Abstract
Deltamethrin pesticide and copper have intensively been used in agriculture and industrial activities and can finally reach the marine ecosystem at high concentrations affecting the health of organisms. In this study, we assessed under laboratory conditions the toxic interactions between deltamethrin and copper and their effects on the fertility rate, cell mitotic division rate, and embryo developmental events of the sea urchin (Paracentrotus lividus). The exposure of sperm to increasing concentrations of deltamethrin (6.10−5 and 6.10−2 μg/L) and copper (50 and 100 μg/L) or to their mixture (6.10−5 μg/L of deltamethrin and 50 μg/L of CuSO4) caused a significant alteration on the fertilizing capability of spermatozoids. Concentration-dependent toxic effects on the early cleavage in P. lividus were observed in groups treated with copper, deltamethrin, and their mixture. The kinetics of early divisions was accelerated and the average size of pluteus larvae was decreased under pollutant treatments. Several developmental anomalies were identified in pluteus, including crossed skeletal tips at the hood apex, joined or atrophied arms, and alteration of general larva shape. In conclusion, the sea urchin represents a suitable and sensitive model for testing the toxicity and the effects of deltamethrin pesticide and copper in sea water. In addition, the sensitivity of various end points to studied contaminants, proved their utility in the infield biomonitoring studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intensive and the chronic use of various pesticides by farmers results in the accumulation of residues in agricultural fields and aquatic environments (Masiá et al. 2013; Weston et al. 2013). One of the most frequently used pesticides is deltamethrin, an insecticide belonging to the classes of pyrethroid compounds, employed against agricultural pests. As a result of year-long application, significant amounts of deltamethrin have been detected in water, sediments, plants, and animals (Pawlisz et al. 1998; Laskowski 2002).
The environmental contamination caused by deltamethrin has led to ecotoxicological problems in non-targeted aquatic organisms involving severe immunotoxicological and metabolic effects (Guardiola et al. 2014), organic morphological abnormalities (Toumi et al. 2013), neurophysiological disturbances (Wolansky and Harrill 2008), and genetic alteration (Ismail and Mohamed 2012).
Copper (Cu) is one of the most important essential metals to the metabolic process, being a cofactor of several cellular enzymes; it can be very toxic to sea urchin at high concentrations (Xu et al. 2011). Sublethal exposure to heavy metals (e.g., Cu, Zn, and Cd) in the laboratory has led to poor embryonic and larval development in the sea urchin (Durkina 1994; Xu et al. 2011). In the natural environment, adult sea urchins are normally exposed to the mixture of pollutants for a prolonged period. The quality and subsequent fertilization ability of sperm and eggs produced by adult sea urchins exposed to mixture of known pollutants may be very different. However, only few studies have been interested in the assessment of the mixture effects of deltamethrin pesticide and copper on aquatic organisms and particularly on sea urchin. The invertebrate sea urchins were frequently and routinely used in aquatic toxicology bioassays (McGibbon and Moldan 1986; Buznikov et al. 2007; Saco−Alvarez et al. 2010; Rial et al. 2013).
As an echinoderm of the class Echinoidea, Paracentrotus lividus is widely distributed in the Mediterranean Sea and particularly, on the Tunisian coastal area. Fundamental data on reproduction and developmental events were deeply investigated. The size at sexual maturity was generally set between 20 and 30 mm according to regions and food supply. Two spawning periods were recorded: in spring (from April to June) and in autumn (from September to November) (Ouréns et al. 2011). This specie (P. lividus) has been used as a particular ecological indicator of marine pollution because its first stages of embryonic development are a very sensitive model to a variety of pollutants (Pesando et al. 2003). Up to now, aquatic toxicology research using sea urchin has mainly focused on investigating individual heavy metal toxicity on fertilization, embryo-larval development, survival, and growth (Xu et al. 2011). Few data are available on the effects of contamination by joint-action of metals and pesticide at the cellular level. Hence, in this work, this typical marine invertebrate, P. lividus, was selected to assess the joint toxicity of copper and deltamethrin under controlled laboratory conditions. The results generated here can serve as a reference for future monitoring studies in this industrial/urban zone located along the east coast of Tunisia, as well as for biomarker studies in other regions of the Mediterranean Sea.
