Abstract
Agricultural activity within coastal watersheds results in estuaries becoming the receiving environment for pesticide inputs. In estuaries, salinity can alter insecticide responses of exposed crustaceans. The acute toxicity of environmentally relevant doses of chlorpyrifos and imidacloprid were examined using the euryhaline amphipod Gammarus lawrencianus at 20 and 30 Practical Salinity Units (PSU). Responses were recorded every 24 h until an incipient (threshold) L(E)C50 was reached. For chlorpyrifos, LC50 ranged from 0.1 to 0.5 µg/L and was two-fold higher at 30 vs. 20 PSU at all time-points over the 96 h exposure. Imidacloprid immobility EC50 ranged from 4 to 40 µg/L over the 144 h exposure. An effect of salinity was only observed at 48 h and the EC50 values showed 1.4 times more potency at 20 PSU compared to 30 PSU. Measured concentrations of both compounds did not differ between salinities. Acetylcholinesterase activity in chlorpyrifos exposed amphipods showed no salinity effect at 96 h. We conclude that salinity level alters G. lawrencianus susceptibility to chlorpyrifos exposure, but not imidacloprid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Estuarine habitats form spatial gradients with vertical salinity stratification and salinity decreasing with increasing distances from the ocean. Pesticides typically enter estuaries via freshwater runoff. However, groundwater from submarine springs and seeps may also be sources of pesticides and may locally modify estuarine salinity (Gallagher et al. 1996; Cuevas et al. 2018). Pesticides can add to the burden of other stressors including varying salinity already influencing euryhaline species (Delorenzo 2015; Cuevas et al. 2018).
Estuarine crustaceans have been observed to be affected by inputs of agricultural insecticides initially applied to control terrestrial arthropods (e.g., Hano et al. 2019). The widespread agrarian uses of the organophosphate, chlorpyrifos, and the neonicotinoid, imidacloprid, is of concern to estuarine species with potentially acutely toxic concentrations measured in rivers (Denning et al. 2004; Lalonde and Garron 2020). Short-term exposures of chlorpyrifos or imidacloprid at constant salinity affected survival and mobility of coastal crustacean species (Hano et al. 2019; Taylor et al. 2019). Despite spatial associations of salinity and insecticides, the effect of salinity as a modifying factor of the acute and sub-acute toxicity of imidacloprid or chlorpyrifos remains unclear (Song and Brown 1998; Hano et al. 2019; Huang et al. 2020).
Salinity can influence insecticide toxicokinetics which in turn changes the apparent toxic potency towards estuarine species (e.g., Saranjampour et al. 2017). Salinity-influenced toxicity differences in the relatively water-soluble (log Kow ~ 0.6) imidacloprid was species-dependent in salt-marsh arthropod exposures (Song and Brown 1998; Morrissey et al. 2015), with few studies examining crustacean species (Delorenzo 2015). The majority of euryhaline species studied showed increased organophosphate toxicity with increasing salinity (Hall and Anderson 1995). For the relativity hydrophobic (log Kow ~ 5.3) compound chlopyrifos, toxicity in a shrimp species was higher in lower salinities (Liu et al. 2001; Pawar et al. 2020). These results suggest salinity effects on pesticide toxicity is compound and species-dependent and further research is required in order to understand this modifying factor of pesticide toxicity (Delorenzo 2015).
Amphipods are frequently used test organisms in estuarine toxicology. They are often chosen for bioassays because they are key species within both freshwater and marine habitats (Podlesińska and Dąbrowska 2019). Gammarus lawrencianus is an ecologically important epibenthic amphipod inhabiting estuaries along the northwestern Atlantic Ocean (Bousfield 1973; Schein et al. 2013). G. lawrencianus is euryhaline, adapted to polyhaline salinities, and dominant in agriculturally impacted estuaries (Steele and Steele 1991; Coffin et al. 2018). There is no representative gammarid for toxicity testing among this region’s estuarine biota; thus, their relative sensitivity to insecticides is unknown (Leight and Van Dolah 1999; Podlesińska and Dąbrowska 2019).
