Abstract
The use of various organophosphates to control mosquito populations is a common practice across the globe. We review the literature (LC50s) on dichlorvos, the primary breakdown product of Dibrom®, and use laboratory and field experiments to determine the lethal and sublethal (bioassays) effects of dichlorvos on two widely distributed and ecologically important estuarine invertebrate species, the marsh grass shrimp, Palaemonetes pugio and the Eastern oyster, Crassostrea virginica. Laboratory results based on LC50s and sublethal acetylcholinesterase (AChE) inhibition activity bioassays indicate that adult grass shrimp are more sensitive (∼ 500 × ) to dichlorvos than juvenile oysters. Although potentially an important factor for intertidal or shallow-dwelling estuarine organisms, the toxicity of dichlorvos was not enhanced in the presence of simulated sunlight for adult P. pugio. The most notable decreases in AChE activity were for grass shrimp and oysters exposed to dichlorvos concentrations above those considered ecologically relevant. In field experiments, both species were deployed in cages in unsprayed (n = 2) and sprayed (n = 3) sites and water samples collected pre- and post-spraying. Quantifiable dichlorvos levels were measured at the two narrowest creek treatment sites following mosquito spraying, suggesting that overspray can occur and there was evidence of a sublethal AChE response at these same sites. However, experiments at the widest creek revealed no measurable dichlorvos or sublethal responses. Results from this research suggest that adult grass shrimp are more sensitive to dichlorvos than juvenile oysters. Spraying near small tidal creeks may have measurable impacts on resident species, while larger (wider) creeks appear to be capable of buffering organisms from transient fluxes of mosquito control agents that may enter the system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Estuaries are among the world’s most productive ecosystems, but they are also important as nursery grounds for many ecologically and economically important species (e.g., Boesch and Turner 1984). Approximately 75% of U.S. shellfish and finfish species of commercial value depend on estuaries during some part of their life history (Chambers 1992). The estuaries of the southeastern United States (e.g., South Carolina) are characterized as being shallow with extensive intertidal areas covered with oysters and a tidal range of 1–3 m (Day et al. 1989; Wiegert and Freeman 1990). By the year 2000, population experts projected that 75% of the population in South Carolina would be living within 50 miles of the coast (Vernberg et al. 1992). This means that a significant portion of the uplands and drainage basins (including tidal creeks and salt marshes) are being developed or will be within the next few years. Tidal creeks form natural pathways between watersheds and estuaries (Mitsch and Gosselink 1993; Lerberg et al. 2000; Weinstein and Kreeger 2000; Holland et al. 2004). Increased runoff from developed areas, including pesticides and sewage, eventually drain into tidal creeks and estuaries. Vernberg et al. (1992) reported significant changes in estuarine systems due to anthropogenic impacts associated with coastal development. The many habitats within estuaries (e.g., mud flats, oyster reefs, and fringing salt marshes) are increasingly impacted due to coastal population expansion and its associated residential, industrial, and recreational development. Numerous papers have suggested that urbanization poses a significant risk to estuarine fauna, particularly crustaceans (e.g., Finley et al. 1999; Fulton et al. 1993; Lerberg et al. 2000; Arnold et al. 2004; Leight et al. 2005).
More than 2,500 mosquitoes species have been reported worldwide (Spielman and D’Antonio 2001), with numerous diseases such as West Nile Virus spread by their feeding mechanism. Rapid coastal development, superimposed on their habitat further adds to their disease-spreading potential by juxtaposing large numbers of people and animals with enhanced breeding grounds. Hence, there is demand for more effective and widespread control measures (Campbell et al. 2002). Most mosquito control spraying is carried out at night and in the early morning when target species are most active, helping to reduce the effects on non-target organisms (e.g., humans, pets, and livestock), but some exposure as a result of overspray and drift is inevitable. Additionally, spraying does not always correspond with high tide. As a consequence exposed areas such as mudflats and intertidal oysters may receive “drift” when young, recently settled (=spat) oysters with thin shells can, therefore, be sprayed directly when at low tide. During daylight hours, when UV-activation (phototoxicity) is a potential complication, as air-exposed, juvenile oysters may be more vulnerable to the effects of these chemicals.
One commonly used Mosquito Control Agent (MCA) is Dibrom® (1,2-dibromo-2,2-dichloroethyl dimethyl phosphate, the trade name for Naled), an organophosphate that is used to manage and control adult mosquito numbers. This is applied both aerially and using truck-mounted sprayers that use an ultra low volume (ULV) application. In 1995, the EPA estimated that approximately 50% of total Naled use in the United States is for mosquito/fly abatement (EPA 1995). Despite its extensive use, there is little information regarding environmental concentrations of Dibrom® in aquatic systems. Additionally, the primary breakdown product of Dibrom® is 2,2-dichlorovinyl dimethyl phosphate (dichlorvos), a more toxic organophosphate. This compound is also manufactured and sold as an insecticide used to control: (1) arthropods primarily in storage areas, green houses, barns, and on livestock, (2) a variety of parasitic worm infections in mammals, and (3) parasitic isopod infestations associated with fish farming in pens (Aquaguard™). Several studies have shown that, in general, crustaceans are more sensitive to dichlorvos than bivalves (e.g., McHenery et al. 1991; Le Bris et al. 1995).
Like other organophosphates, both of these compounds work by interfering with the activity of acetylcholinesterase (AChE), the enzyme responsible for hydrolysis of the neurotransmitter acetylcholine at the neuromuscular junction (e.g., Eto 1974). Inhibition of this enzyme leads to accumulation of the neurotransmitter acetylcholine attached to its receptor, thus over-stimulating the nerve and leading to prolonged muscle contraction, eventually resulting in mortality.
