Abstract
Mercury (Hg) is a global pollutant that affects biota in remote settings due to atmospheric deposition of inorganic Hg, and its conversion to methylmercury (MeHg), the bioaccumulating and toxic form. Characterizing biotic MeHg is important for evaluating aquatic ecosystem responses to changes in Hg inputs. Aquatic insects possess many qualities desired for MeHg biomonitoring, but are not widely used, largely because of limited information regarding percentages of total mercury (THg) composed of MeHg (i.e., MeHg%) in various taxa. Here, we examine taxonomic, spatial, and seasonal variation in MeHg% of stream-dwelling predator and primary-consumer insects from nine streams in the Adirondack region (NY, USA). Predator MeHg% was high (median 94%) and did not differ significantly among five taxa. MeHg% in selected dragonflies (the most abundant predators, Odonata: Aeshnidae and Libellulidae) exhibited little seasonal and spatial variation, and THg concentration was strongly correlated with aqueous (filtered) MeHg (FMeHg; rs = 0.76). In contrast, MeHg% in primary consumers—shredders (northern caddisflies [Trichoptera: Limnephilidae]) and scrapers (flathead mayflies [Ephemeroptera: Heptageniidae]), were lower (medians 52% and 35%, respectively), and differed significantly between taxa, among sites, and seasonally. Correlations of THg with FMeHg were weak (shredders, rs = 0.45, p = 0.09) or not significant (scrapers, p = 0.89). The higher MeHg% of predators corresponded with their higher trophic positions (indicated by nitrogen stable isotopes). Results suggest obligate predators hold the most promise for the use of THg as a surrogate for MeHg biomonitoring with aquatic insects within the Adirondack region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methylmercury (MeHg) is a potent neurotoxin that readily enters the base of aquatic food webs, where it can bioconcentrate and biomagnify to concentrations that can be harmful to humans (Mergler et al. 2007), piscivorous wildlife (Evers et al. 2007), and fish (Sandheinrich and Wiener 2011; Batchelar et al. 2013). Biomonitoring of MeHg is necessary to evaluate responses to recent changes in anthropogenic mercury (Hg) air emissions and atmospheric deposition (Mason et al. 2005; Brigham et al. 2014; Zhang et al. 2016). Many aquatic insect taxa possess qualities that are desirable in a biosentinel organism, as detailed in Beeby (2001). Aquatic insects are important links in MeHg trophic transfer from primary producers to top predators, and their MeHg levels can be strongly related to concentrations in water and fish (Riva-Murray et al. 2011; Haro et al. 2013; Jeremiason et al. 2016). Many taxa have broad geographic distribution (including in fish-less habitats) and high site fidelity, are locally abundant, are relatively easy to collect and identify, and provide time-integrated aquatic exposure information over lifespans of a year or longer (Haro et al. 2013). In addition, aquatic insects are important links in MeHg transfer between aquatic and terrestrial food webs (Cristol et al. 2008; Speir et al. 2014). Finally, the use of aquatic insects as Hg biosentinels can avoid or minimize potentially-confounding factors associated with fish (particularly top predator fish), such as large differences in prey assemblages, food chain length, growth rate, size, age, migration, and fishing pressure among and within waterbodies (Harris and Bodaly 1998; Munthe et al. 2007; Wiener et al. 2007; Yu et al. 2011; Johnson et al. 2015). The benefits of using insects rather than fish was demonstrated in a recent study of biotic total Hg (THg) in lakes (Haro et al. 2013). In that study, THg data from only 10 individual dragonfly naiads per lake allowed for detection of a 20% difference in mean biotic THg among lakes. In contrast, detecting the same 20% difference among lakes required data from 40 individual fish per lake. This and other studies demonstrating positive relations between insect Hg and aqueous Hg (e.g., Riva-Murray et al. 2011; Nelson et al. 2015, 2019) provide support for the use of selected insects as Hg biosentinels in fresh waters.
An important practical consideration in the use of aquatic insects for MeHg biomonitoring is the utility of THg as a surrogate measure for insect tissue concentrations of MeHg, the ecotoxicologically-relevant but more technically-challenging and expensive analytical endpoint. Nearly all Hg in fish muscle is comprised of MeHg (Grieb et al. 1990; Bloom 1992), and this has led to the widespread use of THg to represent fish MeHg (although recent work indicates fish THg is composed of less MeHg than previously thought in some cases (Lescord et al. 2018)). In contrast, the percentage of THg comprised of MeHg (henceforth MeHg%) in aquatic insects has large taxonomic, spatial, and temporal variation (Tremblay et al. 1996; Mason et al. 2000; Gorski et al. 2003), warranting an evaluation of the suitability of THg as a MeHg surrogate in taxa of potential interest for monitoring. Bloom (1992) linked variation in MeHg% of fish muscle to differences in sample-processing methods and concluded that the observed variation could be largely controlled by strict handling and laboratory methods. In contrast, much of the variation in MeHg% among insect taxa is associated with biological factors, especially feeding behavior and trophic position (Tremblay et al. 1996; Tremblay and Lucotte 1997; Mason et al. 2000), as well as environmental factors such as food quality (Tremblay et al. 1996), environmental MeHg concentrations, and inorganic Hg contamination (Lasorsa and Allen-Gil 1995). Thus, a priori characterization of this variation and contributing processes are essential if there is a desire to reduce analytical costs and to simplify laboratory analyses by using THg as a surrogate for aquatic insect MeHg.
The Adirondack Mountain region of New York (hereafter the ‘Adirondack region’) has been identified as a Hg biological ‘hotspot’ (Driscoll et al. 2007; Evers et al. 2007) due to relatively high atmospheric Hg deposition and physical, biological, and chemical characteristics that promote Hg methylation, delivery to aquatic ecosystems, and bioaccumulation (Driscoll et al. 1994; Kamman et al. 2005; Simonin et al. 2008; Yu et al. 2013). Substantial heterogeneity in MeHg availability and bioaccumulation in aquatic habitats occurs across the region (Driscoll et al. 1995; Simonin et al. 2008; Riva-Murray et al. 2011; Riva-Murray et al. 2013a; Burns and Riva-Murray 2018) as a result of spatial variation in (1) landscape methylation potential and delivery to aquatic environments (Driscoll et al. 1995; Dittman and Driscoll 2009; Riva-Murray et al. 2011; Yu et al. 2011), (2) dry and wet Hg deposition (Yu et al. 2013), and (3) fish growth rates and food web characteristics (Yu et al. 2011). In addition, many Adirondack region waterbodies are fish-less or have very low fish densities because of natural factors (e.g., small headwater streams) or the long-term effects of acid deposition (Baker et al. 1996; Simonin et al. 2005; Baldigo et al. 2016). Thus, the potential for improved MeHg monitoring across the region using aquatic insect tissue assessment merits investigation.
Here, we use existing data, from a study of Hg bioaccumulation in streams conducted during 2007–2009 by the USGS National Water Quality Assessment Program (Riva-Murray et al. 2011; Beaulieu et al. 2012; Riva-Murray et al. 2013a), to evaluate THg as a surrogate for MeHg in aquatic insects from the Adirondack region. We consider several predatory and primary consumer taxa, collected during different seasons and from different stream sites, to evaluate trophic, taxonomic, spatial, and temporal variation in MeHg%. Total Hg can, arguably, be a suitable surrogate for MeHg in aquatic insect feeding groups or taxa if at least one of several conditions is met. The occurrence of a sufficiently-high percentage (defined by specific monitoring needs) of the THg concentration as MeHg would support the direct substitution of THg for MeHg, as is commonly done with fish muscle and whole fish. A relatively low percentage of MeHg that is sufficiently consistent across relevant spatial and temporal extents (with degree of consistency defined by specific monitoring needs) would allow for the use of a conversion factor to translate THg to MeHg. Finally, the existence of a predictable relation between THg and MeHg, or the determination of sources of variation (e.g., season, organism size, taxon, environmental characteristics) would provide for development of predictive models and (or) approaches for controlling sources of variation in monitoring.
We hypothesize that aquatic insects that are obligate predators will have higher and more consistent MeHg% than primary consumer insects. We utilize existing stable isotope data for nitrogen (δ15N) to explore whether any observed variation in MeHg% among and within feeding groups can be attributed to variation in trophic position. Finally, we posit that if aquatic insect THg is to be used to monitor effects of changing Hg source conditions, it should be significantly and strongly correlated with concentrations of bioavailable aqueous MeHg (i.e. filtered MeHg [FMeHg]). In a previous study of these streams, strong correlations were found between aquatic insect MeHg and aqueous FMeHg (Riva-Murray et al. 2011). Thus, a similar correlation of THg with FMeHg in a feeding group or taxon would provide additional support for the ability of THg to adequately represent MeHg in that group or taxon.
