatment were 19.6 and 5.5 times, respectively, higher than that under CK. The net export of DOC was reduced by 12–29 % under SAR treatments as compared to CK. Our results indicate the chemical constituents in the soil are more sensitive to SAR than those in the soil solution, and the effects of SAR on soil solution chemistry depend not only on the intensity of SAR but also on the duration of SAR addition. The soil and soil solution chemistry in this region may not be affected by current precipitation (pH≈4.5) in short term, but the soil and soil leachate chemistry may change dramatically if the pH of precipitation were below 3.5 and 3.0, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain has become a global environmental problem since the early 1950s (Wang et al. 2004). Although acid rain and emissions of SO2 and NOx have been controlled in some developed countries since the 1990s (Angéli et al. 2009; Reis et al. 2012), they remain important issues in many developing countries. Rapid development has caused serious acid rain problems in China, and about 40 % of China’s territory is affected by acid rain (Song et al. 2005; Ling et al. 2007; Xie et al. 2009). In recent years, due to the burning of fossil fuel by cars, the emissions of coal-fueled power plants, and agricultural fertilization, large areas in southern China have been hit by acid rain with pH below 4.5 (Liu et al. 2007). The environmental protection and monitoring agencies in Guangdong Province in southern China announced that 50 % of the rainfalls in that province are acid rains (Zhang et al. 2007). The prevalence of acid rain has fueled public concern about the widespread impacts of acid rain on the natural ecosystem in southern China (Liu et al. 2007; Liu et al. 2010), in which acid forest soils are widely distributed and they are usually sensitive to acid rain due to their low cation exchange capacity (CEC), low base saturation (BS), and high leaching potential (Dai et al. 1998; Lu et al. 2014).
The changes in acid forest soil due to acid rain may be irreversible since the influx of H+ ion by acid rain can displace the base cations bound to soil particles and accelerate the depletion of base cations from the soil (Driscoll et al. 2003; Xie et al. 2009). In addition, SO4 2− and NO3 − in the acid rain act as counterions causing the leaching of base cations from soil (Johnson et al. 1982; Driscoll et al. 2003; Ling et al. 2007; Zhang et al. 2007). The depletion of base cations will reduce soil fertility, induce plant nutrient deficiency (Liu et al. 2007), and delay the chemical recovery of the sensitive soils and surface waters from acidification (Driscoll et al. 2003). If the rate of mineral weathering cannot compensate for the depletion of base cations, soil acidification will occur (Johnson et al. 1982), and some toxic ions such as Al, Fe, and other heavy metals will become mobilized from soil. High concentrations of those chemicals have toxic effects on plant growth (Driscoll et al. 2003; Xie et al. 2009). Previous studies in this area paid more attention to the changes of soil chemistry to acid rain (Liu et al. 2007; Liu et al. 2010), while less attention was paid to the soil solution chemistry and the input-output budgets of chemical elements to acid rain.
DOC plays a critical role in the carbon cycle of forest soils. As a relatively labile fraction of the soil organic carbon (Kalbitz et al. 2000), the movement of DOC through soils is an important process for the carbon transport (Neff and Asner 2001). In forest ecosystems, fluxes of DOC from the forest floor into the mineral soil have been estimated at 115∼500 kg C ha−1 year−1, representing up to 35 % of the amount of annual carbon input through litter (Kalbitz and Kaiser 2008). Since DOC is vulnerable to the changing environment, many studies have been carried out in regions suffering from serious acid deposition in the past 25 years (Solinger et al. 2001; Evans et al. 2005; van den Berg et al. 2012). Lateritic red earth, the main soil type in the subtropical zone of China, however, has been largely ignored. It is very important to understand the response of DOC originated from lateritic red earth to acid rain, because this soil is rich in Al and Fe oxides and hydroxides, which are favorable to the adsorption of DOC (Kalbitz et al. 2000). Therefore, the impacts of acid rain on the Al and Fe oxides and hydroxides may further influence the dynamic of DOC in the soil.
Up to now, studies of SAR on soil chemical properties have mostly used reconstructed soil column (i.e., the air-dried, sieved, and mixed thoroughly soil) (Guicharnaud and Paton 2006; Liu et al. 2007; Zhang et al. 2007). This method destroys the natural structures of the soil, exposes the ion exchangeable sites, and often overestimates the impacts of SAR on the concentrations of some chemicals in the experiment (Liu et al. 1990). Therefore, reconstructed soils may not reflect the true response of natural soils to SAR. In addition, few study focused on comparison of the ions influent and effluent which was important to evaluate whether ions were retained in or depleted from the soil. In this study, we designed a field experimental facility with intact soil columns and investigated the responses of soil and soil leachate chemistry to SAR. The primary objectives of this study were (1) to investigate the effects of SAR on the soil and soil leachate chemistry and (2) to understand the regulation mechanisms of the soil chemical responses to SAR.