Materials and methods
Sperm/fertilization assays
Sexually mature specimens of sea urchins (P. lividus), were collected from Monastir littoral (Center of Tunisia), then were immediately transferred to the laboratory in a cooler where they were acclimated in aquaria with clean aerated running seawater until the initiation of the tests which were carried at the same day. The sea urchin embryo test was performed in accordance with the method of McGibbon and Moldan (1986) with brief modifications. In order to induce spawning, 1 mL of 0.5 M KCl was injected through the peristomial membrane of individual sea urchins. Males and females were then placed aboral surface uppermost in 100-mL graduated cylinder containing clean natural seawater which was previously filtered through 0.22-μM filters. The seawater physico-chemical parameters were 8 ± 1 mg/L dissolved oxygen, salinity of 35 ± 0.5 ppt, pH of 7.95 ± 0.1, and temperature of 19 ± 1 °C. Sperm suspension of each individual sea urchin was used for subsequent contamination experimentation. Two groups of sperm suspensions were each individually exposed to 6.10−2 and 6.10−5 μg/L of deltamethrin pesticide. The third and fourth groups were exposed to 50 and 100 μg/L of CuSO4. The fifth group was exposed to a mixture of 6.10−5 μg/L of deltamethrin and 50 μg/L of CuSO4. The sixth group was not contaminated and considered as control. The selection of deltamethrin pesticide concentrations was based on preliminary tests established with a wide range of concentrations and we noted that from the concentration of 6.10−2 μg/L, the embryological event was blocked at the beginning of the segmentation. The concentrations of Cu were chosen based on the work of Fernandez and Beiras (2001). The exposure of sperm suspension was maintained for 30 min in contact with contaminant. The contaminated sperm was then added to the ovocyte suspension in order to test the fertilization rate. After 30 min, 1-mL samples of each suspension were placed in Petri dishes fixed with a few drops of 40 % formaldehyde and stored in the refrigerator overnight if necessary. One hundred eggs were examined under optical microscope and the percentage of successful fertilization was determined, in each suspension sample, as the proportion of eggs with a fertilization membrane.
Cleavage kinetics assay
To assess the toxic effect of copper and deltamethrin pesticide or their mixture on the kinetics of the early cleavage of the fertilized eggs, 1-mL samples were placed into Petri dishes every 10 min for 4 h and fixed by some drops of 40 % formaldehyde. Dishes might be put in the refrigerator if required. The times of the first appearance of egg in stages 2, 4, 8, 16, 32, and 64 cells were noted for each group.
Plutei quality assay
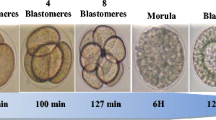
After 72 h, 1-mL sample of each suspension groups were transferred in petri dishes and fixed with some drops of 40 % formaldehyde. One hundred larvae were observed and analyzed under a light microscope and scored as normal and abnormal plutei. In addition, the lengths of larva spicules: from the apex hood to the end of the anal arm (Fig. 1), were measured using an ocular micrometer under light microscope.
Statistical analysis
The results were expressed as means ± SD. SPSS software (version 20.0) was used for statistical analysis. The data were first tested for normality and homogeneity of variance to meet statistical demands. Data from different groups were compared by a one-way analysis of variance (ANOVA) and statistically different sites were identified by Gabriel’s test. All differences were considered significant at p < 0.05. Different letters a, b, c, and d indicated significant differences between groups.
Results
Contamination effects on fertilization rates
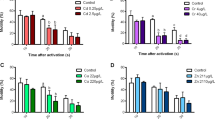
Under normal conditions, the fertilization rate is about 95 %. The treatment of sperm with increasing concentrations of deltamethrin significantly diminished the fertilization rate. The diminution levels were estimated at 8.24 ± 1.36 %, p < 0.05 and 12.94 ± 3.02 %, p < 0.05 in [D1]− and [D2]− treated group, respectively. A similar effect was observed in sperm treated with the mixture of copper and deltamethrin pesticide [D1+Cu1] and the fertilization rate (Fig. 2).