Methods
Study area
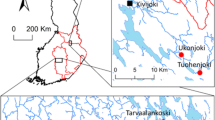
The Adirondack region of New York, in the Appalachian Highlands ecoregion (Fig. 1), receives relatively high amounts of atmospheric Hg, is remote from Hg point sources, has wetlands and soil characteristics that are conducive to methylation of deposited Hg, and has fish Hg concentrations that are elevated in relation to human and wildlife health thresholds and guidelines (Driscoll et al. 2007; Evers et al. 2007; Riva-Murray et al. 2011, 2013b; Beaulieu et al. 2012; Burns and Riva-Murray 2018). The nine study sites for this analysis were located in the upper Hudson River basin in the central portion of the Adirondack region (SI Fig. 1; SI Table 1; Scudder Eikenberry et al. 2011), These consist of eight sites in the Fishing Brook watershed (65.6 km2 drainage area) and one site on the upper Hudson River (493 km2 drainage area). One of the eight Fishing Brook watershed sites was formed by the combination of two original locations on a short stream segment (SI Table 1; Scudder Eikenberry et al. 2011; Bradley et al. 2016.) The environmental settings of the sites varied widely, despite the location of eight of the nine sites within a relatively small area. Some sites were located on low-gradient streams with extensive riparian wetlands, while others were on moderate to high gradient streams with little or no wetlands in the watershed (SI Table 1). Canopy cover ranged from dense to completely open, and one site was located on a ‘flow’, with a low-head impoundment at the lower end. Chemical characteristics of sites also varied widely. Results of a base-flow synoptic survey of these waters conducted during late July 2008 (Burns et al. 2012) provide an indication of this heterogeneity (https://nwis.waterdata.usgs.gov/ny/nwis/qwdata; SI Table 1). Across the 9 sites, pH ranged from 6.0 to 6.8 (median 6.5), dissolved organic carbon ranged from 3.5 to 20.1 mg/L (median 11.8 mg/L), and filtered MeHg (FMeHg) ranged from nondetectable (<0.04 ng/L) to 0.84 ng/L (median 0.31 ng/L). Summaries of other water chemistry data for these sites (Bradley et al. 2011, 2012; Riva-Murray et al. 2011) also show relatively large ranges in conditions. Strong seasonality also has been observed in MeHg concentrations in stream water (both filtered and whole water) in these streams, with higher concentrations occurring later in the growing season (i.e. summer and early fall) as compared with spring (Bradley et al. 2011; Burns et al. 2012; Riva-Murray et al. 2013b).
Sample collection, field processing, and laboratory analysis
Insect MeHg and THg concentration data and nitrogen stable isotope data (δ15N), total weights of composite samples (weight), counts of specimens in composite samples, and stream water FMeHg concentration data were compiled from the National Water Information System (NWIS, http://waterdata.usgs.gov/nwis). Aquatic insect data were from composite samples of larvae and naiads collected May through October in 2007, 2008, and 2009 (most from spring and summer in 2007 and 2008). The FMeHg data were from a single water sample per site per visit collected during non-storm conditions, generally within a week or less of the aquatic insect sampling date. Two sites (FNW and SLL) were sampled more frequently and intensively than others, and these are the only sites from which insect samples were collected in 2009.
In total, the insect data set was composed of 260 composite samples (made up of 6857 individual specimens) in three functional feeding groups (assigned according to Merritt and Cummins 1996) - scrapers, shredders, and predators (44, 90, and 126 composite samples, respectively). The two primary consumer feeding groups each contained one family (scrapers: Ephemeroptera: Heptageniidae [flathead mayflies]; shredders: Trichoptera: Limnephilidae [northern caddisflies]) and the predator feeding group contained five taxa: damselflies (Odonata: Zygoptera), predatory stoneflies (Plecoptera), and three dragonfly (Odonta: Anisoptera) families (Aeshnidae [darners], Libellulidae [common skimmers], and Gomphidae [clubtails]). All insect and water samples were collected and field-processed with trace-metal clean methods (Beaulieu et al. 2012; Riva-Murray et al. 2011). Identification of specimens was done in the field; dragonflies were identified to family, predatory stoneflies to order, and damselflies to suborder.
Specimens were collected with nets and by hand-picking with gloved hands and clean plastic forceps from various available habitats at each site (e.g., rocky substrate, leaf packs, aquatic vegetation, woody debris, streambanks, and depositional zones). To the extent possible, at least 10 (usually more) specimens were collected for each composite sample, multiple composite samples were collected per taxon and site visit, and collection efforts were distributed throughout each stream reach. After collection, specimens were placed in site water in clean plastic jars or new plastic bags and were transported to a field laboratory. Specimens were then separated by taxon (i.e., the 7 taxa indicated above), and some were also separated by size (e.g., smaller versus larger Aeshnidae in a given collection). Specimens were triple-rinsed in deionized water and sorted into single-taxon composite samples consisting of 2–120 specimens each (median = 18). Composite samples were batch-weighed, placed in new plastic vials or plastic bags, labeled, double-bagged, frozen (using dry ice), and shipped to the analytical laboratory.
Each composite sample was analyzed for MeHg, THg, and δ15N. All Hg analyses were conducted at the USGS Mercury Research Laboratory (MRL; Middleton, WI, USA). Following freeze-drying to constant weight and grinding of samples, a 4.57 M nitric acid extraction followed by aqueous-phase ethylation, gas-chromatagraphic separation, and cold vapor atomic fluorescence spectroscopy (CVAFS) was used to determine MeHg in all 260 composite samples, following Hammerschmidt and Fitzgerald (2006). For 159 of the composite samples, THg was determined by CVAFS following bromine monochloride oxidation and stannous chloride reduction of the aforementioned 4.57 M nitric acid extracts (Hammerschmidt and Fitzgerald 2006). The THg analysis was performed on the other 101 composite samples by direct combustion with atomic absorption (a modification of EPA Method 7473 (U.S. EPA 2012). Additional method details can be found at http://wi.water.usgs.gov/mercury-lab. Mean (±1 standard deviation, s.d.) recoveries in standard reference materials (SRMs) NIST2976, NRCC DOLT–3, and NRCC TORT–2 were 90.9% (±27.4%, n = 24), 83.5% (±9.7%, n = 12), and 93.0% (±14.2%, n = 14), respectively, for MeHg, and were 97.8% (±21.5%, n = 21), 93.5% (±6.2%, n = 9), and 103.4% (±10.3%, n = 11), respectively, for THg analyzed by CVAFS. Mean (±1 s.d.) recovery in SRM IAEA-407 was 108.1% (±5.5%, n = 27) for THg analyzed by direct combustion. Detailed QA/QC results are provided in Beaulieu et al. (2012).
Subsamples of freeze-dried tissue were sent to Florida State University’s National High Magnetic Field Laboratory, where they were analyzed for δ15N in the Stable Isotope Geochemistry Laboratory. A ThermoQuest NC2500 Elemental Analyzer interfaced with a Finnegan MAT Delta Plus XP isotope ratio mass spectrometer was used to measure isotope ratios relative to reference gases, as detailed in Riva-Murray et al. (2011). Quality assurances included blind duplicates and standard reference material samples. Typical precision and accuracy for nitrogen isotope ratios was <0.4‰.
Stream water samples were collected as a grab samples from a well-mixed zone of each site, using ultra-trace-metal-clean techniques. Samples were filtered in a field laboratory and were sent to MRL for FMeHg analysis. Collection, processing, and analysis methods are detailed elsewhere (Brigham et al. 2009; Burns et al. 2012).
Data analysis
Concentrations of MeHg and THg (SI Table 2) were used to calculate MeHg% for each composite sample of aquatic insects. Preliminary analysis indicated a difference in calculated MeHg% between the two laboratory methods used for the analysis of THg (i.e., the denominator in the calculation), which could be attributed to the differences in method performance (i.e., the greater SRM recovery for the direct combustion method than for the CVAFS method; means of 108.1 and 98.2%, respectively). Thus, prior to calculation of MeHg%, we applied a recovery-based correction of 90.8% to THg concentrations that were derived from direct combustion. The application of this correction factor, calculated to normalize the mean recovery from direct combustion to that of CVAFS, served to eliminate the influence of the THg analysis method on the regression of MeHg with THg (SI Fig.1).
