Abstract
Humans are exposed to methylmercury (MeHg) principally by consumption of marine fish. The coastal zone supports the majority of marine fish production, and may therefore be an important source of MeHg to humans; however, little is known about the bioaccumulation of MeHg in near-shore marine ecosystems. We examined MeHg in microseston, zooplankton, a decapod crustacean, and four representative species of finfish that differ in trophic status and/or prey selection in Long Island Sound (LIS), a large coastal embayment in the northeastern United States. MeHg biomagnifies in LIS; levels in microseston were 104.2 greater than those in water and 2.3-fold less than zooplankton. MeHg concentrations were related positively to fish length for each species, but often varied considerably among larger individuals. This may be due to differences in the past dietary MeHg exposure of these fish, some of which are migratory. Sedimentary production and mobilization can account for most of the MeHg in microseston of LIS, and by extension, other near-shore locations. Hence, much of the MeHg in higher trophic levels of coastal marine ecosystems, including fishes destined for human consumption, may be attributed to net sedimentary production and dietary bioaccumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Accumulation of toxic methylmercury (MeHg) in aquatic food webs is the primary human health concern related to mercury in the environment. Humans are exposed to MeHg principally by the consumption of fish and fish products (Fitzgerald and Clarkson 1991), and some fish levels may pose a threat to public health. Indeed, transfer of MeHg from a maternal seafood diet to prenatal life stages can inhibit the neurological and cardiovascular development of children (e.g., Grandjean et al. 1997; Sorensen et al. 1999). Additionally, MeHg may adversely affect the cardiovascular health of adults who eat fish (Salonen et al. 1995). Most of the fish consumed by humans is of marine origin ( U.S. EPA 2002), and the coastal zone supports 50–75% of marine fish productivity (Ryther 1969). Thus, bioaccumulation and biomagnification of MeHg in near-shore marine ecosystems are critical processes affecting the exposure of humans who consume fish. Yet, compared to freshwater environments, there is a paucity of knowledge concerning the biogeochemistry and bioaccumulation of MeHg in biologically productive coastal marine systems.
Most MeHg in coastal marine systems results from the bacterial methylation of inorganic mercury (Hg) in sediments. Near-shore sediments are not only a repository for natural and anthropogenically derived inorganic Hg (e.g., Balcom et al. 2004), but they host active communities of sulfate reducing bacteria, the major functional group of organisms mediating the transformation of inorganic Hg to MeHg (Compeau and Bartha 1985). Recent studies have shown that the biogeochemical combination of inorganic Hg and sulfate-reducing bacteria in near-shore deposits results in considerable production and mobilization of MeHg to overlying water (e.g., Gill et al. 1999; Hammerschmidt et al. 2004; Hammerschmidt and Fitzgerald 2006). In Long Island Sound, for example, more than 70% of the MeHg is estimated to be derived from sediments (Balcom et al. 2004).
Aquatic organisms accumulate MeHg from water, sediment, and food. MeHg and inorganic Hg are concentrated from water by unicellular organisms (Mason et al. 1996). Diet is the primary source of MeHg in zooplankton (Tsui and Wang 2004) and fish (Hall et al. 1997). Slow rates of elimination relative to the rate of dietary intake result in the bioaccumulation of MeHg. That is, MeHg concentrations typically increase with age/size of an organism (Wiener and Spry 1996). Relatively slow rates of MeHg depuration also result in its biomagnification during trophic transfers; MeHg increases in concentration with increasing trophic levels in a food web (Wiener et al. 2003). Bioaccumulation and biomagnification often result in fish MeHg concentrations that are 106 to 107 greater than those in surface water (Wiener et al. 2003). Some fish contain levels of MeHg that exceed those deemed safe for human consumption by state, federal, and international agencies (e.g., U.S. EPA 2004).
We examined MeHg in the biota of Long Island Sound (LIS), a large (3200 km2) coastal embayment in the northeastern United States whose productive waters (200–400 g C m−2 y−1; Riley 1956) support active commercial and recreational fisheries. Total Hg in LIS sediments (mean, 140 ng g−1 dry weight; Varekamp et al. 2000) is comparable to that in some lacustrine systems (e.g., Cope et al. 1990; Bodaly et al. 1993) with fish MeHg concentrations that exceed recommended limits for safe consumption (300 ng g−1 wet weight; U.S. EPA 2001). Accordingly, and given that benthic mobilization is the primary source of MeHg in lakes (e.g., Hammerschmidt et al. 2006) and the Sound (Balcom et al. 2004), we posited that MeHg levels in LIS biota also would be elevated. MeHg was examined in surface water, microseston, zooplankton, American lobster Homarus americanus, and four representative species of finfish that differ in trophic status and/or prey selection. These included the alewife Alosa pseudoharengus (a pelagic planktivore), winter flounder Pseudopleuronectes americanus (demersal omnivore), tautog Tautoga onitis (durophagous benthic invertivore), and bluefish Pomatomus saltatrix (piscivore). Each of these is either a commercially or recreationally important species in LIS and other near-shore waters of the eastern United States.
Material and Methods
Sampling
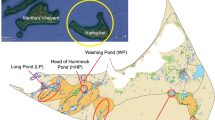
Fish were sampled from the central region of LIS (Fig. 1). LIS is open to the East River and New York Harbor at its western end and the Atlantic Ocean in the east. Fish were collected with the assistance of the Connecticut Department of Environmental Protection (CTDEP) during its biannual Long Island Sound Trawl Surveys. All fish were sampled from LIS at locations between 72.47°W and 73.41°W.