The objective of this study was to examine the acute lethality of chlorpyrifos and imidacloprid to G. lawrencianus at the boundaries of the optimal salinity range of this species (20–30 PSU, Steele and Steele 1991). Exposure of G. lawrencianus to both insecticides at salinities of 20 and 30 PSU is hypothesized to affect median acute lethality estimates. Expectations were that both insecticides would be more toxic in the 30 PSU level relative to the 20 PSU level (Saranjampour et al. 2017). Within environmentally relevant exposure ranges, visually apparent acute toxicity responses were recorded with immobility as a prelude to mortality (EC50) for imidacloprid and mortality (LC50) for chlorpyrifos over time until an apparent incipient (threshold) toxicity was reached. The effect of salinity on a sub-lethal measure of toxicity, acetylcholinesterase activity, was also examined for chlorpyrifos exposed indivudals.
Materials and Methods
Gammarus lawrencianus were collected from Trout–Stanley River estuary, PEI (46°25′15.6" N 63°26′27.6" W), in May 2015 using equipment outlined in Coffin et al. (2018). Individuals were separated from co-occurring G. mucronatus, G. oceanicus, and G. tigrinus. A voucher specimen from the resulting colony was verified (Barcode Index Number: BOLD: AAA5584, http://www.boldsystems.org/) with COI-barcoding at the Canadian Center for DNA Barcoding in Guelph, Ontario (deWaard et al. 2008).
Culture tanks consisted of a series of aerated 38 L aquaria held at 18–20°C. Whisper® Power Filters containing carbon filters and intakes covered in 400 μm Nitex® mesh filtered water. Instant Ocean© salts were mixed with fresh-groundwater to a target 20 PSU as measured using an Instant Ocean hydrometer. Conductivity and pH of the fresh-groundwater source are respectively 811 µS/cm and 8.2 (Leclair et al. 2013). Salinities were measured during amphipod feeding events and adjusted to the target salinity with fresh-groundwater from the University well to compensate for evaporation. Water never exceeded 30 PSU. Amphipods were fed ad libitum a maximum of ~ 3 g of TetraMin® Tropical Flakes twice weekly, supplemented with a sheet of oven-dried Ulva spp. (collected from, 46°26′50.1" N 63°16′37.6" W) monthly during tank cleaning and water replacement. Individuals were born in the tanks as multiple generations were maintained before insecticide exposures. Amphipods were separated using a 2.0 mm sieve and collected onto a 0.5 mm sieve. The average smaller fraction, used for toxicity tests, were 6.1 ± 0.2 (SEM) mm total length (n = 64).
Acute experiments with G. lawrencianus examined insecticide-salinity interactions at 20 and 30 PSU. Experiments were conducted for 24–96 h with chlorpyrifos exposures and 24–144 h with imidacloprid exposures as chlorpyrifos lethality reached steady state earlier than imidacloprid toxicity. Exposure individuals were acclimated to exposure salinities and temperature for 72 h in individual aquaria. Acclimating amphipods were fed flakes, with no feeding during exposures. Ten individuals were placed into aerated 1 L jars with 750 mL of water and covered with a plastic lid. Exposures included four randomly located replicate jars (n = 4) per salinity treatment and insecticide dose. Light was maintained at a 16–8 light–dark cycle. Nominal 15°C temperatures were monitored hourly with a HOBO© TidbiT v2 temperature logger within a freshwater bath surrounding jars. Water temperatures ranged from 14.0 to 14.6°C, for chlorpyrifos and 13.5 to 14.2°C, for imidacloprid experiments. A VWR® sympHony™ conductivity probe and pH probe was used to measure daily salinity and pH conditions, respectively, in the pesticide free jars. The measured salinity ranged from 18.9 to 19.5 PSU and 29.2 to 29.8 PSU in the chlorpyrifos experiment for 20 PSU and 30 PSU nominal treatments, respectively. Salinity in the imidacloprid experiment was 19.1–19.9 and 29.6–29.9 for the 20 PSU and 30 PSU nominal treatments, respectively. Mean pH in the chlorpyrifos experiment ranged from 8.0 to 8.1 and 8.1 to 8.2 in the 20 and 30 PSU treatments, respectively. The mean pH in the imidacloprid experiment ranged from 8.1 to 8.2 in 20 PSU and was consistently 8.2 in the 30 PSU treatment.