Values of MeHg% greater than 100% were retained because these are expected to result from analytical variation (Bloom 1992), and they were observed to be randomly distributed around 100% (SI Fig. 2). Group comparisons to determine statistical significance (α = 0.05) of differences in MeHg% and other variables were done with Wilcoxon rank-sum test and the Kruskal-Wallis nonparametric analysis of variance (ANOVA) for comparisons of two groups and more than two groups, respectively. Statistically-significant global ANOVAs on three or more groups were followed by Tukey’s all-pairwise multiple comparison tests on ranks. Stable isotope nitrogen ratios (δ15N) were similarly compared among groups. The site-specific mean value of nitrogen stable isotopes (δ15N) of shredders (which had the lowest values within sites) was used to derive base-adjusted δ15N (henceforth, δ15Nadj) of scrapers and predators for comparisons of some groups among sites, as described in Anderson and Cabana (2007), and as was done previously for these Adirondack sites (Riva-Murray et al. 2011). Unadjusted δ15N values were used for within-site comparisons. Groups containing fewer than five composite samples were neither used in the above statistical comparisons nor graphically displayed in group comparisons. As an indicator of the overall extent of variation in MeHg% for each feeding group, we calculated percent coefficient of variation (%CV) from medians for each site, season, and year. Linear regression was used within feeding groups to examine the regression of MeHg with THg, after transformation (log10 for predators; square root for scrapers and shredders); two extreme (low) MeHg outliers for predators were not included in the regression. A subsequent analysis of covariance was used to test for the influence of site on the regressions within feeding groups (using data from sites with at least 10 samples). Spearman rank correlation analysis was used to relate insect THg and MeHg with concentrations of FMeHg within feeding groups, using median insect THg and MeHg from each site visit and the single stream water FMeHg value from the corresponding site visit. Similarly, we examined correlations between median MeHg% in each feeding group and FMHg to assess whether or not MeHg% was consistent across a gradient of aqueous MeHg concentrations.
Percent methylmercury in three feeding groups of aquatic insects collected from streams in the Adirondack region (New York, USA) during 2007–09. Numbers of composite samples are indicated above x axis. Boxes show median (horizontal line within box), 25th and 75th percentiles (bottom and top of box, respectively), and 10th and 90th percentiles (bottom and top horizontal lines, respectively). Dots below and above boxes are 5th and 95th percentiles, respectively. Significantly different groups are denoted by different letters above the boxes. Statistics shown are result of analysis of variance on ranked data
Mean specimen weight was calculated for each composite sample from total composite weight and number of specimens in the sample and used as an indicator of size for selected taxa. Statistical analysis of yearly variation in biotic MeHg% was conducted primarily to assess whether to combine data from different years for further analyses of seasonal and spatial variation. More extensive evaluation of annual variation was not possible due to the limited number of samples collected within seasons, sites, and feeding groups across years. All spring collections occurred between 15 and 28 May; all summer collections occurred between 10 July and 10 September, and all fall collections occurred between 6 and 23 October.
Results
The compiled data included scrapers (i.e. mayfly naiads) from 6 sites (n = 44; SI Tables 2 and 3), shredders (i.e., caddisfly larvae) from 8 sites (n = 90; SI Tables 2 and 4), and predators (i.e. dragonfly, damselfly, and stonefly naiads) from 8 sites (n = 126; SI Tables 2 and 5). Five sites had enough composite samples of two or three feeding groups for within-site comparisons among the groups. The overall median MeHg% (Fig. 2; data from all sites, years, and seasons) was highest in predators (94%), intermediate in shredders (52%), and lowest in scrapers (35%). Differences in MeHg% among feeding groups were large and statistically significant, both with all data combined (F = 301.9, p < 0.0001; Fig. 2) and within individual sites (Fig. 3a, SI Table 6). Predators had higher MeHg% than sympatric primary consumers in all five sites, and shredders had higher MeHg% than scrapers in all three sites considered. The higher predator MeHg% corresponded with their higher trophic positions, indicated by significantly higher δ15N within all five sites (Fig. 3b, SI Table 6). In contrast, the pattern of MeHg% between the two primary consumers did not correspond with their relative trophic positions in the two sites compared statistically (Fig. 3b). Instead, scrapers (which had lower MeHg%) occupied higher or similar trophic positions as shredders (based on δ15N results for FNW and F28). Both MeHg and THg were higher in scrapers than in shredders at all three sites (p < 0.0001 for both MeHg and THg at FNW and F28; p = 0.035 and 0.002 for MeHg and THg, respectively at SLL; SI Fig. 3).
Percent methylmercury and nitrogen stable isotopes in sympatric feeding groups of aquatic insects collected from Adirondack streams during 2007–09. Comparison of (a) percent methylmercury, and (b) nitrogen isotope ratios (δ15N) among scrapers (SC), shredders (SH), and predators (PR) within each of five stream sites (i.e., selected sites with ≥5 composite samples of two or more feeding groups). Numbers of composite samples are indicated above x axis. Different letters along top denote significantly different groups within each site. Test statistics are provided in SI Table 5. Boxplot components are defined in the caption of Fig. 2. Site names are provided in SI Table 1. The δ15N values are not base-adjusted because comparisons were made within sites rather than among sites
Annual variation
Analysis of annual variation in MeHg% within sites and seasons was limited to predators (two sites, summer collections; SI Table 7) and shredders (one site, spring collections; SI Table 4). Predator MeHg% was not significantly different between 2007 and 2008 at either SLL (medians 102 and 103%, respectively) or FLL (median 102% in both years). However, predators from SLL had significantly lower MeHg% in 2009 (median 83%) than either previous year (F = 10.8, p = 0.0008). In this case, two of the six samples from 2009 were extreme low outliers for the entire predator data set, due to unusually low MeHg concentrations in these two samples (SI Table 2). Based on these results, predator data from 2007 and 2008 were combined for most of the subsequent analyses, and the limited data from 2009 (i.e., collected from only two sites) were considered separately. Analysis of shredder data collected from SLL during spring 2008 and 2009 showed significantly higher MeHg% in 2009 than in 2008 (p = 0.02; medians 68 and 76% for 2008 and 2009, respectively). Few scrapers were collected in 2009 (3 from 1 site; SI Table 3). Based on the difference in shredder MeHg% in 2009, and the collection of primary consumer samples from only one or two sites that year, we did not include the 2009 scraper and shredder data in analysis of seasonal and spatial variation. Numbers of scraper and shredder samples within sites and seasons did not allow for statistical comparison of 2007 with 2008 in either group. Because of the low numbers, we combined data from 2007 and 2008 for analysis of seasonal and spatial variation.
Seasonal and spatial variation in primary consumers
Seasonal and spatial differences in MeHg% were apparent in scrapers and in shredders collected during 2007–08. The %CVs in mean MeHg% were 38 and 40% for scrapers and shredders, respectively (mean [±1 s.d.] was 40% [±15.0%] for scrapers and 59% [±23.4%] for shredders). Scrapers, collected in all three seasons from three sites, exhibited a greater range of median MeHg% in spring (from 22 to 51%) than in summer (from 38 to 54%) or fall (from 24 to 39%). Statistical comparison of seasonal differences in scraper MeHg%, possible for samples collected from FNW during spring and summer, showed significantly higher MeHg% in summer-collected samples (p = 0.04; SI Fig. 4A) but no corresponding seasonal shift in trophic position (i.e., δ15N; p = 0.73; SI Fig. 4B). A seasonal difference was observed, however, in the size of specimens; those collected in summer were significantly smaller than those collected in spring (p = 0.012; SI Fig. 4C). Further, a pattern of increasing size was apparent from summer (median 0.011 g) to fall (median 0.016 g) to spring (median 0.024 g) when examined with 2007–08 data from all five sites combined (SI Table 3). Sites exhibited large ranges in scraper MeHg% within seasons during 2007–08 (SI Table 3). Site-specific median MeHg% ranged from 20 to 51% in spring (4 sites), from 34 to 74% in summer (6 sites), and from 39 to 64% in fall (3 sites). Two sites (F28 and FNW) with enough composite samples (spring collections) to evaluate statistically had significantly different MeHg% (p = 0.02; Fig. 4), but there was no significant difference in trophic position (i.e., δ15Nadj; p = 0.13) or in specimen size (i.e., mean specimen weight; p = 0.47). Somewhat higher FMeHg concentrations were observed each year at F28 (the site with higher scraper MeHg%) than at FNW (SI Table 8), although this was not statistically evaluated.