Alewife, winter flounder, and bluefish were sampled in May and September 2002. Fish were collected with a 14-m otter trawl that had a 51-mm mesh codend. Four small lobsters also were collected in September 2002. In May 2004, tautog were caught, and an additional nine lobsters obtained from a local lobsterman on the day of their capture from the central region of LIS, south of New Haven, CT. Fish and lobsters were stored on ice and transported within 24 h of capture to the University of Connecticut, where they were weighed and measured for length. Samples of skinless axial muscle were removed from winter flounder, tautog, and bluefish, and the tail muscle and hepatopancreas were dissected from lobsters. Scrupulous trace-metal clean techniques (Hammerschmidt et al. 1999) were used during dissection to minimize Hg contamination, which could bias levels and reduce the percentage of total Hg as MeHg. All dissected tissues and whole alewife were stored frozen ( ≤ −20°C) inside individual plastic bags until lyophilization. The Hg content of forage species, such as alewife in this study, commonly is measured in the whole fish. Freeze-dried whole alewife were homogenized with a stainless steel blender, and lyophilized muscle samples were homogenized inside their plastic storage bags. The age of tautog was estimated by examination of opercular bones (Cooper 1967).
MeHg also was measured in water, microseston, and zooplankton. Water and suspended particulate matter (SPM, >0.2 μm), most of which is phyto- and bacterioplankton (i.e., microseston) in LIS (Lamborg et al. 2004; Hammerschmidt and Fitzgerald 2006), were sampled with trace-metal clean techniques as part of a comprehensive study of the biogeochemical cycling of Hg species in the Sound (Balcom et al. 2004). Water samples were filtered through 0.2-μm polycarbonate membrane filters promptly after collection to separate dissolved and microseston fractions. Zooplankton, nearly all of which were copepods (Acartia sp.), were sampled from four locations along the longitudinal axis of LIS with a 200-μm mesh nylon net in June 2002.
Analytical Procedures
MeHg Extraction and Analysis
MeHg was measured in zooplankton and individual fish after extraction with dilute HNO3. Subsamples (0.1–0.2 g) of lyophilized and homogenized biological material were digested with 7.0 mL of 4.57 M HNO3 in a covered 60°C water bath for 12 h (Hammerschmidt and Fitzgerald 2005). This extraction method is preferable compared to the traditional KOH/methanol techniques because it allows determination of both MeHg and total Hg in the same extract, thereby reducing some random errors (e.g., sample mass determinations, within-sample heterogeneity) associated with analysis of each Hg species in separate tissue subsamples (Bloom 1992). Polycarbonate filters with microseston were digested similarly with 2 M HNO3. MeHg was extracted from filtered surface waters by aqueous distillation (Hammerschmidt and Fitzgerald 2004). All MeHg determinations were made by flow-injection cold-vapor atomic fluorescence spectrometry (CVAFS; Tseng et al. 2004).
The accuracy of our MeHg measurements was quantified by analyses of (1) blanks and calibration standards taken through the digestion process, (2) certified reference materials from the National Research Council of Canada, lobster hepatopancreas (TORT-2), and dogfish liver (DOLT-2), (3) replicate subsamples of fish and zooplankton, and (4) spiked subsamples (before digestion). Our mean measured concentration of MeHg in TORT-2 was 151 ng g−1 dry weight (certified range, 139–165 ng g−1), and that in DOLT-2 was 686 ng g−1 (certified range, 640–746 ng g−1). Method precision (relative standard deviation), estimated from analyses of duplicate and triplicate subsamples, averaged 3.3% (range, 0.1–16%; n = 98). The mean recovery of MeHg was 101% (95% confidence interval, 97–104%) from 26 spiked samples. All MeHg concentrations in biota are reported on a wet-weight basis.
Total Hg Extraction and Analysis
Total Hg was measured in 19 individual fish to verify that MeHg was the dominant Hg species. The 4.57 M HNO3 leachates for MeHg analysis also were used for determination of total Hg after treatment with BrCl for 12 h (Hammerschmidt and Fitzgerald 2005). Hydroxylamine hydrochloride (12% wt:vol) was added to digestates as a prereductant at least 1 h prior to analysis. Digestates were analyzed for total Hg by dual-Au amalgamation CVAFS (Fitzgerald and Gill 1979). Total Hg analyses were calibrated with Hg0 standards removed from the headspace over pure liquid and verified by comparison to analyses of aqueous Hg2+ solutions traceable to the U.S. National Institute of Standards and Technology (NIST). Also, working standard solutions of MeHg were calibrated, after BrCl oxidation, by comparison to NIST-traceable Hg2+ solutions and Hg0 standards. Recovery of added Hg2+ averaged 99% (range, 94–104%) compared to Hg0 standards. Our mean measured concentration of total Hg in TORT-2 was 280 ng g−1 dry weight (certified range, 210–330 ng g−1) and that in DOLT-2 was 2120 ng g−1 (certified range, 1860–2420 ng g−1). The precision of methodically replicated analyses of total Hg averaged 1.9% RSD (range, 0.1–12%). The estimated detection limit for both MeHg and total Hg in a 0.1-g sample of lyophilized fish was about 0.1 ng g−1.