In southeastern U.S. estuaries, shrimp belonging to the genus Palaemonetes and the Eastern oysters (Crassostrea virginica) are amongst the most abundant and ecologically important species (e.g., Anderson 1985; Stanley and Sellers 1986; National Research Council 2004; Coen et al. 1999; Leight et al. 2005). These species play major roles in energy transfer in salt marsh ecosystems from New England to the Gulf of Mexico (e.g., Kneib 1985, 1997; Leight et al. 2005) or as “ecosystem engineers” (Jones et al. 1994) forming important habitats. Crassostrea virginica forms living subtidal and intertidal reefs that are a dominant feature of many Atlantic and Gulf coast estuaries and can be found from Canada to Argentina (reviewed in Kennedy et al. 1996). Historically, it was also an economically important species supporting major fisheries in the Chesapeake Bay, among others (Kirby 2004), Palaemonetes spp. have been used extensively in toxicity testing (e.g., Scott et al. 1992; Key and Fulton 1993; Arnold et al. 2004; Leight et al. 2005) because they are widely distributed, abundant, sensitive to environmental contaminants, and relatively easy to hold and culture in the laboratory. Oysters serve as an ideal indicator species because they are abundant, widely distributed, and easy to culture and handle in the laboratory. The spawning and recruitment seasons (Kennedy et al. 1996; Leight et al. 2005) of both study species also coincide with peak mosquito spraying (May to October) potentially exacerbating observed effects.
In the southeastern United States MCAs are regularly sprayed in and around salt marshes dissected by numerous small to large tidal creeks (e.g., Lerberg et al. 2000; Mallin et al. 2000; Holland et al. 2004). Indirect drift can often lead to the accumulation of biologically significant concentrations of MCAs in water, potentially interfering with the regular functioning or behavior of non-target organisms (Pierce 1998). With small patchy marsh habitats, there is the potential risk for indirect drift into non-target areas. Other factors also affect overspray or drift such as: (1) particle size, (2) wind velocity and direction, (3) humidity, and (4) air temperature. For example, small droplets fall more slowly and are more likely to drift off-target, whereas low temperatures and high relative humidity decrease the drift potential.
The goals of this study were to evaluate the toxicity of dichlorvos using directed laboratory and field experiments on adult daggerblade grass shrimp, Palaemonetes pugio, and the Eastern oyster, Crassostrea virginica, as it relates to mosquito spraying. Acute and sublethal laboratory experiments were employed to provide a basis for comparing the sensitivity of these two species to published values for numerous other vertebrate and invertebrate species. Field experiments were then conducted to determine environmental levels of dichlorvos following spray events and to relate any in situ responses of caged individuals to those observed in the laboratory.
Materials and Methods
Adult Palaemonetes pugio (21–35 mm) were collected from the western branch of Leadenwah Creek, Charleston, South Carolina. This creek has been used as a “reference creek” for numerous studies (e.g., Key et al. 1998a, b; Scott et al. 1999; Leight et al. 2005). Shrimp (exclusive of ovigerous females) were held in aerated 25-L aquaria at 25°C in 30–35 ppt filtered natural seawater. Crassostrea virginica (30–45 mm shell height) were obtained from Auburn University’s oyster hatchery at the Dauphin Island Sea Lab, in Dauphin Island, Alabama. Oysters were held in 114-L aerated tanks at 25°C in 30–35 ppt filtered natural seawater. Specimens were acclimated to laboratory conditions for a period of at least one week prior to use.
Laboratory Experiments
Acute Toxicity Tests
The median lethal concentration or LC50 was determined for adult grass shrimp exposed to dichlorvos. The 96-h definitive test consisted of 10 shrimp/replicate, with animals exposed in 1-L beakers, each of which contained 800 ml of media (N = 3 replicates/treatment). Experiments were set up to determine if there were any phototoxic effects (such that the compound was more toxic during exposure to UV light) using two light regimes (UV and dark), under a bank of sunlight-simulating fluorescent lights (24-h light photoperiod). Nominal concentrations of dichlorvos were 0.004, 0.011, 0.033, 0.1 mg/L, and control. Water quality (temperature, pH, salinity, and dissolved oxygen) was monitored daily using a YSI DO meter (Model 85) and temperature-compensated refractometer throughout the experiment’s duration.
Results from the shrimp acute toxicity tests suggested that there was little or no phototoxicity associated with this compound. Since crustaceans are generally more sensitive to organophosphates than molluscs (e.g., McHenery et al., 1991), UV and dark regimes were not tested in oysters. The 96-h range finding test consisted of five oysters/beaker, exposed in 1-L beakers with 600 ml of media under cool white lights (24-h light photoperiod). There was no replication of treatments in this test. Nominal dichlorvos concentrations were 1,10,100, 1000 mg/L, and two controls. A natural filtered seawater control and a carrier control (containing 6 ml acetone) were used in this test. Because of the high concentrations of dichlorvos used (not environmentally relevant) and the limited mortality observed, a definitive test was not performed on the oysters.
Acetylcholinesterase (AChE) Inhibition
AChE inhibition is widely used as an indicator of organophosphate insecticide exposure (e.g. Galgani and Bocquené 1990; Key and Fulton 1993; Key et al. 1998a, b; Varó et al. 2002). Activity of whole body AChE was measured following Key et al. (1998a, b). Briefly, tissue samples were homogenized in buffer (50 mM Tris-HCl) and incubated for 15 min. A color reagent [5, 5′-dithiobis-(2-nitrobenzoic acid)] and a substrate (acetylthiocholine) were added to the sample and it was read in a spectrophotometer (Ultraspec 4300 pro) for 70 sec at a wavelength of 412 nm. An eserine blank was also assayed to account for nonenzymatic hydrolysis of the substrate. Protein content was also determined using Lowry’s reagent in a spectrophotometric assay. Whole body AChE activity was expressed as nanomoles of product formed per mg of protein per min (nmol/mgP/min).