Percent methylmercury in scraper aquatic insects from two Adirondack streams. Scrapers are flathead mayflies (Ephemeroptera: Heptageniidae) that were collected during spring 2007–08. Numbers of composite samples are indicated above x axis. Different letters above boxes indicate statistically significant difference. Statistic is result of Wilcoxon rank-sum test. Boxplot components are defined in caption of Fig. 2. Site names are provided in SI Table 1
Statistical analysis of within-site seasonal variation in shredder MeHg% for 2007–08 was not possible because of low numbers in summer collections (SI Table 4). However, we note the occurrence of site maximum MeHg% in summer-collected samples at four of the five sites from which samples were collected in both seasons (SI Table 3). Site medians for these five sites ranged from 20 to 68% in spring and from 68 to 98% in summer, and analysis of seasonal differences using medians from all five sites revealed significantly higher MeHg% in summer (p = 0.04). Because of these observed seasonal differences in MeHg%, we did not combine spring and summer when examining variation among sites. Shredder MeHg% ranged widely across sites. Mediansranged from 20 to 68% across eight sites (SI Table 4; spring collections from 2007–08), and within-year ranges were similar (from 20 to 70% in 2007 and from 20 to 68% in 2008; 7 sites each year). Differences among sites (six had enough samples to test) were statistically significant (F = 18.0, p < 0.0001; Fig. 5). The pattern of site-to-site differences generally corresponded with the spatial pattern of FMeHg in stream water collected during spring (SI Table 8). Concentrations of FMeHg were nondetectable at the site with the lowest shredder MeHg% (i.e., <0.04 ng/L at UTR), relatively high at the two sites with the highest shredder MeHg% (0.25 and 0.33 ng/L at SLL; 0.19 and 0.22 at FLL), and generally intermediate at the other sites (SI Table 8).
Comparison of percent methylmercury of shredder aquatic insects (Trichoptera: Limnephilidae) collected in spring 2007 and 2008 from Adirondack streams. Numbers of composite samples are indicated above x axis. Statistics are result of analysis of variance on ranked data. Different letters above boxes indicate statistically significant difference between groups. Boxplot components are defined in caption of Fig. 2. Site names are provided in SI Table 1
Taxonomic, seasonal, and spatial patterns in predators
The predator feeding group consisted of stoneflies (Plecoptera, n = 9), damselflies (Odonata: Zygoptera, n = 7), and three dragonfly families (Odonata: Aeshnidae, Gomphidae, and Libellulidae; n = 74, 12, and 24, respectively). The %CV for predators was 7% (mean [±1 s.d.] 95% [6.6%]), which is more than five times lower than that of either primary consumer. Median MeHg% (data from all sites, seasons, and years combined) was at least 92% in every taxon (Fig. 6; SI Table 5) and did not differ significantly among taxa (F = 0.80, p = 0.53; Fig. 6). Comparisons limited to summer 2007–08 collections from individual sites also showed no significant taxonomic variation (F = 0.34 and p = 0.72 for comparison of Aeshnidae, Gomphidae, and Libellulidae from FLL; p = 0.16 for comparison of Aeshnidae and Libellulidae from FNW). Aeshnidae and Libellulidae , the most numerous and widely-distributed predators in the data set (SI Table 5), were combined (henceforth ‘selected dragonflies’) for analyses of seasonal and spatial variation.
Percent methylmercury in five taxa of predatory aquatic insects collected from Adirondack streams during 2007–09. Numbers of composite samples are indicated above x axis. Absence of significant differences among groups is indicated by same letter above boxes. Statistics are from analysis of variance on ranked data. Boxplot components are defined in the caption of Fig. 2
Selected dragonflies exhibited a wide range of sizes, with mean specimen weight ranging from 0.02 to 0.78 g (median over all composite samples was 0.19 g). There was no significant size difference between the two families (p = 0.92). Results of analysis of covariance (ANCOVA) performed with mean specimen weight (square-root transformed) as the independent variable, and site as a class variable (using data from all collections at FNW and SLL [n = 35 and 29, respectively]) showed a weak, yet significant, negative relation with mean specimen weight and no significant influence of site on MeHg% (model R2 = 0.12, p = 0.02; weight p = 0.01; site p = 0.84). Further inspection revealed that the smallest (mean weight < 0.06 g) were not collected during spring from any sites (SI Table 9). Conducting the ANCOVA without these small specimens eliminated the influence of mean specimen weight (model p = 0.56). Thus, to avoid a possible influence of size on analysis of spatial and seasonal variation, we separated selected dragonflies into ‘smaller’ and ‘larger’ size classes (mean specimen weight <0.06 and ≥0.06 g, respectively) to evaluate seasonal and spatial variation.
The MeHg% of selected dragonflies (larger size class) did not differ significantly among spring, summer, and fall collections from FNW in 2007–08 (F = 0.60, p = 0.56; SI Fig. 5). In contrast, collections from SLL in 2009 exhibited significantly higher MeHg% in spring than summer (p = 0.03; SI Fig. 6; medians 100 and 83%, respecively), and this corresponded with significantly higher δ15N in spring (p = 0.03). To avoid potential seasonal influences on statistical evaluation of site-to-site variation in selected dragonflies, we separated spring collections from summer and fall collections (the latter two seasons combined into ‘summer-fall’).
Median MeHg% in selected dragonflies (larger size class) collected during summer-fall 2007–08 from six sites was relatively high, ranging from 90 to 100% (SI Table 9). The MeHg% of selected dragonflies did not differ significantly among the five sites tested (F = 2.3, p = 0.08; Fig. 7a), despite large differences in trophic position among sites (i.e., δ15Nadj; F = 13.3, p < 0.0001; Fig. 7b), as well as a large FMeHg range across sites (from 0.15 to 0.60 ng/L in summer samples; SI Table 8). These findings were similar for the smaller size class (collected during summer-fall 2007–08 from five sites). Median MeHg% was high at all five sites (from 94 to 104%; SI Table 9), and the three sites tested were not significantly different in MeHg% (F = 2.7, p = 0.10) despite large differences in δ15Nadj (F = 20.0, p < 0.0001). In contrast to the findings based on data from summer-fall 2007–08, a comparison of spring 2009 data (larger size class) between the two sites sampled that year (larger size class) revealed significantly higher MeHg% at SLL than at FNW (p = 0.0012; medians 100 and 86%, respectively; SI Table 9), but no significant difference in trophic position (i.e., δ15Nadj; F = 0.89, p = 0.36). Although there too few composite samples to statistically compare sites within spring 2007–08 we compared medians among the three sites sampled in both spring and summer-fall, and observed a greater range in spring (from 79 to 100%) than in summer-fall (from 94 to 102%), further indicating potentially greater differences in MeHg% among sites in spring than later in the growing season.
Site comparisons of (a) percent methylmercury and (b) base-adjusted nitrogen isotope ratios (δ15Nadj in selected dragonflies collected during summer-fall in 2007–08 from Adirondack streams. Data are from collections of dragonflies (Odonata: Aeshnidae and Libellulidae) having mean specimen weight in composite samples of ≥0.06 g. Numbers of composite samples are indicated above x axis. Statistics are result of analysis of variance on ranked data. Significant differences are indicated by different letters above boxes. Boxplot components are defined in caption of Fig. 2. Site names are listed in SI Table 1
Regressions of MeHg with THg in aquatic insects
Regressions of MeHg with THg (Fig. 8; data from all sites, seasons, and years), were positive and strong for all predators (R2 = 0.92, p < 0.0001, n = 124), selected dragonflies (R2 = 0.91, p < 0.0001, n = 96), and shredders (R2 = 0.80, p < 0.0001, n = 90), and weak for scrapers (R2 = 0.32, p < 0.0001, n = 44). Including site as an interaction term with THg in ANCOVA (limited to sites with at least 10 composite samples of each feeding group collected during 2007–08) indicated a significant interaction of site with THg for shredders and scrapers but no influence for all predators or for selected dragonflies (SI Table 10). Three sites (FNW, FLL, and SLL) had enough shredders and selected dragonflies to compare regression slopes. The slope of the regression for selected dragonflies from the three sites was 1.0 (calculated without a site term, on the basis of ANCOVA results), whereas the slopes for shredders varied widely across the same three sites (0.47, 0.83, and 1.51 for F28, FNW, and SLL, respectively).