Results and Discussion
Bioaccumulation and Biomagnification of MeHg
MeHg biomagnifies in LIS (Table 1). Levels of MeHg in 0.2-μm filtered, oxic waters of LIS average about 0.03 ng L−1, and are comparable to those in other coastal marine systems (Mason et al. 1999; Baeyens et al. 2003; Hammerschmidt and Fitzgerald 2006). Microseston bioconcentrate Hg species from surface water (Mason et al. 1996). The mean concentration of MeHg in LIS microseston is 0.5 ng g−1 wet weight (range, 0.2–1.0 ng g−1), assuming that the water content of microseston averages 90% (Yamaguchi et al. 2005). The increase in MeHg between water and microseston can be expressed as a bioaccumulation factor (BAF, L kg−1), which is the concentration of MeHg in biota (wet weight basis) divided by that in water. The BAF for MeHg in microseston of LIS averages 104.2. This is the greatest amplification step for MeHg in the food web of LIS (Table 1). Comparable BAFs for MeHg in microseston have been observed in other coastal marine (103.5–103.9, Baeyens et al. 2003) and freshwater systems (103.8–105.2; Watras and Bloom 1992; Watras et al. 1998). These studies reported either bioconcentration or bioaccumulation factors for seston based on dry-weight tissue concentrations that we converted to wet weight assuming seston is 90% water.
MeHg accumulated by microseston is transferred to grazing zooplankton. Zooplankton in LIS, mostly Acartia copepods in our samples, contained 0.9–1.4 ng MeHg g−1 (mean, 1.1 ng g−1; Table 1), assuming that the water content of zooplankton is 90%. The average increase in MeHg from microseston to zooplankton in LIS was small (2.3-fold), but comparable to that observed in freshwater environments (Watras and Bloom 1992; Watras et al. 1998). Alewife forage principally on zooplankton (Bowman et al. 2000), and MeHg in whole alewife was about 20-fold greater than that in zooplankton (Table 1). Ultimately, bioaccumulation and trophic transfer resulted in a 106 magnification of MeHg between water and alewife (Table 1). Also, the percentage of total Hg as MeHg (i.e., %MeHg) increased concomitantly with the concentration of MeHg among trophic levels (Table 1). This is consistent with observations of MeHg bioaccumulation and trophic transfer in freshwater systems (Wiener et al. 2003). Winter flounder, American lobster, bluefish, and tautog were not assessed in this biomagnification analysis because only the muscle of these organisms was analyzed for MeHg (Gray 2002). These results indicate that MeHg is biomagnified during trophic transfers of organic material in LIS, and that accumulation of MeHg by microseston is a major factor affecting levels in coastal marine food webs.
It is readily demonstrated that in situ sedimentary production is a primary source of MeHg in LIS microseston. Firstly, the estimated diffusional flux of MeHg from sediments of LIS is 11 ± 4 kg y−1 (Hammerschmidt et al. 2004), and the major source to this system (∼70% of total inputs; Balcom et al. 2004). Lesser inputs from external sources, namely rivers, are balanced roughly by tidal exchange, sedimentation, and photodecomposition within the Sound (Balcom et al. 2004; Hammerschmidt 2005). Secondly, the significance of sedimentary production and mobilization of MeHg to its accumulation at the base of the food web in LIS can be evaluated in the following manner. The concentration of MeHg in microseston would be 0.3–0.7 ng g−1 if all sediment-derived MeHg were accumulated by microseston in LIS (200–400 g C m−2 y−1; Riley 1956). This estimate assumes that the water content of plankton averages 90% and that carbon is 40% of dry material (Redfield et al. 1963). The predicted level of MeHg in microseston agrees quite well with our measurements of MeHg (mean, 0.5 ng g−1), as noted in Table 1. This agreement suggests that most of the MeHg in microseston may be attributed to the sedimentary flux in LIS, and by extension, other comparable coastal marine systems not impacted by large fluvial inputs. This should include many continental shelf regions, where the fraction of MeHg derived from benthic production is presumably even greater than that in LIS (Hammerschmidt and Fitzgerald 2006). Accordingly, levels of MeHg in higher trophic levels of the coastal zone may be related to its production in underlying sediments, and this production may have been enhanced during the past 200 years by anthropogenic loadings of inorganic Hg to near-shore and continental shelf sediments (Varekamp et al. 2002; Balcom et al. 2004).
Hg Speciation in Tissues
Nearly all of the Hg in fish is MeHg. Figure 2 shows that, on average, about 98% (slope of regression) of the Hg in the muscle of LIS fish is MeHg. Alewife are not included in the regression analysis in Figure 2 because whole-fish homogenates of alewife were analyzed, and the %MeHg in these samples (mean, 84%) is considerably less than that in the muscle of the other species (range, 98–101%). The difference in %MeHg between alewife and the other species likely reflects both the distribution of MeHg and inorganic Hg within fish and method of sample preparation. In fish, muscle is the primary repository for MeHg but not inorganic Hg (Wiener and Spry 1996). Nearly all of the Hg in muscle of fish and lobster from LIS is MeHg, in agreement with measurements of MeHg in the muscle of other marine finfish and decapod crustaceans (Bloom 1992; Francesconi and Lenanton 1992; Andersen and Depledge 1997; Baeyens et al. 2003). The Hg content of small forage species, however, is commonly measured after homogenization of the whole fish, such as alewife in this study, which includes some tissues enriched with inorganic Hg compared to muscle (e.g., liver, kidney; Lasorsa and Allen-Gil 1995; Francesconi and Lenanton 1992; Baeyens et al. 2003). Given our clean techniques and scrupulous attention to the avoidance of trace metal contamination, the lower %MeHg in alewife can be attributed to organs enriched with inorganic Hg (Bloom 1992), rather than contamination.