Effects of increasing concentrations of copper, deltamethrin pesticide, and of their mixture on the fertilization rates of the sea urchin (Paracentrotus lividus). [Cu1] = 50 μg/L of CuSO4; [Cu2] = 100 μg/L of CuSO4; [D1] = 6.10−5 μg/L of deltamethrin; [D2] = 6.10−2 μg/L of deltamethrin; [Cu1+D1] = mixture of 6.10−5 μg/L of deltamethrin; and 50 μg/L of CuSO4. Different letters a, b, c, and d indicate significant differences between groups at p < 0.05
Kinetics of early divisions
Under normal conditions, stages 2 and 4 blastomeres are reached, respectively, at 90 and 100 min after the fertilization. Then, divisions continue approximately every 30 min until stage 64 blastomeres. At low concentrations, contaminants can affect the kinetic of the divisions. Indeed, both contaminants at different concentrations, manage to significantly accelerate the first mitotic divisions. This acceleration was accentuated in the presence of a mixture of Cu and deltamethrin (Fig. 3). We also showed that the exposure to the low concentrations of Cu or deltamethrin causes asymmetric and/or asynchronous cell divisions and partial divisions of the cytoplasm were reported (Fig. 4).
Acute effects of copper, deltamethrin, and their mixture on the kinetics of the mitotic cell divisions to reach the different stages of 2, 4, 8, 16, 32, and 64 blastomeres. [Cu1] = 50 μg/L of CuSO4; [Cu2] = 100 μg/L of CuSO4; [D1] = 6.10−5 μg/L of deltamethrin; [D2] = 6.10−2 μg/L of deltamethrin; [Cu1+D1] = mixture of 6.10−5 μg/L of deltamethrin and 50 μg/L of CuSO4. Different letters a, b, c, and d indicate significant differences between groups at p < 0.05
Effects of copper, deltamethrin and their mixture on the first egg segmentation stages of the sea urchin (Paracentrotus lividus). a, b, c control structures at stage 2, 4, and 8 blastomeres, respectively; (a’ asymmetric divisions, b’ asynchronous divisions, c’ partial division of the cytoplasm) of contaminated structures at stage 2, 4, and 8 blastomeres, respectively. (magnification: 10 × 10)
Contamination effects on embryonic development
The control plutei sampled at 72 h presented a low percentage of abnormalities (10 %) among which we reported: general alteration shape, delayed specimens (those that did not reach the pluteus stage at 72 h), and even anomalies of the skeletal rods. More frequent and important abnormalities were observed by adding the two separate contaminants or combined (Fig. 5). The exposure to the increasing concentration of copper: 50 μg/L (Cu1) and 100 μg/L (Cu2) showed a strong negative effect on the morphological abnormalities of larvae estimated at 55.71 ± 5.15 % (p < 0.001) and 50.00 ± 4.545 % (p < 0.001), respectively, and about 89.09 ± 1.71 % (p < 0.001) and 78.49 ± 1.53 % (p < 0.001) in [D1] and [D1+Cu1] treated groups.
Effects of increasing concentrations of copper, deltamethrin pesticide, and of their mixture on the percentage of abnormal plutei of sea urchin. [Cu1] = 50 μg/L of CuSO4; [Cu2] = 100 μg/L of CuSO4; [D1] = 6.10−5 μg/L of deltamethrin; [D2] = 6.10−2 μg/L of deltamethrin; [Cu1+D1] = mixture of 6.10−5 μg/L of deltamethrin and 50 μg/L of CuSO4. Different letters a, b, c, and d indicate significant differences between groups at p < 0.05
Among these abnormalities, we cited crossed skeletal tips at the hood apex, joined or atrophied arms, and alteration of general larva shape (Fig. 6). We also note that for deltamethrin concentration 6.10−2 μg/L, the development was blocked at the blastula stage.
Main pluteus larva abnormalities observed after exposure to increasing concentrations of copper, deltamethrin pesticide, and to their mixture (a Structure of typical pluteus larva under control conditions of laboratory; b pluteus with joined arms; c pluteus with crossed spicules at the hood apex; and d pluteus with general shape alteration) under contaminated conditions
Under control condition, the average size of larva spicules was 386.05 ± 25.48 μm, while it decreased significantly in all treated groups. For the mixture [D1+Cu1], we noted the maximum of larva spicules length reduction; it was estimated at 326.05 ± 17.30 μm; (p < 0.05) (Fig. 7).