PESTANAL® pesticides (≥ 99.7% purity) and other compounds used for measurements and exposures were purchased from MilliporeSigma (Canada) and solvents from Caledon Laboratories Ltd (Canada). Chlorpyrifos and imidacloprid were dissolved in methanol (distilled in glass) as a carrier solvent. Insecticide stocks were serially diluted with methanol for each dose before injection into water (v/v) at a methanol carrier concentration of 0.003% for chlorpyrifos and 0.001% for imidacloprid, respectively. Amphipods were statically exposed to nominal concentrations of chlorpyrifos at 0 (methanol only), 0.02, 0.06, 0.18, 0.53, 1.6, and, 4.8 μg/L and followed for 96 h; or imidacloprid at concentrations of 0, 0.4, 1.2, 3.6, 10.7, 32.0, and 96.0 μg/L for 144 h. The imidacloprid assay was extended to 144 h because mortalities at the highest concentration did not exceed 20% at 96 h and no incipient EC50 was apparent (i.e., steady state not reached). Mortality was defined as lack of pleopod movement whereas immobilization was defined as lack of swimming when probed but movement of pleopods. Immobilization was used for the imidacloprid treatments as immobility occurs before death. Individuals were removed from treatments at each time-point and frozen (− 80°C) with surviving individuals frozen at the end of the experiment. For both experiments, mean mortality at 0 μg/L did not exceed 20% (Fig S1).
To further examine chlorpyrifos effects between salinities, AChE activity was measured. Pooled tissue of five whole individuals selected at random were sampled from 0, 0.02, and 0.06 μg/L chlorpyrifos treatments (n = 3, per salinity) following a procedure modified from Taylor et al. (2019). Amphipods were homogenized in pH 7.4 autoclaved phosphate-buffered saline (8 g sodium chloride, 0.2 g potassium chloride, 1.44 g disodium phosphate, 0.24 g monopotassium phosphate) on ice, using a glass/Teflon homogenizer. The homogenate was centrifuged in an IEC microlite RF refrigerated microcentrifuge (Thermo Electron Corporation) at 7000×g for 10 min at 4°C. The reaction mixture consisted of 400 µM working solution of Amplifu red reagent (20 mM Ampliflu red reagent, 100 U/mL horseradish peroxidase, 20 U/mL choline oxidase, 100 mM acetylcholine chloride, and 50 mM Tris-HCl). The positive controls were 100 U/mL AChE from Electrophorus electricus and 10 µM hydrogen peroxide, independently. The negative controls consisted of 50 mM Tris-HCl buffer (pH 8.0). The reaction was incubated for 30 min in a 96 well plate, and the plate was read in an FL × 800 microplate fluorescence reader (Biotek Instruments Inc.) at 528 nm excitation and 590 nm emission wavelengths. Protein concentrations of homogenized G. lawrencianus were measured using 10.8 mM fluorescamine in acetonitrile at 360 nm excitation and 460 nm emission wavelengths. The activity of AChE was calculated as enzyme activity per mg of protein per minute.
The measured concentrations and toxicant stability over time were measured in separate triplicate jars per salinity at each compound's highest dose concentration. Jars were exposed to the same conditions of temperature and light conditions without amphipods. A volume of 750 mL was extracted from each jar at 24, 48, and 96 h for chlorpyrifos and 24, 72, and 144 h for imidacloprid. Water from individual jars was acidified to pH 2.0 with sulfuric acid, and three sequential extractions were performed in 1 L separatory funnels using 50 mL dichloromethane. The organic phase was separated and filtered through anhydrous sodium sulfate. Extracts were pooled, and evaporated dry under nitrogen gas in a TurboVap® II (Caliper, LifeSciences) and reconstituted in 1 mL methanol. Insecticides were measured by HPLC-UV/Vis using a Varian-Prostar model 240 pump, Varian model 410 autosampler, and a Varian-Prostar model 335 photodiode array detector. Insecticide UV spectrophotometer wavelengths were 290 nm for chlorpyrifos and 270 nm for imidacloprid.