The grass shrimp 24-h test consisted of 15 shrimp/replicate exposed in 1-L beakers, each of which contained 600 ml of media (N = 3 replicates/treatment). Nominal dichlorvos concentrations were 0.195, 0.781, 3.125,12.5, 50 μg/L and two controls (natural filtered seawater and a carrier control, containing 1 ml acetone). The oyster 24-h test consisted of two oysters/replicate, exposed in 1-L beakers, each of which contained 600 ml of media (N = 3 replicates/treatment). Nominal concentrations of dichlorvos were 0.1, 0.29, 0.8, 2, 5 mg/L, and two controls (natural filtered seawater and a carrier control, containing 6 ml acetone).
Field Experiments
Field experiments were conducted at multiple “reference” (=unsprayed) and “treatment” (=sprayed) sites (Table 1). Sites were classified as either “reference” or “treatment” sites based on Charleston County Mosquito Abatement Program’s (CCMAP) spraying routes (Martin Hyatt, CCMAP personal communication) or prior use by other researchers (e.g., Key et al. 1998a, b; Scott et al. 1999; Leighton et al. 2005). All sites were also chosen for their close proximity to roads and accessibility for deploying experiments. The two “reference” sites were Leadenwah Creek and Swinton Creek, neither currently receiving mosquito treatment by CCMAP (Martin Hyatt personal communication). Both sites are located near single-family homes with no large-scale commercial agricultural inputs. The three “treatment” sites were (1) Folly Creek (2) James Island Creek, and (3) Sandy Bay Creek. All three creeks are located along CCMAP spray routes. Creeks widths (measured using perpendicular distance on recent aerial images) varied from 16 to 130 m (Table 1).
All field deployments were coordinated with CCMAP personnel. Deployments were carried out on days with no rainfall when low tide coincided with their nighttime spraying schedule to ensure conditions were similar for each deployment. Treatment sites consisted of areas sprayed with Dibrom® only. Clear plastic (polyurethane) cages, designed to hold multiple shrimp and oysters in separate compartments, were used for field deployments (see Arnold et al. 2004; Bolton-Warberg 2005). These cages retained experimental animals, prevented predation, and maintained sufficient water flow to support both species. Each cage was divided into twelve compartments, and these were deployed intertidally for oysters and subtidally for grass shrimp. At each site, shrimp (24 individuals per cage, N = 5) and oysters (3 individuals per cage, N = 5) were deployed at low tide for a period of 24 h. Shrimp cages were placed subtidally approximately 50 cm off the bottom, while oysters were deployed intertidally to simulate their natural habit.
Both “reference” and “treatment” sites were evaluated prior to any pesticide application to establish baseline levels of mortality and AChE for each site (Table 1, August to November 2004). Water quality parameters (water temperature, dissolved oxygen and salinity) were measured upon deployment and again at retrieval of cages using a YSI (85) DO meter and a temperature-compensated refractometer. Two replicate creek water samples (4-L) for each site were collected at the time of deployment and cage retrieval to quantify background levels of dichlorvos and Naled for each site.
Field Deployments
Prior to a spaying event, grass shrimp and oysters were deployed for 24 h at low tide at each treatment site (see Table 1 for dates). Cages were deployed as described above for baseline assessments with five replicate cages. Two creek water samples (4-L each) were taken at the time of deployment, and water quality parameters, time of day, and tide were recorded. Dibrom® was applied by CCMAP during routine spraying of a site following low tide between 0000 h and 0500 h. Water samples were taken at the first high tide following pesticide application for the second set of applications at two of the sites (Folly and Sandy Bay Creeks).
Field Water Processing and Analyses
Each water sample (∼4-L) was processed using protocols developed by NOAA-NCCOS Center for Coastal Environmental Health and Biomolecular Research Lab (CCEHBR) in Charleston, SC (adapted from Lehotay et al. 1999). Briefly, the samples were filtered twice, first through 90-mm and 45-mm diameter Whatman® glass fiber (GF/F) filters and then through a solid phase extraction cartridge (ENV+ISOLUTE®). The cartridges were dried using a vacuum pump and stored in a freezer (−20°C) until further processing. Samples were eluted in methylene chloride using a Rotovap (Büchi RE121 Rotovapor) and stored in glass vials until GC/MS analysis.
Concentrations of dichlorvos and Naled were determined following EPA Method 8141A (EPA 1994) using a ThermoQuest Trace Gas Chromatograph (GC)-Polaris Q Mass Spectrometer. At a temperature of 60°C, 1-μL injections were made onto a 30-m DB-5 column. Injections were held at 60°C for 2 min and ramped linearly at 20°C/min to 270°C, where they were held for 3 min.
Statistics
Median lethal values (LC50s) were calculated from laboratory tests using PCSAS (Version 9.1, Proc PROBIT command) and Probit® analysis. Values are reported as the LC50 (95% confidence intervals). One-way Analyses of Variance (ANOVAs) were used to determine significant differences (p ≤ 0.05) among treatments for AChE inhibition in both species (using JMP, Version 3.3). If a significant difference was detected, a Dunnett’s Multiple Comparison Test was used to determine which treatments were significantly different from the control. For the field studies, four analyses were performed for each species. First, baseline levels from “reference” and “treatment” sites were compared to levels measured in control shrimp to ensure any changes were not caused by deployment itself. Second, baseline levels from “reference” and “treatment” sites were compared to each other. Third, baseline and spray events were compared using data from all sites. Finally, baseline and spray events were compared within each site.
Results
Acute Toxicity
In shrimp, the highest mortality for either UV or dark regimes (93 ± 3% and 90 ± 6%, respectively, mean ± 1 standard error) occurred in the highest dichlorvos concentration tested (Fig. 1, 0.1 mg/L,). Mortality in control shrimp was less than 10%. The 96-h LC50 values for shrimp in the UV and dark regimes were similar. In the UV regime, the 96-h LC50 value was 0.062 mg/L (0.039–0.109 mg/L), while for the dark regime, the 96-h LC50 value was 0.057 mg/L (0.026–0.216 mg/L) (see Table 2). In oysters, mortality due to dichlorvos occurred in a concentration-dependent fashion (see Fig. 2). Mortality ranged from 0% in control oysters to 100% in those oysters exposed to the 1,000 mg/L dichlorvos treatment. The 96-h LC50 for oysters was 31.62 mg/L (Table 2, 5.04–198.42 mg/L).