Materials and methods
Study site
The intact soil columns were collected from 400-year-old monsoon evergreen broadleaf forest in Dinghushan Biosphere Reserve (112° 30′ 39″ to 112° 33′ 41″ E, 23° 09′ 21″ to 23° 09′ 21″ N), located at the center of Guangdong province in southern China. Annual mean precipitation is about 1678 mm, and nearly 80 % of rainfall is distributed in the wet seasons (from April to September) and the rest 20 % in the dry seasons (from October to March). The yearly average temperature is 21.4 °C, and the relative humidity is 80 % (Zhou et al. 2013). The soil is classified as lateritic red earth, loamy in texture, and acidic (Liang et al. 2013). The soil depth ranges from 30 to 70 cm (Fang et al. 2008). This area suffers from serious acid rain. The frequency of acid rain increased from 62.7 % in 2003 to 97 % in 2009 (Liu et al. 2007; Zhu et al. 2011). Furthermore, the dominant chemical compositions of acid rain have changed from sulfate ions to a combination of sulfate and nitrate ions. The ratio of nitrate ions is increasing, with the current mole ratio of sulfate to nitrate ions as 2:1 (Zhu et al. 2011).
Soil sampling and analysis
The setup of the soil column (20 cm in inner diameter, 25 cm in height) is presented in Fig. 1. A flat plot was chosen, and the soil column was inserted vertically into the soil surface. A plywood flatly placed on the top of the column and repeated tapped with a sledge hammer. After driving the column to a depth of 20 cm, a hole was dug next to the column so that the column and the soil it contained could be lifted out with little disturbance. Thereafter, the soil exceeded the bottom of column was cut using a sharp knife to make sure the soil at the bottom of the column was trimmed. Twenty intact soil columns were collected randomly in the study site in June 2012. The soil in each column was about 7.89 ± 0.28 kg. Plants and litter over the surface of the soil were removed. A piece of paper filter was placed at both ends of the column to prevent the leakage of the soil. At the top of soil column, 5 cm was left to avoid the overflow of SAR solution. At the bottom of soil column, a matched plastic cap with a drainage outlet was used. In order to study whether the soil chemical properties in soil columns were homogeneous, soil samples from the top 20 cm soil layer were collected adjacent to the sampling sites of the soil columns. The intact soil columns were carried quickly to the laboratory and assigned randomly for SAR treatments. Soil samples were analyzed for a number of chemical properties. Those relevant to this study included pH, NO3 −, SO4 2−, K+, Na+, Ca2+, Mg2+, soluble Al, and DOC. Analytical methods of the chemical properties were based on Lu (2000). Soil pH was measured with a glass electrode (Thermo Eutech, USA) in the supernatant after shaking for 30 min with deionized water (1:2.5 soil solution ratio) and sedimentation in a beaker for 30 min. Exchangeable base cations (K+, Na+, Ca2+, and Mg2+) in the soil were extracted with 1 mol L−1 ammonium acetate solution (1:5 soil solution ratio) and adjusted to pH 7.0 and then measured by ICP-Optima 2000 (PerkinElmer Inc., USA). The exchangeable Al3+ in the soil was extracted by the oxalate acid method (Lu 2000), and the extractant solution was analyzed with ICP-Optima 2000 (PerkinElmer Inc., USA). The soluble NO3 − and SO4 2− in the soil were extracted with deionized water (1:5 soil solution ratio). The NO3 − was determined by the phenol disulfonic acid spectrophotometric method (Liu et al. 2007). The SO4 2− was measured by the barium sulfate gravimetric method (Lu 2000). The soil DOC was extracted with deionized water (1:2.5 soil solution ratio), and the extractant solutions were centrifuged for 5 min at 4500 rpm; afterward, the supernatant was filtered through 0.45-μm membrane filters before measurement (Gregorich et al. 2003). The concentration of DOC was measured with TOC-VCSH (Shimadzu Corporation, Japan). The basic soil chemical properties in the intact soil columns were similar before SAR treatments. The initial soil had an average pH about 4.05, a mean soil soluble NO3 − concentration of 41.37 mg kg−1, and a mean SO4 2− concentration of 172.78 mg kg−1. The concentrations of exchangeable K+, Na+, Ca2+, Mg2+, Al3+, and DOC in the original soil were 48.06, 8.01, 228.08, 22.38, 2418.93, and 322.60 mg kg−1, respectively.
Application of SAR to soil column
The experimental design was a random block design with five SAR treatments and four replicates. To simulate acid rain, the soil columns were watered with acidic solutions at different pH levels: CK (pH 4.5, the local lake water), pH 4.0, pH 3.5, pH 3.0, and pH 2.5. The average pH value of precipitation in this region is about 4.39 (Zhu et al. 2011). Considering the pH value of precipitation would probably decrease in the future, we set the pH levels of SAR 0.5 unit lower each time. According to previous acid rain records as well as the tendency of S/N mole ratio in the rainfalls in this area (Zhu et al. 2011), acidic solution was prepared by adding a mixture of H2SO4 and HNO3 in a 2:1 mol ratio to the local lake water. The basic chemical properties of the lake water were shown in the Table 1. SAR treatments began in June 2012 and ended in December 2012. In order to avoid the SAR solution flush the soil directly, a 500-mL input of SAR solution was slowly sprayed at a rate of 6 mL min−1 (equate to the rate of the precipitation infiltrating to the soil in this area) to the top of the column every 2 weeks with a total influent application of 11 times and a total volume of 5500 mL SAR solution. The output samples were collected after SAR treatments at the bottom of the column with a container after filtering. At the end of the experiment, soils in the whole column (0–20 cm) were collected for the chemical analysis.