All statistical analyses were run in R with an α of p < 0.05 (R Core Team 2019). Assumptions of homoscedasticity and residual normality were visually assessed by plotting residuals against fitted values and quantile–quantile plots. The best fitting dose-response probit models (using the package DRC) for each compound were selected using Akaike’s Information Criterion from among two, three or, four parameter models for each day (Ritz et al. 2015). Salinity comparisons between nominal exposure median lethal concentration (LC50 chlorpyrifos) and median effective concentration (EC50, imidacloprid) were made with t-tests using robust sandwich covariance within DRC routines (Zeileis 2006; Ritz et al. 2015). Concentration and AChE contrasts were undertaken using factorial linear models. Significant factors were contrasted with Tukey’s test and sandwich covariance using multcomp (Zeileis 2006; Hothorn et al. 2008).
Results and Discussion
Chlorpyrifos was significantly more acutely lethal at 30 PSU than 20 PSU at all time-points (Fig. 1). Chlorpyrifos LC50 s at 30 PSU were sequentially 1.7, 1.7, 1.5, and 1.5 times as toxic as 20 PSU at 24, 48, 72, and 96 h, respectively. Observations for chlorpyrifos have not been consistent with euryhaline crustaceans. Organophosphate potency increases with increasing salinity (Hall and Anderson 1995); however, crustacean chlorpyrifos exposures show no survival differences between salinity levels or lower lethality with increasing salinities (Bollmohr et al. 2009; Pawar et al. 2020). A potential explanation for differing salinity-toxicity directions with chlorpyrifos is physiological stress at suboptimal salinity (Bollmohr et al. 2009). However, 20 PSU is optimal for both G. lawrencianus and the shrimp Litopenaeus vannamei, with the latter species showing higher lethality at 20 PSU compared to 25 PSU (i.e., opposite to the pattern we observed) (Steele and Steele 1991; Pawar et al. 2020).
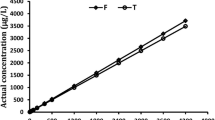
Median concentrations affecting G. lawrencianus at two salinities for a mortality with chlorpyrifos LC50 (± 95% Confidence Interval, CI) and b effective immobilization with imidacloprid EC50 (± CI). n = 4 for all doses per salinity. Significant hourly salinity effects are indicated with an asterisk from contrasts of staggered LC50 or EC50 from nominal exposure probit models. Numbers indicate the number of parameter in the best fitting selected model
In contrast to the chlorpyrifos results, imidacloprid was unexpextedly less acutely effective at 30 PSU than at 20 PSU at most time-points, though this difference was only statistically significant at 48 h (1.4-fold, Fig. 1). Few comparisons of neonicotinoid effects at different salinities exist; however, similar to this study, static imidacloprid exposures of a brine-shrimp (Artemia sp.) showed no salinity-related toxicity differences, whereas, a euryhaline mosquito (Ochlerotatus taeniorhynchus) showed higher lethality at higher salinities (Song and Brown 1998).
Salinity-induced differences in acute toxicity may be explained by differences in solubility, leading to altered uptake (Brecken-Folse et al. 1994; Xie et al. 1997). With the hydrophobic pesticide chlorpyrifos, water solubility decreases with increasing salinity (Xie et al. 1997; Liu et al. 2001). The apparent result of this decreased solubility would be a higher bioconcentration factor (Saranjampour et al. 2017), leading to higher acute lethality, as observed herein. In contrast, imidacloprid is more water-soluble to the extent that it would not be expected to bioconcentrate (Morrissey et al. 2015; Saranjampour et al. 2017).