Acetylcholinesterase (AChE) Activity
For shrimp, the highest concentration at which no change in AChE activity attributable to dichlorvos exposure occurred was 12.5 μg/L (No Observable Effects Concentration or NOEC). There was, however, a significant difference among treatments (F6,12 = 5.7199; p = 0.0051), with significantly lower activities (by Dunnett’s Multiple Comparison Test, 77% inhibition) measured in shrimp exposed to the 50 μg/L dichlorvos treatment (Lowest Observable Effects Concentration or LOEC) compared to control shrimp (see Fig. 3). In contrast for oysters, no significant difference was found between AChE activities in dichlorvos-exposed and control individuals (F6,12 = 1.7616; p = 0.1901). However, it is important to note that oysters exposed to dichlorvos had observed inhibitions that ranged from 28–79%, as compared to control oysters (see Figs. 4–6).
Acetylcholinesterase (AChE) activity in Palaemonetes pugio exposed to experimental concentrations of dichlorvos for 24 h. Bars represent the mean of five shrimp +1SE. *Significant difference from the control. The bold dashed horizontal line represents the average AChE activity measured in all control individuals
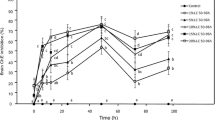
Acetylcholinesterase (AChE) activity in Palaemonetes pugio deployed for 24 h at treatment and reference sites (dates given). Bars represent the mean of five shrimp +1SE. *Significant difference from baseline levels within each site. The bold dashed-horizontal line represents the average AChE activity measured in control individuals
Acetylcholinesterase (AChE) activity in Crassostrea virginica deployed for 24 h at treatment and reference sites (dates given). Bars represent the mean of five oysters +1SE. Asterisks denote a significant difference from baseline levels within each site. The bold dashed-horizontal line represents the average AChE activity measured in control individuals
Field Acute Toxicity
Mortality in field-deployed shrimp was <1% (2 out of 638 shrimp deployed). An average of 20% escaped per cage, equivalent to ∼ five shrimp of 24 deployed per cage (ranging from 0 to 45% per cage). There was no mortality of oysters in field experiments.
Field Acetylcholinesterase Inhibition
In shrimp, average activities measured for all baseline individuals were not significantly different from the average activity measured across all laboratory control individuals (t-test = −0.238; df = 12; p = 0.8126) in this study. Baseline AChE activities were significantly different between treatment and reference sites (F4,20 = 10.6605; p < 0.0001). Shrimp deployed at Swinton Creek had a significantly higher level of activity compared to shrimp deployed in Sandy Bay Creek, while activities in shrimp deployed at Folly and Leadenwah Creek were higher than those of shrimp deployed at James Island and Sandy Bay Creeks. A significant difference was detected between AChE activity in shrimp deployed during spray events and baseline shrimp for all sites (F2, 50 = 3.5521; p = 0.0361), with baseline shrimp having higher AChE activity than those deployed during the second spray event.
For Folly Creek, shrimp deployed during the second spray event had significantly lower AChE activities (56% inhibition of control activities) as compared to shrimp deployed during the baseline study (F2,12 = 9.3237; p = 0.0036). At James Island Creek, there was no significant difference in the AChE activity measured in baseline shrimp compared to the shrimp deployed during spray events (F2, 11 = 3.0476; p = 0.0885). At Sandy Bay Creek, shrimp deployed during the second spray event had significantly higher AChE activities than the shrimp deployed during the baseline study (F2, 11 = 13.3511; p = 0.0011).
In oysters, mean AChE activity measured for baseline individuals at all three treatment sites and those deployed at the two reference sites were significantly greater than the average activity measured across all laboratory control oysters (t-test = 6.404; df = 43; p <0.0001) in this study. Baseline AChE activities in oysters were significantly different among all five field sites (F4, 19 = 7.3114; p = 0.0010). Oysters deployed at Folly Creek and Leadenwah Creek had significantly higher levels of baseline AChE activity compared to the oysters deployed in James Island Creek and Sandy Bay Creek. For all treatment and reference sites, oysters deployed during the second spray events had significantly higher AChE activities compared to baseline/reference oysters and those deployed during the first spray events (F2,51 = 3.3692; p = 0.0422).
For Folly Creek, there was no significant difference in AChE activity measured in baseline oysters compared to the oysters deployed during both spray events (F2,12 = 1.9917; p = 0.1791). At James Island Creek, oysters deployed during the second spray event had significantly higher AChE activities than baseline oysters (F2, 12 = 4.5296; p = 0.0342). At Sandy Bay Creek, oysters deployed during the second spray event had significantly higher AChE activities than baseline oysters (F2, 12 = 5.3416; p = 0.0219).
GC/MS Analysis of Water Samples
Method limits of detection (LOD) and quantitation (LOQ) for dichlorvos were determined from standard curves. The LOD for dichlorvos was 0.1775 mg/L and the LOQ was 0.2 mg/L. Water samples containing concentrations of dichlorvos in between these two values were considered present but not quantifiable. The average spike recoveries for two surrogate standards were 118% and 75%. Concentrations of dichlorvos and Naled were not detected in water samples taken during either the baseline deployments or the first spray event. Several water samples taken during the second spray events contained dichlorvos, although no Naled was detected in any of the samples (see Table 3).
Discussion
Laboratory Experiments
Overall, shrimp mortality was not different between light regimes (dark and UV) at the same concentrations of dichlorvos. This suggests that there was no dichlorvos-related phototoxicity for adult grass shrimp despite reports by the U.S. Public Health Service (1995) that dichlorvos is 5–150X more toxic to aquatic life in the presence of UV radiation. There is evidence that dichlorvos is phototoxic to larval Palaemonetes pugio (Weinstein, unpublished data), and studies have shown that larvae are generally more sensitive to organophosphates than adults (e.g., Key et al. 1998a, b; Forget et al. 1998). The finding that grass shrimp are more sensitive to dichlorvos than oysters, based on their respective LC50 values, is in general agreement with published values. Cusack and Johnson (1990) reported no mortality of the common mussel (Mytilus edulis) exposed to dichlorvos concentrations ranging from 0.001 to 1 mg/L, while mortality in adult common lobsters (Homarus gammarus) began after 25 min exposure to 1 mg/L dichlorvos.