Regressions of methylmercury concentrations with total mercury concentrations in immatures of aquatic insects from Adirondack streams that are (a) predators, and (b) primary consumers (scrapers and shredders). Predators are selected dragonflies (Odonata) in the families Aeshnidae (darners) and Libellulidae (common skimmers); scrapers are flathead mayflies (Ephemeroptera: Heptageniidae), and shredders are northern caddisflies (Trichoptera: Limnephilidae). Data are from composite samples collected from nine sites during 2007–09. The two extreme (low) methylmercury outliers for predators were not included in the regression. Dashed lines are 1:1 lines. Statistics and solid lines are results of linear regression for each feeding group. Recovery-adjusted total mercury concentrations were used for samples analyzed by direct combustion (SI Table 2)
Correlations of aquatic insect THg, MeHg, and MeHg% with FMeHg in water
The strength of correlations of insect THg with FMeHg varied among the feeding groups, despite strong and consistent correlations of insect MeHg with FMeHg in all three groups. In predators (i.e., selected dragonflies) the correlation was strong (rs = 0.78, p = 0.0001; Fig. 9a) and similar to that of their MeHg (rs = 0.77, p = 0.0001; Fig. 9a). The correlation of shredder THg with FMeHg was relatively strong (rs = 0.67, p = 0.0002; Fig. 9b), but much weaker than that of their MeHg (rs = 0.83, p < 0.0001; Fig. 9b). Finally, the correlation of scraper THg with FMeHg was not significant (p = 0.84; Fig. 9c), despite the strong correlation of their MeHg with FMeHg (rs = 0.85, p = 0.0002; Fig. 9c). The relation of MeHg% to FMeHg also differed between primary consumers and predators. Whereas median MeHg% in scrapers and shredders were significantly correlated with FMeHg (rs = 0.89 and p < 0.0001, rs = 0.62 and p = 0.005, respectively), the correlation for selected dragonflies was not significant (p = 0.22).
Relations between aqueous (filtered) methylmercury (MeHg) and concentrations of MeHg and total mercury (THg) in (a) predators (selected dragonflies, Odonata: Aeshnidae and Libellulidae), (b) shredders (northern casemakers, Trichoptera: Limnephilidae), and (c) scrapers (flathead mayflies, Ephemeroptera: Heptageniidae) collected from Adirondack streams during 2007–09. Biotic concentrations are medians for each combination of site, season, and year; water MeHg concentration data are from one composite sample per site visit (most cases) or median of multiple visits in a given season and year. Statistics shown are results of Spearman rank correlation analysis. dw dry weight. ****p < 0.0001, ***p < 0.001, **p < 0.01, ns p = 0.84. Recovery-adjusted total mercury concentrations were used for insect samples analyzed by direct combustion (SI Table 2) prior to calculating median THg
Discussion
Predators and primary consumer insects from these Adirondack streams differ in the extent to which THg is likely to adequately represent MeHg for biomonitoring purposes across the region. This is due to large differences between these two broad groups in MeHg%, in the extent of within-group taxonomic, spatial, and (or) seasonal variation in MeHg%, and in the ability of biotic THg to predict biotic MeHg and to track MeHg in water (i.e., FMeHg). The large range in MeHg% among feeding groups and taxa observed in this study, and the increase in MeHg% with generalized trophic position agree with previous findings for freshwaters in other settings that receive Hg mainly from distant sources (Tremblay et al. 1996; Mason et al. 2000; Gorski et al. 2003; Loukmas et al. 2006). Our finding of higher δ15N in predators than primary consumers demonstrates the importance of biomagnification to MeHg% in aquatic insects from Adirondack streams, as has been proposed to explain such patterns elsewhere (Tremblay et al. 1996; Mason et al. 2000; Loukmas et al. 2006). In contrast, our results indicate trophic magnification of MeHg within primary consumers does not account the observed taxonomic, seasonal, and spatial variation in MeHg% observed in these Adirondack streams.
Taxonomic, seasonal and spatial variation in MeHg% of primary consumers
The primary consumers from these Adirondack streams had MeHg% that varied taxonomically, seasonally, and spatially. Scrapers had lower MeHg% than sympatric shredders, even though they had higher trophic positions and higher MeHg concentrations. The differences in MeHg% could be due to anatomical and physiological differences between the two taxa considered in this study. Higher MeHg concentrations and trophic position of Hepteganiidae (i.e. scrapers) relative to those of Limnephilidae (i.e., shredders) have also been reported elsewhere (Thera et al. 2019). Heptageniidae have been observed to have even higher THg than other aquatic insects from the same site, including predatory aquatic insects (Gorski et al. 2003). In addition, Heptageniidae have a relatively high proportion of chitin in relation to soft tissue (Meyer 1990), particularly compared with Limnephilidae (Cauchie 2002). Lower MeHg% of Heptageniidae could result from this anatomical difference in conjunction with the lower affinity of MeHg for hard tissue than for muscle, and the preferential accumulation of inorganic Hg in sclerotized tissue (e.g. chitin in the insect exoskeleton; Boening 2000; Mason et al. 2000).
The seasonal pattern of lower scraper MeHg% in spring collections than in summer-fall collections also did not correspond with the observed seasonal δ15Nadj patterns, indicating that seasonal variation in MeHg% is not associated with a shift to a higher trophic position later in the growing season. Rather, the higher MeHg% in summer appears more simply attributed to greater dietary intake of MeHg, as indicated by the higher FMeHg in stream water (i.e. more bioavailable MeHg for uptake by periphyton) later in the growing season. Our results for shredders also indicate the potential for seasonal influences on their MeHg%. Although data were insufficient for within-site statistical evaluation, analysis of medians from multiple sites showed higher MeHg% in summer than in spring. This contrasts with the relatively constant MeHg% observed from spring to fall in Limnephilidae and other detritivore-grazers from lakes in Quebec (Canada) and Sweden (Tremblay et al. 1996) but does correspond with the greater aqueous MeHg in summer reported here and previously for Adirondack streams (Bradley et al. 2011; Burns et al. 2012; Riva-Murray et al. 2013b). This pattern suggests that MeHg% in shredders is strongly influenced by seasonal changes in dietary intake of MeHg. The seasonal variation of MeHg% in these primary consumers could also be due to the relatively short life spans of these taxa, since most Heptageniidae and Limnephlidae are univoltine (Thorp and Covich 1991; Myers et al. 2011). In addition, the primary consumers considered here can exhibit seasonal variation in bioenergetics, physiology, habitat, diet, and species composition (Merritt and Cummins 1996; Myers et al. 2011), which can also contribute to seasonal variation in MeHg%.
The wide range in primary consumer MeHg% across sites in this study (within seasons) were similar to those observed elsewhere for Heptageniidae (Boening 2000; Mason et al. 2000; Loukmas et al. 2006) and Limnephilidae (Tremblay et al. 1996; Loukmas et al. 2006). Our findings did not support an interpretation based on spatial differences in trophic position to explain the site-to-site differences in MeHg% of Heptageniidae (we did not have sufficient data to consider this in Limnephilidae). Although located in a small area, these streams vary widely in habitat, food web characteristics, and aqueous MeHg, as reported here and previously (Riva-Murray et al. 2011; Beaulieu et al. 2012; Riva-Murray et al. 2013b). Our findings indicate that differences among sites in primary consumer MeHg% is strongly related to differences in concentrations of aqueous MeHg (i.e. FMeHg) that is available for uptake into periphyton that is scraped off rocks and woody debris, or that is ingested in the process of shredding leaf litter. Primary consumer MeHg% could also be influenced by spatial variation in other factors that influence their bioaccumulation of MeHg, including dietary carbon sources (Jardine et al. 2012; Riva-Murray et al. 2013a), and amino acid content (Thera et al. 2019). Nutritional quality of food is another potential influence on primary consumer MeHg% because poor quality food can in greater exposure to inorganic Hg (Tremblay et al. 1996),
The relatively low and variable MeHg% of the primary consumers considered in this study indicate that THg would not be suitable as a surrogate for MeHg in these groups across the Adirondack region. This contraindication is also supported by our findings concerning the ability of primary consumer THg to predict their MeHg and to track concentrations of MeHg in stream water. The weak regression of scraper THg with MeHg, the strong correlation of MeHg% with FMeHg, and the non-significant correlation of scraper THg with FMeHg further indicate that THg is not likely to adequately represent MeHg in Heptageniidae for monitoring across broad areas, or for monitoring temporal trends in relation to changing environmental concentrations of MeHg. Although THg was strongly correlated to MeHg in shredders when data from all sites were combined, the finding of a significant influence of site on the regression indicates that THg is not likely to perform well as a direct substitution for MeHg (or for use with a constant conversion factor) across multiple sites. This is also indicated by the much weaker correlation of shredder THg than its MeHg with FMeHg, and the significant correlation of shredder MeHg% with FMeHg.