Increasing salinities may affect processes that reduce the quantity of parent pesticide in solution (Liu et al. 2001). While measured concentrations declined over time for both compounds, there were no significant differences between the two salinities (Table 1). Across salinities measured concentrations at 24 h were 113.4% ± 10.2 (SEM; n = 6) of nominal for chlorpyrifos and 104.3% ± 10.2 (SEM, n = 6) for imidacloprid, respectively. Chlorpyrifos showed 29% loss of the active ingredient between 24 and 96 h. Imidacloprid showed 39% loss between 24 and 144 h. Imidacloprid may degrade through reactions with carbonate complexes, and the half-life of chlorpyrifos injected into natural estuary water ranging from 0 to 17.2 PSU is negatively correlated with salinity (Liu et al. 2001; Dell’Arciprete et al. 2012). These results do not suggest that salinity-mediated changes in the concentration of active compounds were responsible for the observed differences in acute effects at differing salinities. Though concentrations were measured without amphipods, insecticides binding to tissues and other materials may mediate additional aqueous reductions, leading to underpredicted toxicity (Brecken‐Folse et al. 1994; Liu et al. 2001).
AChE activity after 96 h of exposure to chlorpyrifos was not significantly different between salinities or dose. However, mean activity was always lower in chlorpyrifos treated amphipods (Table 2). The lack of effects may relate to the use of whole organisms. Basal AChE responses are highly variable within whole-body freshwater G. fossarum, and some estuarine invertebrates do not show lethal responses below 70% inhibition (Fulton and Key 2001; Xuereb et al. 2009). Lobster (Homarus americanus) AChE after 48 h chlorpyrifos exposures showed inhibition which disappeared after a recovery period, and molting (Taylor et al. 2019), which may help explain our results with the 96 h sampling. Additionally, chlorpyrifos affects indirect physiological pathways, like osmoregulation that may contribute to the observed mortality and lack of an AChE salinity effect (Narra 2014).
Our results suggest that agricultural pesticides may produce acute lethality in amphipods within some Atlantic Canadian estuaries. Potentially acutely lethal concentrations of chlorpyrifos (0.60 μg/L), and high imidacloprid concentrations (0.19 μg/L) have occurred in rivers that drain into G. lawrencianus habitats (Bousfield 1973; Lalonde and Garron 2020). Early application levels of imidacloprid in an Atlantic Canadian river reached 11.9 μg/L (Denning et al. 2004). Irrespective of salinity effects, such concentrations are within the acute toxicity range determined herein and could be relevant to estuarine species, in the upper estuary where dilution is low (Hano et al. 2019; Lalonde and Garron 2020).
The 96 h chlorpyrifos LC50 for freshwater G. fasciatus and the 5–20 PSU-inhabiting G. palutris are ~ 0.3 μg/L, three-fold less lethal than the incipient LC50 of ~ 0.1 μg/L for G. lawrencianus at 30 PSU herein (Bousfield 1973; Leight and Van Dolah 1999). However, the ~ 0.2 μg/L chlorpyrifos 96 h LC50 values are near the 0.15 μg/L of grass-shrimp (Palaemon pugio) with both species tested at 20 PSU (Key and Fulton 2006). Thus, G. lawrencianus is a relatively sensitive species for acute chlorpyrifos lethality, and sensitivity likely outweighs the magnitude of salinity effects in risk assessment of the substances tested (Huang et al. 2020).
Estuarine crustaceans are unlikely to experience directly lethal levels of imidacloprid, but toxic exposure inhibits mobility (Hano et al. 2019). The imidacloprid feeding-inhibition EC50 is 19 μg/L after 24 h in freshwater G. pulex, which is twofold less than the dose to fully immobilize G. lawrencianus at 24 h (Agatz et al. 2014). Whereas, the 96 h immobility EC50 for G. pulex is twofold higher than G. lawrencianus (Roessink et al. 2013). There is a paucity of acute imidacloprid data for saltwater crustaceans but with an incipient imidacloprid EC50 of ~ 10 μg/L found in this study, G. lawrencianus would appear to be a relatively sensitive species (Morrissey et al. 2015).