Several studies have investigated the sublethal effects of dichlorvos exposure on crustaceans and molluscs. For example, McHenery and Francis (1990) reported a decrease in AChE activity in lobster larvae exposed to dichlorvos (0.08 to 10 μg/L). Le Bris et al. (1995) reported that dichlorvos relaxed the adductor muscle of two commercial bivalves, the Manila clam, Ruditapes philippianium, and the Japanese oyster, Crassostrea gigas, up to 42 h after exposure to dichlorvos (0.1 and 1 mg/L). In the present study, gaping oysters (i.e., those with relaxed adductor muscles) were observed in two experimental dichlorvos concentrations (10 and 100 mg/L) during the 96-h toxicity test. It is possible that this gaping is due to muscle fatigue as a result of prolonged contraction that is associated with organophosphate exposure. Although it is unlikely that these concentrations would be encountered in the environment, it is possible that lower concentrations could have a similar affect on more sensitive life stages.
Acetylcholinesterase inhibition is indicative of organophosphate poisoning and has been measured in numerous other crustacean species (e.g., Key et al. 1998a, b), molluscs (e.g., Le Bris et al. 1995), and fish species (e.g., Sturm et al. 1999). In crustaceans, lethal effects have been reported at inhibitions of 40% or below (Escartm and Porte 1996), while Lundebye et al. (1997) reported that a 30% reduction in cholinesterase activity was not lethal in the European green crab, Carcinus maenas.
In the present study, shrimp AChE inhibition was highest in those shrimp exposed to 0.05 mg dichlorvos/L (77% inhibition). Coppage and Matthews (1974) reported that an 80% inhibition in activity was critical in short-term organophosphate poisoning in general, with 75% inhibition causing mortality in the pink shrimp, Farfantepenaeus duorarum. Caution should be taken when interpreting inhibition levels, as species-specific differences exist, whereby one level of inhibition is lethal for one species, and not for another.
Although oysters exposed to sublethal concentrations of dichlorvos (0.1 to 5 mg/L) in this study had measurable inhibition of AChE (44–79% inhibition), no treatments yield statistically significantly differences when compared with the control. Le Bris et al. (1995) found depressed levels of AChE activity in oysters and clams exposed to experimental dichlorvos concentrations (0.1 and 1 mg/L). The results in the present study suggest that AChE inhibition may be a good indicator of dichlorvos toxicity in adult grass shrimp and oysters under laboratory conditions, but perhaps not at environmentally relevant concentrations.
Field Experiments
Pesticide drift and deposition into non-target areas are important concerns when mosquito abatement programs are trying to employ Best Management Practices (BMPs) (e.g., Tietze et al. 1995). Numerous studies have looked at the effects of overspray and drift of these compounds (e.g., Hennessey et al. 1992; Snoo and de Wit 1998). In the Florida Keys, Hennessey et al. (1992) reported drift of aerially applied Dibrom® up to 750 m downwind of the target area.
Several studies on the occurrence of aerial applications of fenthion and malathion (MCAs) in salt marsh water indicate that approximately 5–6% of the application rate is deposited in the water (see Wang et al. 1987; Tietze et al. 1994). This amount may be increased with ground-based applications, because the pesticide has less time to disperse.
There are many factors that can contribute to spray drift including, particle size, wind velocity, temperature, and humidity. For example, the smaller the droplet size, the further it will disperse, especially if there is wind. Ground-based applications of Dibrom® in Charleston County are ULV (ultra low volume), which consists of a fine mist (<15 μm), and the swath widths for application are approximately 90 m wide. Thus, there is great potential for drift of this mist into non-target areas, especially when creeks are in close proximity to the road. As mentioned earlier, recently settled oysters can be sprayed directly at low tide, potentially exposing them to relatively high effective concentrations in the field. Here we attempted to measure drift directly using oil-sensitive drift cards, but encountered erratic results (Bolton-Warberg 2005).
In the present study, chemical analysis of water samples revealed quantifiable levels of dichlorvos. Previous studies looking at environmental concentrations of Dibrom® following a spraying event are limited and only one evaluates the presence of measurable concentrations of the breakdown product dichlorvos. This study, carried out in the Florida Keys, reported measurable concentrations of dichlorvos in seawater (0.07–1.0 μg/L) following routine aerial spraying of Dibrom®. However, no Dibrom® was detected in any of the water samples (Pierce 1998). The half-life of dichlorvos can vary in response to a variety of physical parameters. For example, Wells et al. (1990) estimated that the half-life of dichlorvos in seawater (14°C) was six days, while Lartiges and Garrigues (1995) reported the presence of dichlorvos after six months in seawater (25 ppt) with a pH of 8.1 and a temperature of 22°C. With decreasing salinity, its half-life is shortened (Dobson and Tack 1991).
All of the values for AChE activity in the present study are in the same range as those reported in other studies (see Table 2) for grass shrimp and oysters. Acetylcholinesterase inhibition occurred in shrimp deployed at Folly Creek for both spray events (20% and 56% of baseline levels, respectively). These inhibitions are indicative of organophosphate poisoning, and measurable dichlorvos concentrations from the second experiment suggest that the AChE inhibition may be a result of dichlorvos exposure. Other organophosphates in the water may account for this result; however, only dichlorvos and naled were evaluated in this study. In the lab, similar experimental dichlorvos concentrations (0.195 μg/L dichlorvos) did not cause AChE inhibition in shrimp. Laboratory studies generally do not take into account any naturally occurring stressors, such as fluctuations in dissolved oxygen or temperature, or any combined effects, and therefore may not be good estimators of field toxicity. In contrast, at Sandy Bay Creek, elevated AChE levels were observed in grass shrimp deployed in the second spray event where dichlorvos was detected in water samples.