We assumed that MeHg is homogeneous in the axial muscle of fish, so only a relatively small sample of skinless axial muscle was dissected (5–50 g wet weight). We tested whether MeHg concentrations in small subsamples of muscle are representative of those throughout the axial muscle by comparing MeHg in opposite fillets of 13 winter flounder collected in May 2002. There is no significant difference in MeHg levels between right and left fillets of winter flounder (paired t-test, p = 0.20). Accordingly, MeHg is distributed equally in the axial muscle of winter flounder, and by extension, other fish species.
Alewife and Flounder
Alewife and winter flounder have the lowest mean MeHg concentrations of the LIS fish examined (Table 2). Alewife are a schooling pelagic fish, and individuals less than about 300 mm total length forage mostly on copepods and euphausids (Bowman et al. 2000). MeHg in alewife is related to their length, although there is a high degree of variability in MeHg for a given fish size (Fig. 3a). For example, MeHg in alewife having a total length of 270-275 mm ranges from 18 to 65 ng g−1. With data from both May and September 2002 combined, the relation between MeHg in whole alewife (C a , ng g−1 wet weight) and their total length (TL a , mm) can be described by the equation
which has a coefficient of determination (r 2) of 0.20. The trend of increasing MeHg with fish length is significant (p < 0.001), and the p-value does not increase if the two fish having greater than 60 ng g−1 are excluded from the analysis. The relatively low slope of the regression line in Figure 3a suggests that the rate of dietary MeHg intake by alewife is only slightly greater than their rate of MeHg depuration. This might be expected for planktivorous fishes whose diet has a relatively low MeHg content.
The mean concentration of MeHg in axial muscle of winter flounder is comparable to that in whole alewife (Table 2). MeHg in winter flounder varies 10-fold among individuals and is related positively to total length with data from May and September combined (Fig. 3b). The relation between MeHg in axial muscle of winter flounder (C f , ng g−1 wet weight) and their total length (TL f , mm) is described by the regression equation (r 2 = 0.39)
This relationship is influenced strongly by several large fish (>275 mm total length) with relatively high MeHg levels that were sampled in May (Fig. 3b). There is little relation between MeHg and length of winter flounder in September only, but only one fish larger than 275 mm was sampled then. The MeHg content of winter flounder less than 275 mm does not differ significantly (t-test, p = 0.94) between May (mean, 15.0 ng g−1) and September (15.2 ng g−1).
Differences in prey selection of individual fish, and MeHg content of prey, could explain the relatively high variability in concentration of the larger alewife and winter flounder. Euphausids, in contrast to zooplankton, comprise a greater portion of the diet of larger alewife (Bowman et al. 2000). Moreover, winter flounder are omnivorous or opportunistic feeders on benthic invertebrate macrofauna (Pereira et al. 1999; Bowman et al. 2000), and their diet shifts progressively from mostly detritus, polychaetes worms, and small crustaceans (e.g., amphipods and mysid shrimp) to siphons of bivalve mollusks as the fish grow (Steimle et al. 2000). An ontogenic change in the diet of some larger alewife and flounder to prey that contain more MeHg per calorie could result in greater MeHg accumulation relative to body size.
The MeHg content of alewife and winter flounder apparently also is related to their physiological condition. Length-weight relationships for alewife and winter flounder in this study are shown in Figure 4. Seven alewife captured in May 2002 weighed considerably less than others of comparable length. Data points for these fish are shown as open circles and are positioned below the length-weight regression line (Fig. 4a), which is based on values for all other fish sampled. Interestingly, whole-body MeHg concentrations in the “underweight” alewife (mean, 44; range, 32–65 ng g−1) were greater (t-test, p < 0.001) than those in the other clupeids (25; 16–47 ng g−1), suggesting a link between MeHg concentration and physiological condition, a relationship observed for freshwater fish species (e.g., Suns and Hitchin 1990; Cizdziel et al. 2002). This is supported by the results for winter flounder; the three fish with MeHg levels greater than 60 ng g−1 (Fig. 3b) also were underweight compared to others of comparable length (Fig. 4b). Indeed, the weight of these three flounder is 23–29% less than that predicted for their length based on the equation in Figure 4b. The connection between MeHg concentration and physiological condition of alewife and winter flounder sampled in May 2002 may be related to recent spawning and associated changes in diet.
American lobster
MeHg in American lobster is related directly to their size (Fig. 5). With data from both September 2002 and May 2004 combined, the linear relation between MeHg in tail muscle (C l , ng g−1 wet weight) and wet weight of American lobster (W l , g) is significant (r 2 = 0.83, p < 0.0001) and described by the regression equation
The high coefficient of determination for this relationship is surprising given the degree of variability observed between MeHg and size of alewife and winter flounder (Fig. 3). American lobster move throughout LIS, but rarely migrate between the Sound and adjacent continental shelf (Benway et al. 2004). This suggests that our lobsters may be representative of the LIS population, although our sample size was small (n = 13) and limited mostly to the central region of the Sound. The strong relationship between MeHg and lobster size suggests that the MeHg content of their diet (rock crabs, mollusks, polychaetes; Weiss 1970) remains relatively constant throughout their lives.