The dynamic estuarine environment makes agricultural pesticide impact detections challenging as numerous conditions may modify toxicity (Cuevas et al. 2018). Though the 30 PSU level moderately increases chlorpyrifos risks to this estuarine amphipod, salinity does not appear to alter acute imidacloprid risks for tested euryhaline crustaceans (Song and Brown 1998). Nevertheless, further work on other insecticides, especially other neonicotinoids, is needed to clarify the magnitude and direction of salinity level effects on toxicity, including broader salinity gradients (Delorenzo 2015; Saranjampour et al. 2017; Pawar et al. 2020).
References
Agatz A, Ashauer R, Brown CD (2014) Imidacloprid perturbs feeding of Gammarus pulex at environmentally relevant concentrations. Environ Toxicol Chem 33:648–653
Bollmohr S, Schulz R, Hahn T (2009) Interactive effect of salinity decrease, salinity adaptation, and chlorpyrifos exposure on an estuarine harpacticoid copepod, Mesochra parva, in South Africa. Ecotoxicol Environ Saf 72:756–764
Bousfield EL (1973) Shallow-water gammaridean ampipoda of New England. Comstock-Cornell University Press, Ithaca
Brecken-Folse JA, Mayer FL, Pedigo LE, Marking LL (1994) Acute toxicity of 4-nitrophenol, 2,4-dinitrophenol, terbufos and trichlorfon to grass shrimp (Palaemonetes spp.) and sheepshead minnows (Cyprinodon variegatus) as affected by salinity and temperature. Environ Toxicol Chem 13:67–77
Coffin MRS, Courtenay SC, Knysh KM, Pater CC, van den Heuvel MR (2018) Impacts of hypoxia on estuarine macroinvertebrate assemblages across a regional nutrient gradient. FACETS 3:23–44
Cuevas N, Martins M, Costa PM (2018) Risk assessment of pesticides in estuaries: a review addressing the persistence of an old problem in complex environments. Ecotoxicology 27:1008–1018
Dell’Arciprete ML, Soler JM, Santos-Juanes L, Arques A, Mártire DO, Furlong JP, Gonzalez MC (2012) Reactivity of neonicotinoid insecticides with carbonate radicals. Water Res 46:3479–3489
Delorenzo ME (2015) Impacts of climate change on the ecotoxicology of chemical contaminants in estuarine organisms. Curr Zool 61:641–652
de Waard JR, Ivanova NV, Hajibabaei M, Hebert PDN (2008) Assembling DNA barcodes: analytical protocols. In: Martin CC (ed) Methods in molecular biology: environmental genetics, vol 410. Humana Press, Totowa, NJ, pp 275–293
Denning A, Ernst WR, Julien GR, Doe KG, Cook A, Bernier M, Jackman P, Loiser C (2004) An assessment of buffer zone effectiveness in reducing pesticide runoff from potato fields in Prince Edward Island (2001–2002). Environment Canada Surveillance Report: EPS-5-AR-04–05, Atlantic Region.