Effects of copper, deltamethrin, and their mixture on the spicule length of the pluteus. [Cu1] = 50 μg/L of CuSO4; [Cu2] = 100 μg/L of CuSO4; [D1] = 6.10−5 μg/L of deltamethrin; [D2] = 6.10−2 μg/L of deltamethrin; [Cu1+D1] = mixture of 6.10−5 μg/L of deltamethrin and 50 μg/L of CuSO4. Different letters a, b, c, and d indicate significant differences between groups at p < 0.05
Discussion
In the marine ecosystem, organisms are subject to a variety of contaminant molecules that rarely occur independently (Monserrat et al. 2007; Jebali et al. 2013; 2014). Among these, interactions between pesticides and metals have received increasing attention over recent years. The assessment of biological effects of complex mixtures of pollutants by the use of biomarkers and bioassay tools has been recommended by several authors (Woodworth et al. 1999; Jebali et al. 2012). The characterization of these biological responses to chemical exposure under controlled conditions is necessary in biomonitoring studies in order to check the biological marker response-chemical concentration relationship. Thus, in this study, we assessed the toxic interactions between deltamethrin and copper in the early stages of the development of the sea urchin, P. lividus, from fertilization to the pluteus larva. Indeed, a decrease in the fertilization rate was observed, the divisions of the segmentation appeared to be accelerated, and numerous larvae abnormalities were detected.
As clearly indicated in our results, in contrast to copper exposure, deltamethrin and the mixture of deltamethrin and copper [Cu1+D1] had serious effects on the fertilization rate of sea urchin. The results presented here strongly suggested that deltamethrin pesticide was more toxic than copper at the tested concentrations while no additive effects on the fertilization rate were observed in the group treated with mixture of [Cu1+D1]. The toxic effect of deltamethrin is complex and depends on several factors including its non-polar nature, its capability to act on several intracellular targets, and the deficiency in the urchin cell enzyme system to hydrolyze pyrethroids. Copper is an essential trace element found in small amounts in a variety of cells and tissues. In this study, the increasing concentrations of copper had no significant negative effects on the fertilization rate of sea urchin (P. lividus). In opposition to the work of Lee and Xu (1984) which revealed that the pretreatment of semen of P. lividus with Cu did not affect fertilization and the results of Gambardella et al. (2013) which showed that exposing sea urchin sperm to various engineered nanoparticles did not affect their fertilizing ability, our results are in agreement with all the other works that confirmed that exposing sea urchin sperm to different chemical pollutants enormously reduced the rate of fertilization. A study carried by Warnau et al. (1996) showed that the exposure of P. lividus sperm to Hg, Cu, Cd, and Ag caused a significant decrease in fertilization rate without affecting the offspring quality. Similar results were found by Pesando et al. (2004) when exposing the sperm of the same species to other pesticides such as lindane and dieldrin.
Concerning the ovocytes fertilized by sperm treated with copper, deltamethrin, and with the mixture, the first mitotic divisions of eggs were altered. Thus, the kinetics of the mitotic divisions of cell to reach the different stages of 2, 4, 8, 16, 32, and 64 blastomeres were shorter than those of controls. These interesting results revealed that the acceleration in the rates of mitotic divisions was related to the high quantitative and qualitative embryo anomalies. Moreover, the effects induced by the low concentrations of copper, deltamethrin, or to their mixture to contaminated egg mitotic divisions, indicated that animals displayed high sensitivity to these compounds. Pesando et al. (2003, 2004), noted that the exposure of the unfertilized eggs to different pesticides caused a significant delay of the first divisions but did not indicate the time of the first appearance of each stage.