Soil solution sampling and analysis
To evaluate the net loss of the chemical component, the volume of soil solution was measured after SAR treatments. There were no significant differences in the volume of leachate among treatments in each sampling time. After that, the soil leachate passed through a 0.45-μm membrane. The solutions were stored at 4 °C, and chemical components were measured as soon as possible. The analytical indicators included pH, NO3 −, SO4 2−, K+, Na+, Ca2+, Mg2+, soluble Al, soluble Fe, and DOC in the soil solution. Analytical methods of the chemical components were based on Lu (2000). Soil solution pH was measured directly with a glass electrode (Thermo Eutech, USA). The soluble NO3 − was determined by the phenol disulfonic acid spectrophotometric method (Liu et al. 2006). The soluble SO4 2− was measured by the barium sulfate gravimetric method (Lu 2000). K+, Na+, Ca2+, Mg2+, soluble Al, and soluble Fe in the soil solution were analyzed with ICP-Optima 2000 (PerkinElmer Inc., USA). The concentration of DOC was measured with TOC-VCSH (Shimadzu Corporation, Japan).
Data analysis
Data analyses were carried out using the SPSS 10.0 software (SPSS Inc., Chicago, IL, USA). The significant differences of the chemical properties in the soil and the net export of chemical components from soil under SAR treatments were tested using one-way ANOVA with LSD comparison. The differences of change between the initial and the final chemical components in the soil were tested using the t test. Repeated measure ANOVA with LSD test was performed to examine the effects of SAR treatments on the soil leachate chemistry. Statistically significant differences were set at α = 0.05. Artworks were carried out using the SigmaPlot 12.5 graphics program (Systat Software Inc., CA, USA).
Results and discussion
pH
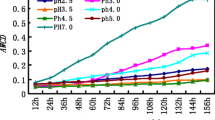
Not all of the differences in the soil pH levels between treatments were statistically significant at the end of the experiment (Table 2). Although the soil pH levels decreased at all levels of SAR treatment as compared to the initial pH level (Table 3), significant decrease in soil acidity occurred only at the pH of SAR ≤ 3.5 (Table 2). Furthermore, pH values of the soil solution also dropped significantly under pH <3.5 treatments after the forth acid rain addition (P < 0.01; Fig. 2). These results suggested that the soil, to some extent, possessed a buffering capacity for acidity. However, when a high addition of H+ exceeded a certain threshold value (pH = 3.5 in this study), soil buffering capacity would be depleted, and soil acidification would occur. This finding was consistent with the observations of Liu et al. (2007) and Dai et al. (1998) who reported that the acidity threshold was around the initial soil pH level. Soil pH level was not affected by pH 4.0 treatment (Table 2), suggesting that although the lateritic red earth belongs to acidic soil, further acidification may only occur with very low pH precipitation (Johnson et al. 1982). According to the above results, we can predict that if the pH value of acid rain in the near future in this region is still above the threshold value (pH 3.5), soil acidification will remain a slow process. Conversely, soil will acidify quickly once the pH value of the precipitation is below this threshold value. It should be emphasized here that this prediction was just based on the short-term leaching experiment. The solution pH under each treatment decreased gradually as the experiment proceeded (Fig. 2). This tendency suggested that soil acidification might also occur if the soil was exposed to low intensity but chronic acid rain addition.
NO3 − and SO4 2−
Concentration of soluble NO3 − in the soil increased significantly as the pH level of SAR below 3.5 (Table 2). Given extremely high acid inputs caused increase in nitrogen mineralization and nitrification (Johnson et al. 1982). Chronic nitrogen and sulfur amendments also increased the NO3 − content at the Harvard Forest (Magill et al. 1997). Compared with the initial soil, however, the concentrations of NO3 − in the soil reduced significantly at all levels of SAR treatments, which indicated that precipitation (acid and nonacid addition) enhanced the leaching of NO3 − in the soil. The concentration of NO3 − in the soil solution increased first, and then decreased, and thereafter stayed relatively constant for all treatments (Fig. 3). The initial increase of NO3 − concentration in the soil solution was likely a consequence of the directly leaching of the NO3 − from the native soil, since the NO3 − was susceptible to leach and the soil had a relatively higher NO3 − concentration (Lu et al. 2014). In addition, some dead fine roots might remain in the soil, even though plants and litter on the surface of the tested soil were removed. Rapid mineralization of dead fine roots could fuel nitrification resulting in very high soil solution NO3 − concentration (Sherman and Fahey 1994). The following decrease in NO3 − concentration might have resulted from the soil adsorption. The soil in this study site was rich in Al and Fe oxides (Johnson et al. 1982; Kalbitz et al. 2000), and free Fe and Al oxides created significant sources of positive charge across a large range of pH levels (Ji 1997), which in turn enhanced the adsorption of NO3 − and decreased its leaching loss. The relatively constant NO3 − concentration at the later stage of leaching suggested that the soil began to equilibrate with the NO3 − input (Hodson and Langan 1999). As compared with CK, the NO3 − concentrations in the soil solution were not affected by SAR with pH ranged from 3.0 to 4.0 throughout the experiment. It was also not affected by pH 2.5 treatment during the first nine leaching, but it was significantly greater than that under CK during the later stage of leaching. This suggested that the effect of SAR on NO3 − leaching depended not only on the intensity of SAR but also on the duration of SAR addition. The net export of NO3 − under pH 2.5 treatment was about 41–48 % higher than that under other SAR treatments (except pH 3.0 treatment, P < 0.05, Fig. 4). This result was in agreement with the findings observed in the same area (Lu et al. 2014). The concentration of SO4 2− in the soil solution was variable throughout the experiment. It decreased first, and then increased, and thereafter switched between decrease and increase. The initial decline in the SO4 2− concentration was probably due to the temporal adsorption