Humans often eat the hepatopancreas (i.e., “green tomalley”) of lobster in addition to muscle. MeHg levels in the hepatopancreas of the nine lobsters sampled in May 2004 are relatively low (20–59 ng g−1) and averaged only 23% of that in tail muscle (range, 13–41%). MeHg, unlike more lipophilic organic contaminants, does not concentrate in fatty tissues (Niimi 1983), and lipids comprise more than 40% of American lobster hepatopancreas by weight (Floreto et al. 2000). In addition, the hepatopancreas may be a site of detoxification for Hg, as it is for other heavy metals (Ahearn et al. 2004). In contrast to the Hg content of tail muscle (99% MeHg), MeHg averages only 28% of total Hg in the hepatopancreas. This suggests that either MeHg is demethylated in the hepatopancreas or complexes of inorganic Hg are sequestered preferentially in this organ.
Bluefish and Tautog
Bluefish are an apex predator in LIS, and their average MeHg concentration is greater than that of alewife and winter flounder (Table 2), both of which, in addition to numerous other bony fishes and cephalopods, are common prey for this schooling piscivore (Fahay et al. 1999; Pereira et al. 1999). In September 2002, a strong relationship was observed between the MeHg concentration (C b , ng g−1 dry weight) and total length (TL b , mm) of bluefish (Fig. 6a), which is described by the regression equation (r 2 = 0.96)
The relationship for September bluefish is noteworthy given both the extreme migratory distance of this species, seasonally between the Middle and South Atlantic Bights (Fahay et al. 1999), and the presumably variable MeHg contents of their prey, geographically, and possibly ontogenically.
Bluefish sampled in May 2002 have significantly more MeHg than those of the same size captured in September, although there is no relationship between MeHg in muscle and total length of bluefish sampled in May (Fig. 6a). Bluefish typically migrate in schools of like-sized individuals from southerly coastal waters into LIS in May, and the total length of the 14 May bluefish ranges from 460 to 620 mm (mean, 547 mm). Seven bluefish caught in September are within this size range, and although their total length (mean, 540 mm) is comparable to that of the May bluefish (t-test, p = 0.79), the MeHg content of the May bluefish (mean, 276 ng g−1 wet weight) is considerably greater than that of comparably sized bluefish caught in September (mean, 136 ng g−1; t-test, p < 0.0001). The difference in MeHg concentration of bluefish between these periods is related most probably to recent or past variations in the MeHg content of their prey, potentially as a result of migration. This is in contrast to results for the September bluefish only, which suggest that the MeHg content of their prey is relatively constant.
Durophagous tautog, which feed mostly on blue mussels and small decapod crustaceans (Steimle et al. 2000), have the greatest mean MeHg concentration of the fish species examined in LIS (Table 2). The relation between MeHg in tautog (C t , ng g−1 wet weight) and total length (TL t , mm) is described by the equation (r 2 = 0.69)
The relatively high levels in tautog are surprising because MeHg biomagnifies and typically increases in food webs according to trophic position (Wiener et al. 2003), resulting in apex piscivores (e.g., bluefish in LIS) having the greatest levels within a system. One explanation for enhanced levels in tautog compared to bluefish is their longer lifespan and opportunity to accumulate MeHg. Indeed, the mean age of tautog collected for this study is nearly 10-fold greater than the average estimated age of bluefish (Table 2).
Bluefish accumulate MeHg much more rapidly than tautog. The mean MeHg content of 2–3-year-old bluefish in LIS is 230 ng g−1 (n = 22). The ages of LIS tautog having a similar MeHg concentration are between 8 and 10 years. This comparison indicates that bluefish accumulate MeHg 3–5 times more rapidly than tautog. A greater rate of bioaccumulation by bluefish may be attributed to differences in the MeHg content of prey. Although levels in their diets are unknown, it is likely that MeHg in the prey of bluefish (e.g., alewife, ∼25 ng g−1) is greater than that in the shellfish diet of tautog. For example, northern quahog clams Mercenaria mercenaria from nearby New York Harbor (Fig. 1), a surrogate for blue mussels in LIS, have only about 2 ng MeHg g−1 (Hammerschmidt 2005). Yet, although bluefish accumulate MeHg at a much greater rate than tautog, the considerably longer lifespan of tautog, up to 34 years (Cooper 1967), permits an extended period for MeHg accumulation, and LIS tautog have a mean MeHg concentration that is comparable to bluefish (Table 2). The significance of fish longevity to MeHg accumulation is evident in the U.S. Environmental Protection Agency warning against human consumption of tilefish Lopholatilus chamaeleonticeps (U.S. EPA 2004), which has a diet and extended lifespan comparable to tautog. The results of this work confirm that species longevity, in addition to trophic position, must be considered when assessing both the bioaccumulation of MeHg by near-shore fishes and the associated potential exposure of humans who consume these fish.
Comparison with New York Bight
Bluefish and tautog captured in LIS can be compared with those from nearby New York Bight. Deshpande and co-workers (2000) measured total Hg in 14 axial muscle composites (i.e., three fish of similar size per composite) each of bluefish and tautog sampled from near-shore waters of the New York Bight Apex, along the northern New Jersey-Atlantic Ocean coast. The average MeHg content of the New Jersey bluefish (mean total length, 561 mm) was 102 ng g−1 wet weight, assuming all total Hg was MeHg. This concentration is about 30% less than that estimated for a LIS bluefish having this length in September (151 ng g−1, equation 4) and almost 3-fold less than the mean level of MeHg in comparably sized bluefish sampled from LIS in May (276 ng g−1; mean length, 547 mm). Differences in bluefish MeHg between the two locations may be related to geographical variations in the synthesis and mobilization of MeHg from sediments (Hammerschmidt et al. 2004; Hammerschmidt and Fitzgerald 2006), which as noted, is a major factor affecting the bioaccumulation in lower trophic levels, and regional differences in planktonic productivity that influence the MeHg:biomass ratio in food webs (Pickhardt et al. 2002). It is most probable, however, that bluefish migration patterns, and associated past differences in dietary exposure, are the major sources of MeHg variation between these locations. This is supported by the considerable difference in bluefish MeHg between May and September in LIS (Fig. 6a).