Predators
Predator MeHg% was typically high (≥90%), consistent among taxa, and exhibited minimal seasonal and site-to-site variation. The generally high MeHg% in predatory aquatic insects considered here is similar to the high MeHg% reported in fish muscle (Grieb et al. 1990; Bloom 1992) and whole fish (Bodaly and Fudge 1999; Mason et al. 2000; Wiener et al. 2012), which is the basis for the common substitution of THg for MeHg in fish (although recent work shows lower fish MeHg% in some cases [Lescord et al. 2018]). In this study, we mainly focused on two families of active, obligate predators (i.e., Aeshnidae and Libellulidae or “selected dragonflies”). The relatively high MeHg% in these dragonflies has been observed in other studies (Myers et al. 2011; Buckland-Nicks et al. 2014; Nelson et al. 2015, 2019; Rolfhus et al. 2015), as has the similar MeHg% in these two families (Buckland-Nicks et al. 2014; Nelson et al. 2015), which supports their possible use interchangeably for MeHg biomonitoring with THg data. Although we found similar MeHg% in Gomphidae as in the other dragonfly families, we had few composite samples of these burrowing dragonflies overall, and others have reported somewhat lower MeHg% in this family than what we have found here (Haro et al. 2013) or have observed lower MeHg% in Gomphidae than in other dragonfly families (including Aeshnidae and Libellulidae) from the same locations (Buckland-Nicks et al. 2014). Further investigation of the influence of dragonfly habitat and feeding behavior would help establish which families can be used interchangeably for MeHg monitoring with THg data; this can be especially useful where distributions of individual taxa are limited or patchy.
We had a large range of sizes of the selected dragonflies and observed a weak negative relation between mean specimen weight and MeHg% as well as significantly higher MeHg% in smaller naiads than larger naiads collected during summer from one site (but not from the other two sites considered). Exploring size in relation to MeHg% in dragonfly naiads was beyond the scope of the current study, but others have reported a positive relation of dragonfly naiad size with both concentration and variability of MeHg (Buckland-Nicks et al. 2014). Haro et al. (2013) recommend focusing on later instars for biomonitoring with Gomphidae because they integrate over a longer time period (at least two years) and provide sufficient mass for analysis of individual specimens. Our results indicate that targeting particular instars will also serve to reduce the variation in MeHg% and will enhance the ability of THg to represent MeHg in dragonflies.
The limited seasonal and spatial variation of MeHg% in predators (selected dragonflies) in this study corresponds with their higher trophic positions than the primary consumers, as well as their relatively long aquatic life stages (2–4 years, Peckarsky et al. 1990; Thorp and Covich 1991). The only seasonal difference in selected dragonfly MeHg% occurred in samples collected during 2009 from one site (SLL), when MeHg% in spring-collected samples was significantly higher than in summer-collected samples. This differs from the slight increase from spring through fall in MeHg% of predators reported elsewhere (Tremblay et al. 1996), and from our finding of no difference between MeHg% of selected dragonflies collected during 2007–08 from two other sites. Although possibly limited within sites, seasonality may be particularly important because of its potential influence on spatial patterns. In this study, we observed a greater range of site median MeHg% in spring than in summer, and the only significant difference between sites in MeHg% of selected dragonflies occurred during spring of 2009. This suggests the potential for greater variability in MeHg% among sites during spring than later in the growing season, possibly as a result of more pronounced differences in food availability, bioenergetics, and possibly different relative abundances of particular species within the dragonfly families considered here. These factors should be considered further when determining how best to design MeHg monitoring strategies with THg in dragonflies. Our ability to explore seasonality was limited by the numbers of samples collected within sites across seasons. We analyzed MeHg and THg in composites of multiple specimens, but the larger instars have sufficient mass for individual analyses (Haro et al. 2013) which would provide a larger number of analyses for biomonitoring. Furthermore, a power analysis (e.g., Haro et al. 2013) would be useful to determine the optimal number of samples required to detect change in MeHg by use of THg in dragonflies for particular monitoring goals.
When limited to summer-fall collections of selected dragonflies (larger size class), we found that THg is composed of at least 90% MeHg across diverse streams, is a strong predictor of dragonfly MeHg, and performs well in tracking aqueous concentrations of MeHg (i.e. FMeHg). The finding of little spatial variation in MeHg% corresponds with reports for dragonflies from other relatively pristine systems (Tremblay et al. 1996). The current study indicates that MeHg% in these dragonflies is robust to large differences among sites in food chain length (as indicated by stable isotope results) and environmental characteristics of sites, including a large gradient of bioavailable MeHg (i.e., FMeHg), and differences in dietary carbon source (Riva-Murray et al. 2013b).
Relatively high MeHg% was observed in damselflies and predatory stoneflies, although we note that there were relatively small numbers of samples of each in our dataset. Similarly-high MeHg% has been reported for stoneflies from Maryland streams (Mason et al. 2005), and for damselflies (Odonata: Zygoptera) from South Carolina streams (calculated from data presented in Beaulieu et al. 2012). Although damselfly naiads often occupy similar habitats as Aeshnidae and Libellulidae dragonfly naiads, predatory stonefly naiads also are active, long-lived (2–4 years, Peckarsky et al. 1990), obligate predators that will typically be found in higher gradient streams that do not support dragonflies (Merritt and Cummins 1996). Additional investigation of MeHg% in predatory stoneflies would be particularly valuable for Hg biomonitoring because of the relatively high MeHg% in these important predators from habitats that are not typically occupied by dragonflies or damselflies.
Implications for the use of aquatic insect THg to monitor MeHg across the Adirondacks and other remote settings
The heterogeneity of this small portion of the Adirondack region in terms of Hg-sensitivity of aquatic systems reflects the heterogeneity of the broader Adirondack region in terms of Hg methylation potential, environmental characteristics such as concentrations of MeHg and DOC, and food web characteristics that influence Hg bioaccumulation in Adirondack waters (Driscoll et al. 1995; Simonin et al. 2008; Yu et al. 2011; Burns et al. 2012; Burns and Riva-Murray 2018). Studies in other regions and regional extents have shown spatial heterogeneity in these factors (Kamman et al. 2005; Evers et al. 2007; Simonin et al. 2008; Clayden et al. 2013, 2014; Depew et al. 2013; Eagles-Smith et al. 2016). Our results suggest this heterogeneity will produce large variation in MeHg% of primary consumers across the Adirondacks and similar regions and will have limited influence on MeHg% in aquatic insects that are obligate predators.
Conclusions
The potential for THg to serve as a reliable surrogate for MeHg in aquatic insects varies among feeding groups and taxa. By documenting these patterns in relation to indicators of trophic position and environmental MeHg patterns, we provide critical information to help inform the best use of THg as a surrogate for MeHg in aquatic insects and we highlight important areas for further research. Results of the current study, in a remote forested setting, indicate that MeHg comprises a relatively high percentage of THg in aquatic insects that are obligate predators, particularly Aeshnidae and Libellulidae dragonflies. Depending upon monitoring objectives and requirements, THg is more likely to be a suitable surrogate for MeHg in predatory aquatic insects than in primary consumers. We caution that these results would most likely apply to remote settings such as the Adirondack region that receive Hg primarily from atmospheric deposition, and not to locations that have industrial, mining and (or) legacy sources of Hg. Because aquatic insects are good biosentinel organisms, the use of THg in certain aquatic insects to represent spatial and temporal patterns of MeHg could greatly enhance their use for Hg monitoring. Additional investigation will be necessary to more thoroughly define the spatial, temporal, and taxonomic extent to which THg is an appropriate surrogate for MeHg in particular taxa, aquatic habitats, and geographic regions. There are many reasons why primary consumer taxa also could function as good Hg biosentinels but results of the current study indicate that, absent additional information on the consistency of MeHg% in particular taxa, settings, and time periods, and (or) the development of predictive models from which to derive MeHg from THg, the continued analysis of MeHg is warranted for monitoring with these groups.