of SO4 2−. Previous studies reported that SO4 2− adsorption capacity was positively correlated with the contents of Al and Fe oxides (Johnson et al. 1986; Singh et al. 1980). Although we did not determine the Al and Fe oxides directly, several studies found that the soil in this study site was rich in Al and Fe oxides (Johnson 1982; Kalbitz et al. 2000). The responses of the soluble Al and Fe in this study might also offer indirect evidence since the concentrations of soluble Al and Fe were not affected by SAR treatments at the initial stage of leaching (see below, Fig. 6). The following increase of SO4 2− concentration in the soil solution (from t3 to t5) might have resulted from desorption of SO4 2− from the Al and Fe oxides. The reasons for the following variation in SO4 2− were not clear, but Selim et al. (2004) reported that the mobility of SO4 2− in forest soil was most likely controlled by kinetic reactivity of SO4 2− of both reversible and irreversible mechanisms. The variability of SO4 2− concentration in the soil solution indicated that soil had experienced a series of transformations in order to buffer the added acidity. There was no significant difference in SO4 2− concentration for all treatments during the first three leaching (Fig. 3). However, the SO4 2− concentrations under pH 3.0 and pH 2.5 treatments were significantly greater than that under CK after the fourth leaching. Although the differences in SO4 2− concentration between CK and pH 4.0 as well as pH 3.5 treatments were not significant, these differences became increasingly apparent at the later stage of leaching (from t8 to t11). This result suggested that the amount of SO4 2− leaching depended not only on the intensity of SAR but also on the duration of SAR addition. At the end of the experiment, the net leaching loss of SO4 2− increased dramatically under pH 3.0 and pH 2.5 treatments (P < 0.05; Fig. 4). Furthermore, the soil soluble SO4 2− concentration also increased when the pH levels of SAR ≤3.0 (Table 2). It is generally believed that SO4 2− acts as a conservative ion, and the export of SO4 2− equals input in deposition (Dillon et al. 1982). However, recent study found that SO4 2− export from catchments in both Europe and North America exceeded input in deposition (Watmough et al. 2005). Liu et al. (2007), who used the soil sampled from the same study site as ours, also found that SO4 2− concentrations in both the soil and soil leachate increased under the most acidic treatment (pH 3.05). The increased SO4 2− in the soil under the most intensive acid treatment could be derived from the weathering of S-bearing minerals (Driscoll et al. 2001) and the mineralization of organic S in soil (Watmough et al. 2005).
Net export of the chemical constituents under different SAR treatments (means ± SD). Net export = (the total effluent of a certain chemical constituent − the total influent of the chemical constituent)/the weight of soil in each soil column. Positive values indicate that a net leaching loss of the soil chemical constituents. Asterisks (*) indicates that there is significant difference at P < 0.05 level between the SAR treatment and the control
Base cations
At the end of the experiment, concentrations of the exchangeable K+, Ca2+, and Mg2+ in the soil under SAR treatments were about 48∼58, 59∼92, and 63∼85 %, respectively, lower than the initial values (Table 3). This was consistent with Liao et al. (1997), who reported that dramatic depletion of exchangeable K+, Ca2+, and Mg2+ occurred after application of acid solution to the lateritic red earth. The concentration of exchangeable Na+ in the soil was not affected by SAR treatments, but its concentration increased for all treatments at the end of the experiment (Table 3). This indicated that the input of Na+ through SAR addition was accumulated in the soil (Fig. 4). In general, the K+ concentration in the soil solution for SAR treatments (except for pH 2.5) was slightly lower than that under CK (P > 0.05) during the first eight leaching (Fig. 5). However, as the experiment proceeded, it was higher than CK and tended to increase with increasing the acidity of SAR. This suggested that the sensitivity of K+ to continued input of SAR had enhanced (Zhang et al. 2007). The responses of Ca2+ and Mg2+ to SAR were similar to that of K+ (Fig. 5). As compared to CK, the concentrations of Ca2+ and Mg2+ in the soil solution increased significantly under pH 2.5 treatment (P < 0.05). Although repeated measure ANOVA showed that the concentrations of Ca2+ and Mg2+ in the soil solution were not affected by SAR with a pH above 3.0, their concentrations in the soil solution were slightly higher than CK after the eighth leaching. This suggested that the effects of SAR on base cations leaching depended not only on the intensity of SAR but also on the duration of SAR addition (Zhang et al. 2007). Therefore, chronic application of low intensity acid rain may progressively deplete the nutrient elements (such as Ca2+ and Mg2+) in the evergreen broad-leaved forest. The net leaching loss of the Ca2+ and Mg2+ under pH 2.5 treatment was about 56 and 50 %, respectively, higher than that under CK (Fig. 4). A positive relationship between base cations and acid anions (NO3 − and SO4 2−) (r = 0.336 to 0.564, P < 0.01, N = 220) in this study suggested that higher leaching losses of acid anions were likely responsible for the greater losses of Ca2+ and Mg2+, as accompanying ions (Johnson et al. 1982; Driscoll et al. 2003; Ling et al. 2007; Zhang et al. 2007). Acid rain led to the losses of Ca2+, but the concentrations of exchangeable Ca2+ in the soil under pH 2.5 treatment were still higher than other treatments (P < 0.05; Table 2). This may be partially attributed to the ability of the soil to release Ca2+ through mineral weathering to mitigate soil acidification, thereby increasing its potential contribution to soil buffering capacity. Liu et al. (2007) also reported that after 2 years of SAR treatments, the increase of exchangeable Ca2+ in the soil was faster under the intensive acid treatment as the H+ accelerated its weathering. On the contrary, Lu et al. (2009, 2014) reported that the exchangeable Ca2+ decreased with the increment of acidity in the same area. The opposite results may be attributed to the difference in soil acidity. As mineral weathering can be intensified by increasing soil acidity (Liu et al. 2007), the lowest soil pH under his treatment was 3.74, while it was 3.18 in our study (Table 2).