In our assay, exposing P. lividus sperm to increasing concentrations of copper, and/or deltamethrin caused a significant increase in the embryo anomalies. The most common anomalies in the plutei stages compared to those of controls were fusion or atrophy of anal and oral arms, crossed skeletal tips, decrease of the larva spicule size, and alteration of the general larva shape. These anomalies observed in pluteus larvae were similar to those identified by Pesando et al. (2003, 2004), Carballeira et al. (2012a), b), and Gambardella et al. (2013) in sea urchin larvae exposed to different chemical pollutants. Skeletal formation is essential in the morphogenesis of living organisms and any decrease or perturbation in this event will affect larval survival and population continuity. In this work, we showed that the addition of copper from 50 μg/L and deltamethrin from 6.10−5 μg/L or their mixture led to a significant decrease of the larva spicule lengths. These results are in agreement with those of Pétinay et al. (2009) that postulate that copper led to significant decrease in growth from 30 μg/L with which the larva spicules reached 464 versus 495 μm with the reconstituted seawater without copper addition. The sea urchin embryo test (SET) has been also, used as a rapid, sensitive, and cost−effective biological tool for the evaluation of mixture of pollutants in marine sediment and seawater quality (Bellas and Paredes 2011; Khosrovyan et al. 2013). Moreover, the sea urchin P. lividus during the first stages of development is a very sensitive model to a variety of effluents in coastal areas including the effluents from land-based aquaculture farms (Carballeira et al. 2012a, b) and industrial and urban agglomeration (Burgess et al. 1995; Meriç et al. 2005).
Conclusion
In the present study, the toxicity of increasing concentrations of copper and deltamethrin pesticide on the fertility rate, cell mitotic division rate, and embryo developmental events of the sea urchin P. lividus was determined. The exposure of sperm to copper, deltamethrin, or to their mixture causes a significant decrease in the fertilization rate, being more sensitive to deltamethrin exposure than to copper. Ovocytes fertilized by contaminated sperm show a high increase in mitotic division and asymmetric and or asynchronous cell divisions. In addition, the embryo anomaly frequency and particularly, the larva spicule size was highly increased by the exposure to mixture of deltamethrin and copper. Therefore, the joint effects of trace metals and pesticide should be taken into account in the risk assessment of multiple pollutions in the marine environment. The measurements of the fertilization rate, kinetics of the egg mitotic divisions, embryo anomaly frequencies, and larva spicule sizes are a very sensitive and useful bioassay in the field biomonitoring of multiple pollutions of marine ecosystem.
References
Bellas, J., & Paredes, E. (2011). Advances in the cryopreservation of sea-urchin embryos: potential application in marine water quality assessment. Cryobiology, 62(3), 174–180.
Burgess, R. M., Ho, K. T., Tagliabue, M. D., Kuhn, A., Comeleo, R., Comeleo, P., Modica, G., & Morrison, G. E. (1995). Toxicity characterization of an industrial and a municipal effluent discharging to the marine environment. Marine Pollution Bulletin, 30(8), 524–535.
Buznikov, G. A., Nikitina, L. A., Rakic, L. M., Milosevic, I., Bezuglov, V. V., Lauder, J. M., & Slotkin, T. A. (2007). The sea urchin embryo, an invertebrate model for mammalian developmental neurotoxicity, reveals multiple neurotransmitter mechanisms for effects of chlorpyrifos: therapeutic interventions and a comparison with the monoamine depleter, reserpine. Brain Research Bulletin, 74, 221–231.
Carballeira, C., De Orte, M. R., Viana, I. G., DelValls, T. A., & Carballeira, A. (2012a). Assessing the toxicity of chemical compounds associated with land-based marine fish farms: the sea urchin embryo bioassay with Paracentrotus lividus and Arbacia lixula. Arch Archives of Environmental Contamination and Toxicology, 63, 249–261.
Carballeira, C., Ramos-Gómez, J., Martín-Díaz, L., & DelValls, T. A. (2012b). Identification of specific malformations of sea urchin larvae for toxicity assessment: application to marine pisciculture effluents. Marine Environmental Research, 77, 12–22.
Durkina, V. B. (1994). Development of the progeny of sea urchin Strongylocentrotus intermedius exposed to copper and zinc. Biological Morya Marine Biology, 20, 305–310.
Fernandez, N., & Beiras, R., (2001). Combined toxicity of dissolved mercury with copper, lead and cadmium on embryogenesis and early larval growth of the Paracentrotus lividus Sea-Urchin. Ecotoxicology, 10: 263–271.
Gambardella, C., Aluigia, M. G., Ferrandoa, S., Gallusa, L., Ramoinoa, P., Gattib, A. M., Rottignia, M., & Falugi, C. (2013). Developmental abnormalities and changes in cholinesterase activity in sea urchin embryos and larvae from sperm exposed to engineered nanoparticles. Aquatic Toxicology, 130–131, 77–85.