References
Anderson C, Cabana G (2007) Estimating the trophic position of aquatic consumers in riverine food webs using nitrogen stable isotopes. J North Am Benthol Soc 26:273–285
Baker JP, Van Sickle J, Gagen CJ, DeWalle DR, Sharpe WE, Carline RF, Baldigo BP, Murdoch PS, Bath DW, Kretser WA, Simonin HA, Wiggington Jr PJ (1996) Episodic acidification of small streams in the northeastern United States: effects on fish populations. Ecol Appl 6:422–437. https://doi.org/10.2307/2269380
Baldigo BP, Roy KM, Driscoll CT (2016) Response of fish assemblages to declining acidic deposition in Adirondack Mountain lakes, 1984–2012. Atmos Environ 146:223–235. https://doi.org/10.1016/j.atmosenv.2016.06.049
Batchelar KL, Kidd KA, Drevnick PE, Munkittrick KR, Burgess NM, Roberts AP, Smith JD (2013) Evidence of impaired health in yellow perch (Perca flavescens) from a biological mercury hotspot in northeastern North America. Environ Toxicol Chem 32:627–637. https://doi.org/10.1002/etc.2099
Beaulieu KM, Button DT, Scudder Eikenberry BC, Riva-Murray K, Chasar LC, Bradley PM, Burns DA (2012) Mercury Bioaccumulation Studies in the National Water-Quality Assessment Program–Biological Data from New York and South Carolina, 2005-2009. Reston (VA): U.S. Geological Survey; 2012. Data Series Report No.: 705. https://doi.org/10.3133/ds705
Beeby A (2001) What do sentinels stand for? Environ Pollut 112:285–298. https://doi.org/10.1016/S0269-7491(00)00038-5
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017. https://doi.org/10.1139/f92-113
Bodaly RA, Fudge RJP (1999) Uptake of mercury by fish in an experimental boreal reservoir. Arch Environ Contam Toxicol 37:103–109. https://doi.org/10.1007/s002449900494
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351
Bradley PM, Burns DA, Harvey JW, Journey CA, Brigham ME, Riva-Murray K (2016) Hydraulic and biochemical gradients limit wetland mercury supply to an Adirondack stream. SOJ Aquat Res 1:1–9
Bradley PM, Burns DA, Riva-Murray K, Brigham ME, Button DT, Chasar LC, Marvin-DiPasquale M, Lowery MA, Journey CA (2011) Spatial and seasonal variability of dissolved methylmercury in two stream basins in the eastern United States. Env Sci Technol 45:2048–2055. https://doi.org/10.1021/es103923j
Bradley PM, Journey CA, Brigham ME, Burns DA, Button DT, Riva-Murray K (2012) Intra- and inter-basin mercury comparisons: importance of basin scale and time-weighted methylmercury estimates. Environ Pollut 172:42–52
Brigham ME, Sandheinrich MB, Gay DA, Maki RP, Krabbenhoft DP, Wiener JG (2014) Lacustrine responses to decreasing wet mercury deposition rates—results from a case study in northern Minnesota. Environ Sci Technol 48:6115–6123. https://doi.org/10.1021/es500301a
Brigham ME, Wentz DA, Aiken GR, Krabbenhoft DP (2009) Mercury cycling in stream ecosystems. 1. Water column chemistry and transport. Environ Sci Technol 43:2720–2725. https://doi.org/10.1021/es802694n
Buckland-Nicks A, Hillier KN, Avery TS, O’Driscoll NJ (2014) Mercury bioaccumulation in dragonflies (Odonata: Anisoptera): examination of life stages and body regions. Environ Toxicol Chem 33:2047–2054. https://doi.org/10.1002/etc.2653
Burns DA, Riva-Murray K (2018) Variation in fish mercury concentrations in streams of the Adirondack region, New York: a simplified screening approach using chemical metrics. Ecol Indic 84:648–661. https://doi.org/10.1016/j.ecolind.2017.09.031
Burns DA, Riva-Murray K, Bradley PM, Aiken GR, Brigham ME (2012) Landscape controls on total and methyl Hg in the upper Hudson River basin, New York, USA. J Geophys Res Biogeosci 117:G01034. https://doi.org/10.1029/2011JG001812
Cauchie H-M (2002) Chitin production by arthropods in the hydrosphere. Hydrobiologia 470:63–96
Clayden MG, Kidd KA, Wyn B, Kirk JL, Muir DCG, O’Driscoll NJ (2013) Mercury biomagnification through food webs is affected by physical and chemical characteristics of lakes. Environ Sci Technol 47(21):12047–12053. https://doi.org/10.1021/es4022975
Clayden MG, Kidd KA, Chetelat J, Hall BD, Garcia E (2014) Environmental, geographic and trophic influences on methylmercury concentrations in macroinvertebrates from lakes and wetlands across Canada. Ecotoxicology 23:273–284. https://doi.org/10.1007/s10646-013-1171-9
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SLK, Hallinger KK, Monroe AO, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335–335. https://doi.org/10.1126/science.1154082
Depew DC, Burgess NM, Campbell LM (2013) Modelling mercury concentrations in prey fish: derivation of a national‐scale common indicator of dietary mercury exposure for piscivorous fish and wildlife. Environ Pollut 176:234–243. https://doi.org/10.1016/j.envpol.2013.01.024
Dittman JA, Driscoll CT (2009) Factors influencing changes in mercury concentrations in lake water and yellow perch (Perca flavescens) in Adirondack lakes. Biogeochemistry 93:179–196. https://doi.org/10.1007/s10533-009-9289-9
Driscoll CT, Blette V, Yan C, Schofield CL, Munson R (1995) The role of dissolved organic carbon in the chemistry and bioavailability of mercury in remote Adirondack lakes. Water Air Soil Pollut 80:499–508. https://doi.org/10.1007/978-94-011-0153-0_54
Driscoll CT, Han Y-J, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK (2007) Mercury contamination in forest and freshwater ecosystems in the northeastern United States. Bioscience 57:17–28. https://doi.org/10.1641/B570106
Driscoll CT, Han Y-J, Schofield CL, Munson R, Holsapple J (1994) The mercury cycle and fish in Adirondack lakes. Env Sci Technol 28:136A–143A. https://doi.org/10.1641/B570106
Eagles-Smith C, Wiener JG, Eckley CS, Willacker JJ, Evers DC, Marvin-DiPasquale M, Obrist D, Fleck JA, Aiken GR, Lepak JM, Jackson AK, Webster JP, Stewart AR, Davis JA, Alpers CN, Ackerman JT (2016) Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci Total Environ 568:1213–1226. https://doi.org/10.1016/j.scitotenv.2016.05.094
Evers DC, Han Y-J, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T (2007) Biological mercury hotspots in the northeastern United States and southeastern Canada. BioScience 57:29–43. https://doi.org/10.1641/B570107
Gorski PR, Cleckner LB, Jiurley JP, Soerszen ME, Armstrong DE (2003) Factors affecting enhanced mercury bioaccumulation in inland lakes of Isle Royale National Park, USA. Sci Total Environ 304:327–348. https://doi.org/10.1016/S0048-9697(02)00579-X
Grieb TM, Driscoll CT, Gloss SP, Schofield CL, Bowie GL, Porcella DB (1990) Factors affecting mercury accumulation in fish in the upper Michigan peninsula. Environ Toxicol Chem 9:919–930. https://doi.org/10.1002/etc.5620090710
Hammerschmidt CR, Fitzgerald WF (2006) Bioaccumulation and trophic transfer of methylmercury in long Island sound. Arch Environ Contam Toxicol 51:416–424. https://doi.org/10.1007/s00244-005-0265-7
Haro RJ, Bailey SW, Northwick RM, Rolfhus KR, Sandheinrich MB, Wiener JG (2013) Burrowing dragonfly larvae as biosentinels of methylmercury in freshwater food webs. Environ Sci Technol 47:8148–8156. https://doi.org/10.1021/es401027m
Harris RC, Bodaly RA (1998) Temperature, growth, and dietary effects on fish mercury dynamics in two Ontario lakes. Biogeochemistry 40:175–187. https://doi.org/10.1023/A:1005986505407
Jardine TD, Kidd KA, Rasmussen JB (2012) Aquatic and terrestrial organic matter in the diet of stream consumers: implications for mercury bioaccumulation. Ecol Appl 22:843–855. https://doi.org/10.1890/11-0874.1
Jeremiason JD, Reiser TK, Weitz RA, Berndt ME, Aiken GR (2016) Aeshnid dragonfly larvae as bioindicators of methylmercury contamination in aquatic systems impacted by elevated sulfate loading. Ecotoxicology 25:456–468. https://doi.org/10.1007/s10646-015-1603-9
Johnson BM, Lepak JM, Wolff BA (2015) Effects of prey assemblage on mercury bioaccumulation in a piscivorous sport fish. Sci Total Environ 506-507:330–337. https://doi.org/10.1016/j.scitotenv.2014.10.101
Kamman NC, Burgess NM, Driscoll CT, Simonin HA, Goodale W, Linehan J, Estabrook R, Hutcheson M, Major A, Scheuhammer AM, Scruton DA (2005) Mercury in freshwater fish of northeast North America—a geographic perspective based on fish tissue monitoring databases. Ecotoxicology 14:163–180. https://doi.org/10.1007/s10646-004-6267-9