The net export of Ca2+ (15.69 mg kg−1) and Mg2+ (0.24 mg kg−1) under pH 2.5 treatment was 1.56, 1.96, 1.89, 1.46 and 1.49, 1.66, 1.59, 1.45 times higher than that under CK, pH 4.0, pH 3.5, and pH 3.0 treatments, respectively (Fig. 4). It seems that the Ca2+ was more sensitive than Mg2+ to acid rain based on the variation of bivalent cation concentrations. Similarly, Liu et al. (2008) and Bergkvist (1987) reported that the sensitivity of base cations in the lateritic red earth to acid rain was in the order of Ca2+ > Mg2+ > K+ > Na+. Soil is not passive to acidic precipitation, but possesses a buffering capacity for acidity (e.g., base cations exchange and mineral weathering) to resist acid rain (Guicharnaud and Paton 2006; Liu et al. 2008), and the losses of base cations could be compensated to some extent by acid induced increases in soil weathering (Johnson et al. 1982; Yu et al. 2001).
Soluble Al and Fe
Compared with CK, the concentration of exchangeable Al3+ in the soil increased with increasing the acidity of SAR (Table 2). At the end of the experiment, its concentration under pH <3.5 treatment was about 18∼43 % greater than the initial values (Table 3). The increase in exchangeable Al3+ indicated that rock weathering was stimulated by the application of SAR with a pH below 3.5. Mulder and Stein (1994) also observed that soil exchangeable Al3+ increased significantly in highly acidic forest soil (pH <4.0) in Europe. Exchangeable Al3+ increased substantially when leached with the most acid solutions (pH 3.5) for two Chinese forest soils (Liao et al. 1997). The soluble Al in the soil solution was not affected by SAR at the initial leaching, but it increased significantly under pH 2.5 and under pH 3.0 treatments after the fifth leaching and eighth leaching, respectively (Fig. 6). This suggested that there was time delay between the input of acid solution and the release of soluble Al. Throughout the experiment, the soluble Al in the soil solution was not affected by SAR with a pH above 3.5. However, the differences in soluble Al concentration between CK and pH 4.0 as well as pH 3.5 treatments were increasingly pronounced and tended to increase with increasing the acidity of SAR as the experiment proceeded. This indicated that the cumulative effects of low intensity acid rain addition enhanced the sensitivity of soluble Al to acid rain (Bowman et al. 2008). At the end of the experiment, the net export of soluble Al under pH 2.5 treatment was about 19.6 times higher than that under CK (Fig. 4). Similarly, the mobility of soluble Fe in the soil solution was not affected by SAR at the early stage of leaching, but it increased significantly from 0.04 mg L−1 at fifth leaching to 1.20 mg L−1 by the end of the experiment under pH 2.5 treatment (Fig. 6). The net export of soluble Fe under pH 2.5 treatment was 5.5 times higher than that under CK (Fig. 4). The concentrations of soluble Fe and Al increased in the soil and soil solution when the pH of SAR decreased to 3.0 and 2.5, respectively. This suggested that the releases of soluble Al and Fe were pH dependent. A good exponential correlation between the SAR pH and the leaching of soluble Al concentration as well as Fe further supported this view (Fig. 7). Similar results were also obtained by other studies (Abrahamsen 1983; Liu et al. 2007). There was little change in the concentration of Al and Fe in the soil solution during the early stages of acid rain addition. However, as the experiment proceeded, the concentrations of Al and Fe in the soil solution increased under SAR treatments, especially under pH 2.5 treatment. This phenomenon might be attributed to the following reasons. First, it was time dependent for the Al and Fe to release from a kinetically constrained Al and Fe pool to a phase easy to mobilize (Larssen et al. 1999; Bowman et al. 2008). Second, during acidification, soils underwent a transition through different ranges of buffering associated with the weathering and liberation of different elements. Most soils were buffered by base cations (Bowman et al. 2008; Ulrich and Pankrath 1983), but when the base cations on the exchange sites were very low, the Al and Fe buffer systems dominated to neutralize a rapid increase of acidity (Bowman et al. 2008; Ulrich and Pankrath 1983; Louzao et al. 1990).