However, and in contrast to the bluefish, levels of MeHg in tautog are comparable between LIS and the northern New Jersey-Atlantic Ocean coast. Tautog sampled from the New Jersey shore (mean total length, 310 mm) had an average MeHg concentration of 81 ng g−1 (Deshpande et al. 2000), again assuming all total Hg was MeHg. This is comparable to the estimated MeHg level in LIS tautog having the same total length (78 ng g−1; equation 5). The differences and similarities in MeHg accumulation by bluefish and tautog between these two locations illustrate the complexity of MeHg cycling and bioaccumulation within and among coastal marine systems. Together, and in accord with individual fish species in this study, these results suggest that the MeHg content of coastal marine fish, some of which are highly migratory, is related to their past dietary exposure to MeHg, which may vary geographically and ontogenically. Clearly, there is a need for more comprehensive and detailed examinations of factors and processes affecting the bioaccumulation of MeHg in near-shore food webs and levels of MeHg in coastal marine fishes consumed by humans.
Conclusions
MeHg in biota of coastal marine ecosystems is related to its production in underlying sediments. In situ sedimentary production is a major source of MeHg in near-shore systems (Mason et al. 1999; Balcom et al. 2004), and most of the MeHg in microseston of LIS can be attributed to the sedimentary flux. Uptake from water by microseston is the greatest bioaccumulation step for MeHg in the food web of LIS, and MeHg biomagnifies during subsequent dietary transfers. Many of the fish species in LIS are migratory, so their MeHg contents are not dependent entirely on the production and accumulation of MeHg in LIS. The MeHg content of near-shore fish reflects their life-long dietary exposure in coastal waters and embayments such as LIS, which they may inhabit permanently or seasonally.
Anthropogenic loadings of inorganic Hg to near-shore deposits may have increased the accumulation of MeHg in biota. We have found that MeHg production in coastal marine sediments is limited by the availability of inorganic Hg (Hammerschmidt and Fitzgerald 2004; Hammerschmidt and Fitzgerald 2006), which is influenced strongly by Hg loadings and partitioning with sedimentary organic material. Anthropogenic sources have increased annual loadings of inorganic Hg to LIS at least 5-fold since the Industrial Revolution (Varekamp et al. 2002; Balcom et al. 2004), and it is likely that comparable increases in the delivery of inorganic Hg have occurred in other semi-industrialized near-shore regions. If factors affecting the bacterial methylation of inorganic Hg have not changed over this same time period, then it is reasonable to infer that synthesis and subsequent bioaccumulation of MeHg coastal marine systems have increased relative to pollutant Hg enrichment. However, increased loadings of allochthonous and autochthonous organic matter may have reduced the bioavailability and associated potential for methylation of the pollutant inorganic Hg (Hammerschmidt and Fitzgerald 2004).
Each of the fish species analyzed for MeHg in this study were selected to represent a particular trophic level/feeding mode in near-shore marine ecosystems. Surprisingly, given the level of Hg contamination in LIS sediments, MeHg concentrations in LIS fish are low. All alewife, winter flounder, and American lobster sampled for this study have MeHg concentrations less than the U.S. EPA-recommended criterion of 300 ng g−1 for safe consumption (U.S. EPA 2001), and only five of 32 tautog (16%) and five of 46 bluefish (11%) exceed this level. It is noteworthy, however, that many of the bluefish sampled in this study may be smaller, and have subsequently lower MeHg levels, than those either typically kept by fishermen or sampled in other studies. Without regard for fish size, the average concentration of MeHg in bluefish captured in LIS (137 ng g−1 wet weight) is considerably less than that determined from other regional (New Jersey fish markets, 260 ng g−1, Burger et al. 2005; New Jersey coast and estuaries, 410 ng g−1, Ashley and Horwitz 2000; Florida estuary, 640 ng g−1, Strom and Graves 2001) and national assessments (340 ng g−1, U.S. Department of Health and Human Services and U.S. EPA 2006). It is evident, given the MeHg levels observed in some fishes of near-shore food webs, that bioaccumulation of MeHg in the coastal zone, as well as the open ocean, should be investigated more comprehensively.