This type of response has been referred to as a “hormetic” response (Stebbing 1982), where a toxic substance acts as a stimulant in small doses, but is an inhibitor in large doses, Stebbing (1982) also reported that prolonged exposure at these low concentrations caused the typical decrease associated with inhibitors. It is possible that prolonged grass shrimp and oyster exposures to dichlorvos might also yield depressed AChE levels similar to those associated with organophosphate exposures. Similarly, increased AChE activities were observed in oysters in the second spray events at two other sites (James Island and Sandy Bay Creek). However, no dichlorvos was detected in water samples from James Island Creek, whereas dichlorvos. was measured from Sandy Bay Creek as mentioned above. Parallel effects have been observed for amphipods (e.g., Hyale nilssoni, Murison et al. 1997) and lobster (Homarus gammarus) larvae (McHenery et al. 1991) exposed to dichlorvos so that this is indeed a widely observed phenomenon for a diverse array of responses (e.g., growth) and compounds in the toxicological literature (Stebbing 1982; Calabrese and Baldwin 1998).
At one of our reference sites, Leadenwah Creek, water samples had low, but detectable dichlorvos concentrations. However, no significant differences were found in AChE activity at this site versus those observed in the control for either species employed here. Although no spraying in the area was reported by CCMAP, dichlorvos can be introduced through many other common household uses (e.g., flee collars, farm insecticides), thus accounting for its presence.
Both natural and anthropogenic stressors may affect the sensitivity of bioassays such as acetylcholinesterase in field studies. Laboratory studies that take into account natural stressors such as variations in pH, salinity, and dissolved oxygen may provide a more accurate interpretation of sublethal responses measured in the field. Two other bioassays (lysosomal destabilization and lipid peroxidation) have been tested by us as potential indicators of dichlorvos exposure. However, no clear responses were observed in either laboratory or field experiments (Bolton-Warberg 2005).
Summary and Conclusions
This study is one of the few to date that has examined dichlorvos and Naled in both field and lab trials using these two important and broadly distributed estuarine species along both the western Atlantic and Gulf of Mexico coasts. The results from this research suggest that adult grass shrimp are more sensitive to dichlorvos than juvenile oysters. No dichlorvos-induced phototoxicity was detected for adult grass shrimp in the 96-h acute toxicity tests. Previous studies have shown that acetylcholinesterase (AChE) inhibition is a good biomarker of organophosphate exposure, particularly for chemical contaminants. AChE inhibition is a sensitive sublethal response to dichlorvos in the laboratory for both species. Ideally biomarkers should not be sensitive to natural stressors and it is important to choose one that is sensitive to the test contaminant.
Chemical analysis of water samples demonstrated that detectable levels of dichlorvos were measured at several sites, suggesting that occasional overspray from routine mosquito spraying may occur. There were no clear sublethal responses in the field attributable to dichlorvos exposure for the two species evaluated. With such wide variations in sensitivity to dichlorvos, it is possible that other more sensitive taxa (or life history stages) would be more susceptible to exposure from mosquito spraying. For example, larvae in general are more sensitive to organophosphate exposure than adults of the same species. Spraying near small tidal creeks may have measurable impacts on resident species, while larger creeks appear to be capable of buffering organisms from transient fluxes of contaminants that may enter the system perhaps due to flushing rates.
At present, spraying is carried out at night, with little/no wind and no rain, to reduce the effects on non-target organisms. At this time, mosquitoes are most active and the pesticides are more likely to be effective. Other BMPs such as no spraying over bridges, spraying at high tide, implementing buffer zones, increasing droplet size, and limiting the number of applications that can be made at each location may help to minimize the effects on no-target species. Spraying at high tide would allow for tidal flushing to reduce the amount of time that organisms would be exposed to the chemical. Payne et al. (1988) assessed buffer zone width for ground-based applications of permethrin (a mosquito adulticide), by choosing an acceptable low mortality and then determining the distance (downwind) at which this value is obtained (20 m for permethrin). Using spray additives, larger orifice sizes, and low pressure serves to increase the droplet sizes and pesticide effectiveness. Larger droplets have a lower potential to drift off-target. Drift-reduction nozzles are available that produce large droplets at low pressures. At present, the amount of times a given location is sprayed is dependent on mosquito densities and equipment availability. These types of practices can help reduce the amount of spray drift occurring.