DOC
The DOC concentrations in the soil solution under SAR treatments were about 7 mg L−1 lower than that under CK during the first seven leaching (except the third and fourth of leaching under pH 2.5). Afterward, it decreased rapidly and stayed relatively constant as CK (Fig. 8). Liao et al. (1997) and Hodson and Langan (1999) also observed that DOC concentration in the soil solution not only decreased rapidly in short time but also decreased with increasing acidity of acid rain. The rapid decrease in DOC concentration might be due to the highly biodegradability of DOC (Kalbitz et al. 2000). SAR treatments reduced about 12–29 % of the DOC export as compared to CK (Fig. 4). This reduction, however, was not associated with an increase in soil DOC concentrations under SAR treatments (Table 2). This may be partially attributed to the increased exchangeable Al3+ and Fe which could form coprecipitation with part of DOC in the soil under SAR treatments, and this kind of metal-DOC precipitate was too stable to be extracted by deionized water (Jansen et al. 2003; Kalbitz and Kaiser 2008). Also, the addition of acidic solution decreased the solubility of organic matter because of the increase in intermolecular and intramolecular bonding, which involved a rise in molecular aggregation (Louzao et al. 1990). Although we could not confirm whether part of DOC in the soil was immobilized, the positive relationship between DOC concentration and the soil leachate pH (r = 0.251, P < 0.01, N = 220) was also observed in other studies (Kalbitz et al. 2000; Filep and Rékási 2011; Xiao et al. 2013). This indicated that inputs of acid rain could increase the potential of the soil to retain DOC. SanClements et al. (2012) also reported that acid deposition decline was a driving factor for the increasing of DOC concentrations in lakes and streams.
Conclusion
From the results presented in this study, it could be concluded that soil acidification would occur when the pH level of SAR was lower than 3.5 in this area. Concentrations of NO3 − and Ca2+ in the soil increased dramatically when the pH level of SAR fell 3.5. Concentrations of SO4 2− in the soil increased significantly when the pH level of SAR below 4.0. The effects of SAR on the soil solution chemistry were increasingly pronounced as the experiment proceeded (except for Na+ and DOC). The net exports of the NO3 −, SO4 2−, Mg2+, and Ca2+ under pH 2.5 treatment were 42–86 % greater than that under CK. SAR treatments also led to the soil to release soluble Al and Fe, and the net leaching losses of soluble Al and Fe under pH 2.5 treatment were 19.6 and 5.5 times higher than that under CK. SAR treatments reduced about 12–29 % of the net leaching loss of DOC as compared to CK. These results indicate that the soil chemical constituents are more sensitive than the soil leachate constituents to SAR, and the effects of SAR on soil solution chemistry depend not only on the intensity of SAR but also on the duration of SAR addition. Current precipitation may not pose great risks to the soil and soil leachate chemistry in short term, as the pH of precipitation in this region is about 4.5, well above the threshold value for acid rain to have an effect on soil acidification. However, if the acid rain becomes more severe, it may affect the chemical properties of soil (pH<3.5) and soil leaching solution (pH <3.0) dramatically. Short-term application of intensive acid rain will accelerate soil acidification, leading to depletion of nutrient elements (such as Ca2+ and Mg2+) and mobilization of toxic metals (soluble Al and Fe) in the evergreen broad-leaved forest. Long-term application of low intensity acid rain may progressively cause detrimental effects on the soil and soil solution chemistry, since the sensitivity of the soil solution chemistry to continued input of low intensity acid rain enhanced as the experiment proceeded. Therefore, the effects of chronic acid rain addition on the soil and soil chemistry are worthy of further study.
References
Abrahamsen, G. (1983). Sulphur pollution: Ca, Mg and Al in soil and soil water and possible effects on forest trees. In B Ulrich, J Pankrath (Eds.), Effects of accumulation of air pollutants in forest ecosystems (pp. 207–218) Springer Netherlands.
Angéli, N., Dambrine, E., Boudot, J. P., Nedeltcheva, T., Guérold, F., Tixier, G., Probst, A., Party, J. P., Pollier, B., & Bourrié, G. (2009). Evaluation of streamwater composition changes in the Vosges Mountains (NE France): 1955–2005. Science of the Total Environment, 407(14), 4378–4386.
Bergkvist, B. (1987). Leaching of metals from a spruce forest soil as influenced by experimental acidification. In Acidic precipitation (pp. 1955–1970) Springer Netherlands.
Bowman, W. D., Cleveland, C. C., Halada, L., Hresko, J., & Baron, J. S. (2008). Negative impact of nitrogen deposition on soil buffering capacity. Nature Geoscience, 1(11), 767–770.
Dai, Z., Liu, Y., Wang, X., & Zhao, D. (1998). Changes in pH, CEC and exchangeable acidity of some forest soils in southern China during the last 32–35 years. Water, Air, and Soil Pollution, 108(3–4), 377–390.
Dillon, P., Jeffries, D., & Scheider, W. (1982). The use of calibrated lakes and watersheds for estimating atmospheric deposition near a large point source. Water, Air, and Soil Pollution, 18(1–3), 241–258.