References
Ahearn GA, Mandal PK, Mandal A (2004) Mechanisms of heavy-metal sequestration and detoxification in crustaceans: A review. J Comp Physiol B 174:439–452
Andersen JL, Depledge MH (1997) A survey of total mercury and methylmercury in edible fish and invertebrates from Azorean waters. Mar Environ Res 44:331–350
Ashley J, Horwitz RJ (2000) Assessment of PCBs, selected organic pesticides and mercury in fishes from New Jersey: 1998–1999 monitoring program. Patrick Center for Environmental Research, The Academy of Natural Sciences, Philadelphia, PA
Balcom PH, Fitzgerald WF, Vandal GM, Lamborg CH, Langer CS, Rolfhus KR, Hammerschmidt CR (2004) Mercury sources and cycling in the Connecticut River and Long Island Sound. Mar Chem 90:53–74
Baeyens W, Leermakers M, Papina T, Saprykin A, Brion N, Noyen J, De Gieter M, Elskens M, Goeyens L (2003) Bioconcentration and biomagnification of mercury and methylmercury in North Sea and Scheldt Estuary fish. Arch Environ Contam Toxicol 45:498–508
Benway J, Giannini C, Gottschall K, Howell P, Pacileo D (2004) Assessment and monitoring of the American lobster resource and fishery in Long Island Sound. Report to Natl Mar Fish Serv NA16FW1238
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017
Bodaly RA, Rudd JWM, Fudge RJP, Kelly CA (1993) Mercury concentrations in fish related to size of remote Canadian shield lakes. Can J Fish Aquat Sci 50:980–987
Bowman RE, Stillwell CE, Michaels WE, Grosslein MD (2000) Food of northwest Atlantic fishes and two common species of squid. NOAA Tech Mem NMFS-NE-155 National Marine Fisheries Service, Woods Hole, Massachusetts
Burger J, Stern AH, Gochfeld M (2005) Mercury in commercial fish: Optimizing individual choices to reduce risk. Environ Health Perspect 113:266–271
Cizdziel JV, Hinners TA, Pollard JE, Heithmar EM, Cross CL (2002) Mercury concentrations in fish from Lake Mead, USA, related to fish size, condition, trophic level, location, and consumption risk. Arch Environ Contam Toxicol 43:309–317
Compeau G, Bartha R (1985) Sulfate-reducing bacteria: Principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol 50:498–502
Cooper RA (1967) Age and growth of the tautog, Tautoga onitis (Linnaeus) from Rhode Island. Trans Am Fish Soc 96:134–142
Cope WG, Wiener JG, Rada RG (1990) Mercury accumulation in yellow perch in Wisconsin seepage lakes: Relation to lake characteristics. Environ Toxicol Chem 9:931–940
Deshpande AD, Draxler AFJ, Zdanowicz VS, Schrock ME, Paulson AJ, Finneran TW, Scharack BL, Corbo K, Arlen L, Leimburg EA, Dockum BW, Pikanowski RA, May B, Rosman L (2000) Contaminant levels in muscle of four species of recreation fish from the New York Bight Apex. NOAA Tech Mem NMFS-NE-157 National Marine Fisheries Service, Woods Hole, Massachusetts
Fahay MP, Berrien PL, Johnson DL, Morse WW (1999) Essential fish habitat source document: Bluefish, Pomatomus saltatrix, life history and habitat characteristics. NOAA Tech Mem NMFS-NE-144 National Marine Fisheries Service, Woods Hole, Massachusetts
Fitzgerald WF, Clarkson TW (1991) Mercury and monomethylmercury: Present and future concerns. Environ Health Perspect 96:159–166
Fitzgerald WF, Gill GA (1979) Subnanogram determination of mercury by two-stage gold amalgamation applied to atmospheric analysis. Anal Chem 51:1714–1720
Floreto EAT, Prince DL, Brown PB, Bayer RC (2000) The biochemical profiles of shell-diseased American lobsters, Homarus americanus Milne Edwards. Aquaculture 188:247–262
Francesconi KA, Lenanton RCJ (1992) Mercury contamination in a semi-enclosed marine embayment: Organic and inorganic mercury content of biota, and factor influencing mercury levels in fish. Mar Environ Res 33:189–212
Gill GA, Bloom NS, Capellino S, Driscoll CT, Mason R, Rudd JWM (1999) Sediment-water fluxes of mercury in Lavaca Bay, Texas. Environ Sci Technol 33:663–669
Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorenson N, Dahl R, Jorgensen P (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 19:417–428
Gray JS (2002) Biomagnification in marine systems: The perspective of an ecologist. Mar Poll Bull 45:46–52
Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM (1997) Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Pollut 100:13–24
Hammerschmidt CR (2005) The biogeochemistry of methylmercury in coastal marine sediments. PhD dissertation, University of Connecticut
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol 38:1487–1495
Hammerschmidt CR, Fitzgerald WF (2005) Methylmercury in mosquitoes related to atmospheric mercury deposition and contamination. Environ Sci Technol 39:3034–3039
Hammerschmidt CR, Fitzgerald WF (2006) Methylmercury cycling in sediments on the continental shelf of southern New England. Geochim Cosmochim Acta 70:918–930
Hammerschmidt CR, Wiener JG, Frazier BE, Rada RG (1999) Methylmercury content of eggs in yellow perch related to maternal exposure in four Wisconsin lakes. Environ Sci Technol 33:999–1003
Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Visscher PT (2004) Biogeochemistry of methylmercury in sediments of Long Island Sound. Mar Chem 90:31–52
Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Tseng C-M (2006) Biogeochemical cycling of methylmercury in lakes and tundra watersheds of arctic Alaska. Environ Sci Technol 40:1204–1211
Lamborg CH, Fitzgerald WF, Skoog A, Visscher PT (2004) The abundance and source of mercury-binding organic ligands in Long Island Sound. Mar Chem 90:151–163
Lasorsa B, Allen-Gil S (1995) The methylmercury to total mercury ratio in selected marine, freshwater, and terrestrial organisms. Water Air Soil Pollut 80:905–913
Mason RP, Reinfelder JR, Morel FMM (1996) Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ Sci Technol 30:1835–1845