Lasorsa B, Allen-Gil S (1995) The methylmercury to total mercury ratio in selected marine, freshwater, and terrestrial organisms. In: Porcella DB, Huckabee JW, Wheatley B (eds) Mercury as a global pollutant. Springer, Dordrecht, https://doi.org/10.1007/978-94-011-0153-0_97
Lescord GL, Johnston TA, Branfireun BA, Gunn JM (2018) Percentage of methylmercury in the muscle tissue of freshwater fish varies with body size and age and among species. Environ Toxicol Chem 37:2682–2691. https://doi.org/10.1002/etc.4233
Loukmas JJ, Quinn SO, Bloomfield J (2006) Total and methyl mercury in the Neversink Reservoir watershed. Albany NY: New York State Department of Environmental Conservation Report No.:11/21/06
Mason RP, Abbott ML, Bodaly RA, Bullock Jr OR, Driscoll CT, Evers DC, Murray ELS, Swain EB (2005) Monitoring the response to changing mercury deposition. Environ Sci Technol 39:14A–22A. https://doi.org/10.1021/es053155l
Mason RP, Laporte JM, Andres S (2000) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297. https://doi.org/10.1007/s002449910038
Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH (2007) Methylmercury exposure and health effects in humans: a worldwide concern. Ambio 36:3–11. https://doi.org/10.1579/0044-7447
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America, 3rd ed. Kendall/Hunt Publishing Co, Dubuque, IA
Meyer E (1990) Levels of major body compounds in nymphs of the stream mayfly Epeorus sylvicola (Pict.) (Ephemeroptera: Heptageniidae). Archiv Fur Hydrobiologie 117:497–510
Munthe J, Bodaly RA, Branfireun BA, Driscoll CT, Gilmour CG, Harris R, Horvat M, Lucotte M, Maim O (2007) Recovery of mercury-contaminated fisheries. Ambio 36:33–44. https://doi.org/10.1579/0044-7447
Myers LW, Kondratieff BC, Mihuc TB, Ruiger DE (2011) The mayflies (Ephemeroptera), stoneflies (Plecoptera), and caddisflies (Trichoptera) of the Adirondack Park (New York State). Trans Am Entomol Soc 137:63–140
Nelson SJ, Chen CY, Kahl JS (2019). Dragonfly larvae as biosentinels of Hg bioaccumulation in Northeastern and Adirondack lakes: relationships to abiotic factors. Ecotoxicology https://doi.org/10.1007/s10646-019-02149-4
Nelson SJ, Webber HM, Flanagan Pritz CM (2015) Citizen scientists study mercury in dragonfly larvae: dragonfly larvae provide baseline data to evaluate mercury in parks nationwide. National Park Service Natural Resource Report NPS/NRSS/ARD/NRR—2015/938, Fort Collins, CO
Peckarsky BL, Fraissinet PR, Penton MA, Conklin Jr DJ (1990) Freshwater macroinvertebrates of north-eastern North America. Cornell University Press, Ithaca, NY
Riva-Murray K, Chasar LC, Bradley PM, Burns DA, Brigham ME, Smith MJ, Abrahamsen TA (2011) Spatial patterns of mercury in macroinvertebrates and fishes from streams of two contrasting forested landscapes in the eastern United States. Ecotoxicology 20:1530–1542. https://doi.org/10.1007/s10646-011-0719-9
Riva-Murray K, Bradley PM, Chasar LC, Button DT, Brigham ME, Scudder Eikenberry BC, Lutz MA (2013a) Influence of dietary carbon on mercury bioaccumulation in streams of the Adirondack Mountains of New York and the Coastal Plain of South Carolina, USA. Ecotoxicology 22:60–71. https://doi.org/10.1007/s10646-012-1003-3
Riva-Murray K, Bradley PM, Scudder Eikenberry BC, Knightes CD, Journey CA, Brigham ME, Button DT (2013b) Optimizing stream water mercury sampling for calculation of fish bioaccumulation factors. Environ Sci Technol 47:5904–5912. https://doi.org/10.1021/es303758e
Rolfhus KR, Wiener JG, Haro RJ, Sandheinrich MB, Bailey SW, Seitz BR (2015) Mercury in streams at Grand Portage National Monument (Minnesota, USA): assessment of ecosystem sensitivity and ecological risk. Sci Total Environ 514:192–201. https://doi.org/10.1016/j.scitotenv.2015.01.079
Sandheinrich MB, Wiener JG (2011) Methylmercury in freshwater fish—Recent advances in assessing toxicity of environmentally relevant exposures. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota: interpreting tissue concentrations. 2nd ed.CRC/Taylor and Francis, Boca Raton, FL, pp. 169–190. https://doi.org/10.1201/b10598-6
Scudder Eikenberry BC, Riva-Murray K, Smith MJ, Bradley PM, Button DT, Clark JM, Burns DA, Journey CA (2011) Environmental settings of streams sampled for mercury in New York and South Carolina, 2005–09. Reston (VA): U.S. Geological Survey Open-File Report No.: 2011–1318. https://doi.org/10.3133/ofr20111318
Simonin HA, Colquhoun JR, Paul EA, Symula J, Dean HJ (2005) Have Adirondack stream fish populations changed in response to decreases in sulfate deposition? Trans Am Fish Soc 134:338–345. https://doi.org/10.1577/T03-138.1x.2007.12.032
Simonin HA, Loukmas JJ, Skinner LC, Roy KM (2008) Lake variability: key factors controlling mercury concentrations in New York State fish. Environ Pollut 154:107–115. https://doi.org/10.1016/j.envpol
Speir SL, Chumchal MM, Drenner RW, Cocke WG, Kewis ME, Whitt HJ (2014) Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environ Toxicol Chem 33:2506–2509. https://doi.org/10.1002/etc.2700
Thera JC, Kidd KA, Bertolo RF, O’Driscoll NJ (2019) Tissue content of thiol-containing amino acids predicts methylmercury in aquatic invertebrates. Sci Tot Environ 688:567–573. https://doi.org/10.1016/j.scitotenv.2019.06.225
Thorp JH, Covich AP (1991) Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego
Tremblay A, Lucotte M (1997) Accumulation of total mercury and methyl mercury in insect larvae of hydroelectric reservoirs. Can J Fish Aquat Sci 54:832–841
Tremblay A, Lucotte M, Meili M, Cloutier L, Pichet P (1996) Total mercury and methylmercury contents of insects from boreal lakes: ecological, spatial, and temporal patterns. Water Qual Res J Can 31:851–873
U.S. EPA (2012) Selected analytical methods for environmental remediation and recovery (SAM). U.S. Environmental Protection Agency Report No.: EPA/600/R-12/555, Washington, D.C.
Wiener JG, Bodaly RA, Brown SS, Lucotte M, Newman MC, Porcella DB, Reash RJ, Swain EB (2007) Monitoring and evaluating trends in methylmercury accumulation in aquatic biota. In: Harris R, Krabbenhoft DP, Mason RF, Murray MW, Reash RJ, Saltman T (eds) Ecosystem responses to mercury contamination: indicators of change. Society of Environmental Toxicology and Chemistry, Pencsacola, pp. 87–122 https://doi.org/10.1201/9780849388897.ch4
Wiener JG, Sandheinrich MB, Bhavsar SP, Bohr JR, Evers DC, Monson BA, Schrank CS (2012) Toxicological significance of mercury in yellow perch in the Laurentian great Lakes region. Environ Pollut 161:350–357. https://doi.org/10.1016/j.envpol.2011.09.025
Yu X, Driscoll CT, Huang J, Holden TM, Blackwell BD (2013) Modeling and mapping of atmospheric mercury deposition in Adirondack Park, New York. PLoS ONE 8:e59322. https://doi.org/10.1371/journal.pone.0059322
Yu X, Driscoll CT, Montesdeoca M, Evers D, Duron M, Williams K, Schoch N, Kamman NC (2011) Spatial patterns of mercury in biota of Adirondack, New York lakes. Ecotoxicology 20:1543–1554. https://doi.org/10.1007/s10646-011-0717-y
Zhang Y, Jacob DJ, Horowitz HM, Chen L, Amos HM, Krabbenhoft DP, Slemr F, Louis VLS, Sunderland EM (2016) Observed decrease in atmospheric mercury explained by global decline in anthropogenic emissions. Proc Natl Acad Sci USA 113:526–531. https://doi.org/10.1073/pnas.1516312113
Acknowledgements
This work was supported by the New York State Energy Research and Development Authority, with cooperative matching funds from the U.S. Geological Survey (USGS). The USGS National Water Quality Assessment Program funded the original collection and laboratory analysis of insect samples. The authors appreciate the advice and insight provided by John De Wild (USGS, Mercury Research Laboratory) and Daniel Button (USGS, Ohio Water Science Center); suggestions on an earlier draft by Barbara Eikenberry (USGS), (Wisconsin Water Science Center) and several anonymous reviewers are greatly appreciated. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Funding
This study was funded by New York State Energy Research and Development Authority (NYSERDA) under Agreement #40515.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Riva-Murray, K., Bradley, P.M. & Brigham, M.E. Methylmercury—total mercury ratios in predator and primary consumer insects from Adirondack streams (New York, USA). Ecotoxicology 29, 1644–1658 (2020). https://doi.org/10.1007/s10646-020-02191-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02191-7