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20:37–45
Gallagher DL, Dietrich AM, Reay WG, Hayes MC, Simmons GM Jr (1996) Ground water discharge of agricultural pesticides and nutrients to estuarine surface water. Groundw Monit Remediat 16:118–129
Hall LW, Anderson RD (1995) The influence of salinity on the toxicity of various classes of chemicals to aquatic biota. Crit Rev Toxicol 25:281–346
Hano T, Ito K, Ohkubo N, Sakaji H, Watamabe A, Takashima K, Sato T, Sugaya T, Matsuki K, Onduka T et al (2019) Occurrence of neonicotinoids and fipronil in estuaries and their potential risks to aquatic invertebrates. Environ Pollut 252:205–215
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Huang X, Cui H, Duan W (2020) Ecotoxicity of chlorpyrifos to aquatic organisms: a review. Ecotoxicol Environ Saf 200:110731
Key PB, Fulton MH (2006) Correlation between 96-h mortality and 24-h acetylcholinesterase inhibition in three grass shrimp larval life stages. Ecotoxicol Environ Saf 63:389–392
Lalonde B, Garron C (2020) Temporal and spatial analysis of surface water pesticide occurrences in the Maritime region of Canada. Arch Environ Contam Toxicol 79:12–22
Leclair LA, MacDonald GZ, Phalen LJ et al (2013) The immunological effects of oil sands surface waters and naphthenic acids on rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 142–143:185–194
Leight AK, Van Dolah RF (1999) Acute toxicity of the insecticides endosulfan, chlorpyrifos, and malathion to the epibenthic estuarine amphipod Gammarus palustris (bousfield). Environ Toxicol Chem 18:958–964
Liu B, McConnell LL, Torrents A (2001) Hydrolysis of chlorpyrifos in natural waters of the Chesapeake Bay. Chemosphere 44:1315–1323
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro M, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303
Narra MR (2014) Tissue-specific recovery of oxidative and antioxidant effects of chlorpyrifos in the freshwater crab Barytelphusa guerini. Arch Environ Contam Toxicol 67:158–166
Pawar AP, Sanaye SV, Shyama S, Sreepada RA, Dake AS (2020) Effects of salinity and temperature on the acute toxicity of the pesticides, dimethoate and chlorpyrifos in post-larvae and juveniles of the whiteleg shrimp. Aquac Reports 16:100240
Podlesińska W, Dąbrowska H (2019) Amphipods in estuarine and marine quality assessment—a review. Oceanologia 61:179–196
R Core Team (2019) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS ONE 10:e0146021
Roessink I, Merga LB, Zweers HJ, Van den Brink PJ (2013) The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environ Toxicol Chem 32:1096–1100
Saranjampour P, Vebrosky EN, Armbrust KL (2017) Salinity impacts on water solubility and n-octanol/water partition coefficients of selected pesticides and oil constituents. Environ Toxicol Chem 36:2274–2280
Schein A, Courtenay SC, Kidd KA, Campbell A, van den Heuvel MR (2013) Food web structure within an estuary of the southern Gulf of St. Lawrence undergoing eutrophication. Can J Fish Aquat Sci 70:1805–1812
Song MY, Brown JJ (1998) Osmotic effects as a factor modifying insecticide toxicity on Aedes and Artemia. Ecotoxicol Environ Saf 41:195–202
Steele DH, Steele VJ (1991) Effects of salinity on the survival, growth rate, and reproductive output of Gammarus lawrencianus (crustacea, amphipoda). Mar Ecol Prog Ser 78:49–56
Taylor LJ, Mann NS, Daoud D, Clark KF, van den Heuvel MR, Greenwood SJ (2019) Effects of sublethal chlorpyrifos exposure on postlarval american lobster (Homarus americanus). Environ Toxicol Chem 38:1294–1301
Xie WH, Shiu WY, Mackay D (1997) A review of the effect of salts on the solubility of organic compounds in seawater. Mar Environ Res 44:429–444
Xuereb B, Chaumot A, Mons R, Garric J, Geffard O (2009) Acetylcholinesterase activity in Gammarus fossarum (crustacea amphipoda). Intrinsic variability, reference levels, and a reliable tool for field surveys. Aquat Toxicol 93:225–233
Zeileis A (2006) Object-oriented computation of sandwich estimators. J Stat Softw 1:1–16
Acknowledgments
Thanks to Griffen Wakelin, Christina Pater, and a Natural Sciences and Engineering Research Council of Canada (NSERC-SGTP 463277-14) grant.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knysh, K.M., Courtenay, S.C., Grove, C.M. et al. The Differential Effects of Salinity Level on Chlorpyrifos and Imidacloprid Toxicity to an Estuarine Amphipod. Bull Environ Contam Toxicol 106, 753–758 (2021). https://doi.org/10.1007/s00128-021-03157-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03157-z