Mason RP, Lawson NM, Lawrence AL, Leaner JJ, Lee JG, Shue G-R (1999) Mercury in the Chesapeake Bay. Mar Chem 65:77–96
Niimi AJ (1983) Biological and toxicological effects of environmental contaminants in fish and their eggs. Can J Fish Aquat Sci 40:306–312
Pereira JJ, Goldberg R, Ziskowski JJ, Berrien PL, Morse WW, Johnson DL (1999) Essential fish habitat source document: Winter flounder, Pseudopleuronectes americanus, life history and habitat characteristics. NOAA Tech Mem NMFS-NE-138 National Marine Fisheries Services, Woods Hole, Massachusetts
Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD (2002) Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc Nat Acad Sci 99:4419–4423
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of seawater. In: Hill MN (ed), The sea, vol. 2. Interscience, New York, pp 26–77
Riley GA (1956) Oceanography of Long Island Sound; 1952–1954: IX. Production and utilization of organic matter. Bull Bingham Oceanogr Collect 18:324–341
Ryther JH (1969) Photosynthesis and fish production in the sea. Science 166:72–76
Salerno DJ, Burnett J, Ibara RM (2001) Age, growth, maturity, and spatial distribution of bluefish, Pomatomus saltatrix (Linnaeus), off the northeast coast of the United States, 1985–96. J Northw Atl Fish Sci 29:31–39
Salonen JT, Seppänen K, Nyyssönen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R (1995) Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 91:645–655
Sorensen N, Murata K, Budtz-Jorgensen E, Weihe P, Grandjean P (1999) Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 10:370–375
Steimle FW, Pikanowski RA, McMillan DG, Zetlin CA, Wilk SJ (2000) Demersal fish and American lobster diets in the Lower Hudson-Raritan estuary. NOAA Tech Mem NMFS-NE-161 National Marine Fisheries Service, Woods Hole Massachusetts
Strom DG, Graves GA (2001) A comparison of mercury in estuarine fish between Florida Bay and the Indian River lagoon, Florida, USA. Estuaries 24:597–609
Suns K, Hitchin G (1990) Interrelationships between mercury levels in yearling yellow perch, fish condition and water quality. Water Air Soil Pollut 50:255–265
Tseng C-M, Hammerschmidt CR, Fitzgerald WF (2004) Determination of methylmercury in environmental matrixes by on-line flow injection and atomic fluorescence spectrometry. Anal Chem 76:7131–7136
Tsui MTK, Wang W-X (2004) Uptake and elimination routes of inorganic mercury and methylmercury in Daphnia magna. Environ Sci Technol 38:808–816
US Department of Health and Human Services, US Environmental Protection Agency (2006) Mercury levels in commercial fish and shellfish. http://www.cfsan.fda.gov/∼frf/sea-mehg.html(accessed February 7, 2006)
US Environmental Protection Agency (2001) Water quality criterion for the protection of human health: Methylmercury. EPA-823-R-01-001, Washington, DC
US Environmental Protection Agency (2002) Estimated per capita fish consumption in the United States, August 2002. EPA-821-C-02-003, Washington, DC
US Environmental Protection Agency (2004) National listing of fish advisories. EPA-823-F-04-016, Washington, DC
Varekamp JC, Buchholtz ten Brink MR, Mecray EL, Kreulen B (2000) Mercury in Long Island Sound sediments. J Coast Res 16:613–626
Varekamp JC, Kreulen B, Buchholtz ten Brink MR, Mecray EL (2002) Mercury contamination chronologies from Connecticut wetlands and Long Island Sound sediments. Environ Geol 43:268–282
Watras CJ, Bloom NS (1992) Mercury and methylmercury in individual zooplankton: Implications for bioaccumulation. Limnol Oceanogr 37:1313–1318
Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP (1998) Bioaccumulation of mercury in pelagic freshwater food webs. Sci Tot Environ 219:183–208
Weiss HM (1970) The diet and feeding behavior of the lobster, Homarus americanus, in Long Island Sound. PhD dissertation, University of Connecticut, Storrs, CT. 80 pp
Wiener JG, Spry DJ (1996) Toxicological significance of mercury in freshwater fish. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds), Environmental contaminants in wildlife: Interpreting tissue concentrations. Lewis Publishers, Boca Raton, pp 297–339
Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM. (2003) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds), Handbook of Ecotoxicology. Lewis Publishers, Boca Raton, pp 409–463
Yamaguchi A, Watanabe Y, Ishida H, Harimoto T, Maeda M, Ishizaka J, Ikeda T, Takahashi MM (2005) Biomass and chemical composition of net-plankton down to greater depths (0–5800 m) in the western North Pacific Ocean. Deep-Sea Res I 52:341–353
Acknowledgments
We thank Deb Pacileo, Kurt Gottschall, Penny Howell, Paul Stacey, Larissa Graham, lobsterman D. J. King, the captain and crew of the R/V John Dempsey, and the CTDEP for help with fish and lobster sampling. Kurt Gottschall and Deb Pacileo provided age data for the tautog. Sheean Haley and Amy Smith assisted with sampling and identification of zooplankton, and Prentiss Balcom and Grace Vandal helped with measurements of MeHg in dissolved and SPM water fractions. We are grateful to two anonymous reviewers for providing helpful comments on an earlier version of the manuscript. This study was supported by a STAR grant (R827635) and graduate student fellowship (U91591801) from the U.S. EPA, and the Postdoctoral Scholar Program at the Woods Hole Oceanographic Institution, with funding provided by the Doherty Foundation. The work, however, does not necessarily reflect the views of the U.S. EPA or CTDEP, and no official endorsement should be inferred.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hammerschmidt, C.R., Fitzgerald, W.F. Bioaccumulation and Trophic Transfer of Methylmercury in Long Island Sound. Arch Environ Contam Toxicol 51, 416–424 (2006). https://doi.org/10.1007/s00244-005-0265-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0265-7