Driscoll, C. T., Lawrence, G. B., Bulger, A. J., Butler, T., Cronan, C., & Eagar, C. (2001). Acidic deposition in the northeastern US: sources and inputs, ecosystems effects, and management strategies. BioScience, 51(3), 180–198.
Driscoll, C. T., Driscoll, K. M., Mitchell, M. J., & Raynal, D. J. (2003). Effects of acidic deposition on forest and aquatic ecosystems in New York State. Environmental Pollution, 123(3), 327–336.
Evans, C. D., Monteith, D. T., & Cooper, D. M. (2005). Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environmental Pollution, 137(1), 55–71.
Fang, Y., Gundersen, P., Mo, J., & Zhu, W. (2008). Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences, 5(2), 339–352.
Filep, T., & Rékási, M. (2011). Factors controlling dissolved organic carbon (DOC), dissolved organic nitrogen (DON) and DOC/DON ratio in arable soils based on a dataset from Hungary. Geoderma, 162(3–4), 312–318.
Gregorich, E., Beare, M., Stoklas, U., & St-Georges, P. (2003). Biodegradability of soluble organic matter in maize-cropped soils. Geoderma, 113(3), 237–252.
Guicharnaud, R., & Paton, G. I. (2006). An evaluation of acid deposition on cation leaching and weathering rates of an Andosol and a Cambisol. Journal of Geochemical Exploration, 88(1–3), 279–283.
Hodson, M., & Langan, S. (1999). A long-term soil leaching column experiment investigating the effect of variable sulphate loads on soil solution and soil drainage chemistry. Environmental Pollution, 104(1), 11–19.
Jansen, B., Nierop, K. G. J., & Verstraten, J. M. (2003). Mobility of Fe(II), Fe(III) and Al in acidic forest soils mediated by dissolved organic matter: influence of solution pH and metal/organic carbon ratios. Geoderma, 113, 323–340.
Ji, G. (1997). Electrostatic adsorption of anions. In T. R. Yu (Ed.), Chemistry of variable charge soils (112–139). New York: Oxford University Press.
Johnson, D. W., Turner, J., & Kelly, J. M. (1982). The effects of acid rain on forest nutrient status. Water Resources Research, 18(3), 449–461.
Johnson, D. W., Cole, D. W., Van Miegroet, H., & Horng, F. (1986). Factors affecting anion movement and retention in four forest soils. Soil Science Society of America Journal, 50(3), 776–783.
Kalbitz, K., & Kaiser, K. (2008). Contribution of dissolved organic matter to carbon storage in forest mineral soils. Journal of Plant Nutrition and Soil Science, 171(1), 52–60.
Kalbitz, K., Solinger, S., Park, J. H., Michalzik, B., & Matzner, E. (2000). Controls on the dynamics of dissolved organic matter in soils: a review. Soil Science, 165(4), 277–304.
Larssen, T., Vogt, R. D., Seip, H. M., Furuberg, G., Liao, B., Xiao, J., et al. (1999). Mechanisms for aluminum release in Chinese acid forest soils. Geoderma, 91(1), 65–86.
Liang, G., Liu, X., Chen, X., Qiu, Q., Zhang, D., Chu, G., Liu, J., Liu, S., & Zhou, G. (2013). Response of soil respiration to acid rain in forests of different maturity in southern China. PloS One, 8, e62207.
Liao, B., Seip, H. M., & Larssen, T. (1997). Response of two Chinese forest soils to acidic inputs: leaching experiment. Geoderma, 75(1), 53–73.
Ling, D. J., Zhang, J. E., Ouyang, Y., & Huang, Q. C. (2007). Role of simulated acid rain on cations, phosphorus, and organic matter dynamics in Latosol. Archives of Environmental Contamination and Toxicology, 52(1), 16–21.
Liu, K. H., Mansell, R. S., & Rhue, R. D. (1990). Cation removal during application of acid solutions into air-dry soil columns. Soil Science Society of Ammerica Journal, 54(6), 1747–1753.
Liu, J., Zhou, G., & Zhang, D. (2007). Effects of acidic solutions on element dynamics in the monsoon evergreen broad-leaved forest at Dinghushan, China. Environmental Science and Pollution Research - International, 14(3), 215–218.
Liu, L., Zhou, Y. Y., Song, C. Y., & Li, F. S. (2008). Release of basic cations in red soil under simulated acid rain and buffering mechanism. Research of Environmental Sciences, 21(2), 49–55.
Liu, K. H., Fang, Y. T., Yu, F. M., Liu, Q., Li, F. R., & Peng, S. L. (2010). Soil acidification in response to acid deposition in three subtropical forests of subtropical China. Pedosphere, 20, 399–408.
Louzao, M. J., Leirós, M. C., & Guitián, F. (1990). Study of buffering. Systems in soils from Galicia, N.W. Spain. Water, Air, and Soil Pollution, 49(1–2), 17–33.
Lu, R. K. (2000). The analysis method of soil agricultural chemistry. Beijing: China agricultural science and technology press.
Lu, X. K., Mo, J. M., Gundersern, P., Zhu, W. X., Zhou, G. Y., Li, D. J., & Zhang, X. (2009). Effect of simulated N deposition on soil exchangeable cations in three forest types of subtropical China. Pedosphere, 19(2), 189–198.