References
Anderson G (1985) Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (Gulf of Mexico) -grass shrimp. U.S. Fish and Wildlife Service Biological Report. 82(11.35). U.S. Army Corps of Engineers TR EL-8-24, 19pp
Arnold GL, Luckenbach MW, Unger MA (2004) Runoff from tomato cultivation in the estuarine environment: biological effects of farm management practices. J Exp Mar Biol Ecol 298:323–346
Boesch DF, Turner RE (1984) Dependence of fishery species on salt marshes; the role of food and refuge. Estuaries 7:460–468
Bolton-Warberg M (2005) Effects of the organophosphate insecticide dichlorvos on the daggerblade grass Shrimp, Palaemonetes pugio and the eastern oyster, Crassostrea virginica with reference to mosquito spraying. M.S. Thesis, College of Charleston, p 123
Calabrese EJ, Baldwin LA (1998) Hormesis as a biological hypothesis. Environ Health Perspect 106:357–362
Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ (2002) West Nile Virus, Lancet Infect Dis 2:519–529
Chambers JR (1992) Coastal degradation and fish population losses. In: Stroud RH (ed) Stemming the tide of coastal fish habitat loss. National Coalition for Marine Conservation. Savannah, Georgia, p 285
Coen LD, Luckenbach MW, Breitburg DL (1999) The role of oyster reefs as essential fish habitat: a review of current knowledge and some new perspectives. In: Benaka LR (ed) Fish habitat: essential fish habitat and rehabilitation. American Fisheries Society, Symposium 22, Bethesda, MD. p 438–454
Coppage DL, Matthews E (1974) Short-term effects of OP pesticides on cholinesterases of estuarine fishes and pink shrimp. Bull Environ Contain Toxicol 11:483–488
Cusack R, Johnson G (1990) A study of dichlorvos (Nuvan; 2, 2 dichloroethenyl dimethyl phosphate), a therapeutic agent for the treatment of salmonids infected with sea lice (Lepeophtheirus salmonis). Aquaculture 90:101–112
Day JW, Hall CA, Kemp WM, Yanez-Arancibia A (1989) Estuarine ecology. John Wiley and Sons, New York, p 576
Dobson DP, Tack TJ (1991) Evaluation of the dispersion of treatment solutions of dichlorvos from marine salmon pens. Aquaculture 95:15–32
EPA (1995) Summary Report for Naled. Environmental Fate and Effects Division. Office of Prevention, Pesticides and Toxic Substances, p 26
Escartín E, Porte C (1997) The use of cholinesterase and carboxylesterase activities from Mytilus galloprovincialis in pollution monitoring. Environ Toxicol Chem 16:2090–2095
Eto E (1974) Organophosphorous pesticides and biological chemistry. CRC Press, Cleveland, OH, p 387
Finley DB, Scott GI, Daugomah JW, Layman SL, Reed LA, Sanders M, Sivertsen SK, Strozier ED (1999) Case study: Ecotoxicological assessment of urban and agricultural nonpoint source runoff effects on the grass shrimp, Palaemonetes pugio. In: Ecotoxicology and risk assessment for wetlands. Lewis MA, Mayer FL, Powell RL, Nelson MK, Klaine SJ, Henry MG, Dickson GW (eds). SETAC Press, USA, pp 243–274
Forget J, Pavilion JF, Menasria MR, Bocquene G (1998) Mortality and LC50 values for several stages of the marine copepod Tigriopus brevicornis (Müller) exposed to the metals arsenic and cadmium and the pesticides atrazine, carbofuran, dichlorvos and malathion. Ecotoxicol Environ Saf 40:239–244
Fulton MH, Scott GI, Fortner A, Bidleman TF, Ngabe B (1993) The effects of urbanization on small high-salinity estuaries of the southeastern United States. Arch Environ Contam Toxicol 25:476–484
Hennessey MK, Nigg HN, Habeck DH (1992) Mosquito (Diptera: Culicidae) adulticide drift into wildlife refuges of the Florida Keys. Environ Entomol 21:714–721
Holland AF, Sanger DM, Gawle CP, Lerberg SB, Santiago MS, Riekerk GHM, Zimmerman LE, Scott GI (2004) Linkages between tidal creek ecosystems and the landscape and demographic attributes of their watersheds. J Exp Mar Biol Ecol 298:151–178
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Kennedy VS, Newell RIE, Eble AF (1996) The Eastern Oyster: Crassostrea virginica. Maryland Sea Grant, College Park, Maryland, p 734
Key PB, Fulton MH (1993) Lethal and sub-lethal effects of chlorpyrifos exposure on adult and larval stages of the grass shrimp, Palaemonetes pugio. J Environ Sci Health 28:621–640
Key PB, Fulton MH, Layman SL, Scott GI (1998a) Azinphosmethyl exposure to grass shrimp (Palaemonetes pugio) life stages with emphasis on larval acetylcholinesterase activity. Bull Environ Contam Toxicol 60:645–650
Key PB, Fulton MH, Scott GI, Layman SL, Wirth EF (1998) Lethal and sub-lethal effects of malathion on three life stages of the grass shrimp, Palaemonetes pugio. Aquat Toxicol 40:311–322
Kirby MX (2004) Fishing down the coast: historical expansion and collapse of oyster fisheries along coastal margins. Proc Natl Acad Sci 101:13096–13099
Kneib RT (1985) Predation and disturbance by grass shrimp, Palaemonetes pugio Holthius in soft-substratum benthic invertebrate assemblages. J Exp Mar Bio Ecol 93:215–223
Kneib RT (1997) Early life stages of resident nekton in intertidal marshes. Estuaries 20:214–230
Lartiges SB, Garrigues PP (1995) Degradation kinetics of organophosphorous and organonitrogen pesticides in different waters under various environmental conditions. Environ Sci Technol 29:1246–1254
Le Bris H, Maffart P, Bocquené G, Buchet V, Galgani F, Blanc G (1995) Laboratory study on the effect of dichlorvos on two commercial bivalves. Aquaculture 138:139–144
Lehotay SJ, Harman-Fetcho JA, McConnell LL (1999) Agricultural pesticide residues in oysters and water from two Chesapeake Bay tributaries. Mar Pollut Bull 37:32–44
Leight AK, Scott GI, Fulton MH, Daugomah JW (2005) Long term monitoring of grass shrimp Palaemontes spp. population metrics at sites with agricultrual runoff influences. Integr Comp Biol 45:143–150
Lerberg SB, Holland AF, Sanger DM (2000) Responses of tidal creek macrobenthic communities to the effects of watershed development. Estuaries 23:838–853