Lu, X., Mao, Q., Gilliam, F. S., Luo, Y., & Mo, J. (2014). Nitrogen deposition contributes to soil acidification in tropical ecosystems. Global Change Biology, 20(12), 3790–3801.
Magill, A. H., Aber, J. D., Hendricks, J. J., Bowden, R. D., Melillo, J. M., & Steudler, P. A. (1997). Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecological Applications, 7(2), 402–415.
Mulder, J., & Stein, A. (1994). The solubility of aluminum in acidic forest soils: long-term changes due to acid deposition. Geochimica et Cosmochimica Acta, 58(1), 85–94.
Neff, J. C., & Asner, G. P. (2001). Dissolved organic carbon in terrestrial ecosystems: synthesis and a model. Ecosystems, 4(1), 29–48.
Reis, S., Grennfelt, P., Klimont, Z., & Ammann, M. (2012). From acid rain to climate change. Science, 338(30), 1153–1154.
SanClements, M. D., Oelsner, G. P., McKnight, D. M., Stoddard, J. L., & Nelson, S. J. (2012). New insights into the source of decadal increases of dissolved organic matter in acid-sensitive lakes of the Northeastern United States. Environmental Science & Technology, 46(6), 3212–3219.
Selim, H., Gobran, G., Guan, X., & Clarke, N. (2004). Mobility of sulfate in forest soils. Journal of Environmental Quality, 33(2), 488–495.
Sherman, R. E., & Fahey, T. J. (1994). The effects of acid deposition on the biogeochemical cycles of major nutrients in miniature red spruce ecosystems. Biogeochemistry, 24(2), 85–114.
Singh, B., Abrahamsen, G., & Stuanes, A. (1980). Effect of simulated acid rain on sulfate movement in acid forest soils. Soil Science Society of America Journal, 44(1), 75–80.
Solinger, S., Kalbitz, K., & Matzner, E. (2001). Controls on the dynamics of dissolved organic carbon and nitrogen in a Central European deciduous forest. Biogeochemistry, 55(3), 327–349.
Song, Y. Z., Qin, B. Q., Yang, L. Y., Hu, W. P., & Luo, L. C. (2005). The chemical characteristic of wet deposition and preliminary analysis of surface water acidification along the coastal area of Taihu Lake. Journal of Nanjing Institute of Meteorology, 28(5), 593–600.
Ulrich, B., & Pankrath, J. (1983). Effects of accumulation of air pollutants in forest ecosystems. Springer Science & Business Media.
van den Berg, L. J. L., Shotbolt, L., & Ashmore, M. R. (2012). Dissolved organic carbon (DOC) concentrations in UK soils and the influence of soil, vegetation type and seasonality. Science of the Total Environment, 427–428(15), 269–276.
Wang, J. Q., Wu, J. B., Li, R. Z., & Qian, J. (2004). Research development and some problems discuss on acid rain in China. Advances in Water Science, 15(4), 526–530.
Watmough, S. A., Aherne, J., Alewell, C., Arp, P., Bailey, S., Clair, T., Dillon, P., Duchesne, L., Eimers, C., & Fernandez, I. (2005). Sulphate, nitrogen and base cation budgets at 21 forested catchments in Canada, the United States and Europe. Environmental Monitoring and Assessment, 109, 1–36.
Xiao, K., Zhou, J., Liu, X., Wu, J., & Xu, J. (2013). Leaching of dissolved organic carbon (DOC) as affected by plant residue composition and soil pH. In J Xu, J Wu, Y He (Eds.), functions of natural organic matter in changing environment (pp. 475–478) Springer Netherlands.
Xie, Z., Du, Y., Zeng, Y., Li, Y., Yan, M., & Jiao, S. (2009). Effects of precipitation variation on severe acid rain in southern China. Journal of Geographical Sciences, 19(4), 489–501.
Yu, Y. C., Ding, A. F., Hu, J., & Meng, L. (2001). Effects of simulated acid rain on soil acidification and base ions transplant. Journal of Nanjing Forestry University, 25(2), 39–42.
Zhang, J. E., Ouyang, Y., & Ling, D. J. (2007). Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere, 67(11), 2131–2137.
Zhou, G., Peng, Y., Li, Y., Liu, S., Zhang, Q., Tang, X., Liu, J., Yan, J., Zhang, D., & Chu, G. (2013). A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Global Change Biology, 19(4), 1197–1210.
Zhu, S. J., Wang, X. M., Long, X. J., Dong, H. Y., Huang, Z. L., & Cheng, Y. J. (2011). Characteristics of inorganic chemical components in rainfall of the Dinghu Mountain. Acta Scientiarum Naturalium Universitatis Sunyatseni, 50(5), 135–141.
Acknowledgments
We thank Prof. Dafeng Hui and Lucy Hui for helpful comments on an earlier version of the manuscript. We also thank two anonymous reviewers for their valuable contributions. This work was financially supported by National Natural Science Foundation of China (31428001, 41430529) and “Strategic Priority Research Program-Climate Change: Carbon Budget and Related Issues” of the Chinese Academy of Sciences (XDA05050205).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, Q., Wu, J., Liang, G. et al. Effects of simulated acid rain on soil and soil solution chemistry in a monsoon evergreen broad-leaved forest in southern China. Environ Monit Assess 187, 272 (2015). https://doi.org/10.1007/s10661-015-4492-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4492-8