Lundebye AK, Curtis T, Braven J, Depledge M (1997) Comparative properties of channel catfish (Ictalurus punctatus) and blue crab (Callinectes sapidus) acetylcholinesterase. Comp Biochem Physiol, Part C: Toxicol Pharmacol 91:293–300
Mallin MA, Burkholder JM, Cahoon LB, Posey MH (2000) North and South Carolina coasts. Mar Pollut Bull 41:56–75
Mayer FL Jr, Ellersieck, MR (1986) Manual of Acute Toxicity: Interpretation and Data Base for 410 Chemicals and 66 Species of Freshwater Animals. Resour Publ No. 160, U.S. Dept. Interior, Fish Wildl. Serv. Washington, DC, 505pp
McHenery JG, Francis C (1990) Toxicity of dichlorvos to stage 4 Homarus gammarus larvae. Scottish Fisheries Working Paper 8/90
McHenery JG, Saward D, Seaton DD (1991) Lethal and sub-lethal effects of the salmon debusing agent dichlorvos on the larvae of the lobster (Homarus gammarus L.) and herring (Clupea harengus L.). Aquaculture 98:331–347
Mitsch WJ, Gosselink JG (1993) Wetlands, 2nd edition. Van Nostrand Reinhold, New York p 72
National Research Council (2004) Nonnative oysters in Chesapeake Bay. National Academies Press. Washington, DC, p 325
Payne NJ, Helson BV, Sundaram KMS, Fleming RA (1988) Estimating buffer zone widths for pesticide applications. Pestic Sci 24:147–161
Pierce RH (1998) Effects of mosquito control measures on non-targeted organisms in the Florida Keys National Marine Sanctuary. Mote Marine Laboratory Technical Report No 609
Scott GI, Fulton MH, Crosby MC, Key PB, Daugomah JW, Walden JT, Strozier ED, Louden CJ, Chandler GT, Fidleman TF, Jackson KL, Hampton TW, Hoffman T, Shultz A, Bradford M (1992) Agricultural nonpoint runoff effects on estuarine organisms: correlating laboratory and field bioassays and ecotoxicological biomomtoring. Final Report. U.S. EPA, Gulf Breeze, FL, p 281
Scott GI, Fulton MH, Moore DW, Wirth EF, Chandler GT, Key PB, Daugomah JW, Strozier ED, Devane J, Clark JR, Lewis MA, Finley DB, Ellenberg W, Karnaky JJ Jr (1999) Assessment of risk reduction strategies for the management of agricultural nonpoint source pesticide runoff in estuarine ecosystems. Toxicol and Ind Health 15:200–213
Snoo GR, de Wit PJ (1998) Buffer zones for reducing pesticides drift to ditches and risks to aquatic organisms. Ecotoxicol Environ Saf 41:112–118
Spiehnan A, D’Antonio M (2001) Mosquito: a natural history of our most persistent and deadly foe. Hyperion. New York, p 247
Stanley DW, Sellers MA (1986) Species profile: life histories and environmental requirements of coastal fishes and invertebrates (Gulf of Mexico)-American Oyster. U.S. Fish Wildl Serv Biol Rep 82(11.64) U.S. Army Corps of Engineers, TR EL-82-4, p 25
Stebbing ARD (1982) Hormesis- the stimulation of growth by low levels of inhibitors. Sci Total Environ 22:213–234
Sturm A, da Silva de Assis HC, Hansen PD (1999) Cholinesterases of marine teleost fish: Enzymological characterization and potential use in biomonitoring of neurotoxic contamination. Mar Environ Res 47:389–398
Tietze NS, Hester PG, Shaffer KR (1994) Mass recovery of malathion in simulated open field mosquito adulticides tests. Arch Environ Contain Toxicol 26:473–477
Tietze NS, Hester PG, Shaffer KR (1995) Acute effects of Permanone registered 31–66 (permethrin-piperonyl butoxide) on nontarget minnows and grass shrimp. J Am Mosq Control Assoc 11 476–479
U.S. Department of Interior, Fish and Wildlife Service (1980) Handbook of Acute Toxicity of Chemicals to Fish and Aquatic Invertebrates. Resource Publication No.137. Washington, DC: U.S. Government Printing Office
US Public Health Service (1995) Hazardous Substance Data Bank. Washington, D.C
Vernberg FJ, Vernberg WB, Blood E, Fortner A, Fulton MH, McKellar H, Mitchner W, Scott GI, El-Fiji K (1992) Impacts of urbanization on high salinity estuaries of the Southeastern U.S. Netherlands. J Deep Sea Res 30:239–248
Verschueren K (1983) Handbook of environmental data on organic chemicals, 2nd ed., Van Nostrand Reinhold Company, New York, NY, 839pp
Wang TC, Lenahan RA, Tucker Jr JW (1987) Deposition and persistence of aerially-applied fenthion in a Florida estuary. Bull Environ Contam Toxicol 38:226–231
Weinstein MP, Kreeger DA (2000) Concepts and controversies in tidal marsh ecology. Kluwere Academic Publishers, Boston, p 875
Wells DE, Robson JN, Finlayson DM (1990) Fate of dichlorvos (DDVP) in sea water following treatment for salmon louse, Lepeophtheirus salmonis, infestations in Scottish fish farms. Scottish Fisheries Working Paper 13/90
Wiegert RG, Freeman BJ (1990) Tidal salt marshes of the southeast Atlantic Coast: A community profile. U.S. Fish Wildl Serv Biol Rep 85:1–67
Acknowledgments
We wish to acknowledge Amy Ringwood for technical assistance and Keith Walters for statistical advice throughout this study. Special thanks to Pete Key, Marie DeLorenzo, Jen Hoguet, Chuck Keppler, Kevin Crawford, Martin Hyatt, Ed Harne, staff at the Center for Coastal Environmental Health and Biomolecular Research Lab (CCEHBR) and the Shellfish Research Section, Marine Resources Research Institute, SCDNR, for their laboratory and field assistance. This research was made possible through grant funds to L.D.C. from the SC Maine Recreational Fisheries Stamp Program, CICEET (NA17OZ2507), and the South Carolina Sea Grant Consortium (nos. NA86RG0052 and NA16RG2250) and to J.E.W. through The Citadel Foundation. This is contribution no. 294 from the Grice Marine Laboratory, College of Charleston, and from SCDNR no. 591.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bolton-Warberg, M., Coen, L.D. & Weinstein, J.E. Acute Toxicity and Acetylcholinesterase Inhibition in Grass Shrimp (Palaemonetes pugio) and Oysters (Crassostrea virginica) Exposed to the Organophosphate Dichlorvos: Laboratory and Field Studies. Arch Environ Contam Toxicol 52, 207–216 (2007). https://doi.org/10.1007/s00244-005-0325-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0325-z