Guardiola, F. A., Gónzalez-Párraga, P., Meseguer, J., Cuesta, A., & Esteban, M. A. (2014). Modulatory effects of deltamethrin-exposure on the immune status, metabolism and oxidative stress in gilthead seabream (Sparus aurata L.). Fish & Shellfish Immunology, 36, 120–129.
Ismail, M. F., & Mohamed, H. M. (2012). Deltamethrin-induced genotoxicity and testicular injury in rats: comparison with biopesticide. Food and Chemical Toxicology, 50, 3421–3425.
Jebali, J., Banni, M., & Boussetta, H. (2012). Biochemical biomarkers in aquatic ecotoxicology: fundamental mechanisms, application and perspectives. In J. A. Daniel (Ed.), Advance in environmental research (Vol. 23, pp. 143–168). New York: Nova Science Publisher. 323p.
Jebali, J., Chicano-Gálvez, E., Banni, M., Guerbej, H., Boussetta, H., López-Barea, J., & Alhama, J. (2013). Biochemical responses in seabream (Sparus aurata) caged in-field or exposed to benzo(a)pyrene and paraquat. Characterization of glutathione S-transferases. Ecotoxicology and Environmental Safety, 88, 169–177.
Jebali, J., Chicano-Gálvez, E., Fernandez-Cinal, R., Banni, M., Chouba, L., Boussetta, H., López-Barea, J., & Alhama, J. (2014). Proteomic analysis in caged Mediterranean crab (Carcinus maenas) and chemical contaminant exposure in Téboulba Harbour, Tunisia. Ecotoxicology and Environmental Safety, 100, 15–26.
Khosrovyan, A., Rodríguez-Romero, A., Salamanca, M. J., Del Valls, T. A., Riba, I., & Serrano, F. (2013). Comparative performances of eggs and embryos of sea urchin (Paracentrotus lividus) in toxicity bioassays used for assessment of marine sediment quality. Marine Pollution Bulletin, 70(1–2), 204–209.
Laskowski, D. A. (2002). Physical and chemical properties of pyrethroids. Reviews of Environmental Contamination and Toxicology, 174, 49–170.
Lee, H. H., & Xu, C. H. (1984). Effects of metals on sea urchin development: a rapid bioassay. Marine Pollution Bulletin, 15(1), 18–21.
Masiá, A., Campo, J., Vázquez-Roig, P., Blasco, C., & Picó, Y. (2013). Screening of currently used pesticides in water, sediments and biota of the Guadalquivir River Basin (Spain). Journal of Hazardous Materials, 263, 95–104.
McGibbon, S., & Moldan, A. G. S. (1986). Routine toxicity testing of toxicants using a sea urchin gamete bioassay. Marine Pollution Bulletin, 17(2), 68–72.
Meriç, S., De Nicola, E., Iaccarino, M., Gallo, M., Di Gennaro, A., Morrone, G., Warnau, M., Belgiorna, V., & Pagano, G. (2005). Toxicity of leather tanning wastewater effluents in sea urchin early development and in marine microalgae. Chemosphere, 61(2), 208–217.
Monserrat, J. M., Martínez, P. E., Geracitano, L., Amado, L. L., Gaspar Martins, C. M., Leães Pinho, G. L., Chaves, I. S., Ferreira-Cravo, M., Ventura-Lima, J., & Bianchini, A. (2007). Pollution biomarkers in estuarine animals: critical review and new perspectives. Comparative Biochemistry and Physiology, Part C, 146, 221–234.
Ouréns, R., Fernández, L., & Freire, J. (2011). Geographic, population, and seasonal patterns in the reproductive parameters of the sea urchin, Paracentrotus lividus. Marine Biology, 158(4), 793–804.
Pawlisz, J., Busnarda, J., McLauchlin, A., Caux, P. Y., & Kent, R. A. (1998). Canadian water quality guidelines for deltamethrin. Environmental Toxicology and Water Quality, 13, 175–210.
Pesando, D., Huitorelb, P., Dolcinia, V., Angelinic, C., Guidettid, P., & Falugic, C. (2003). Biological targets of neurotoxic pesticides analysed by alteration of developmental events in the Mediterranean sea urchin, Paracentrotus lividus. Marine Environmental Research, 55, 39–57.
Pesando, D., Robert, S., Huitorel, P., Gutknecht, E., Pereira, L., Girard, J. P., & Ciapa, B. (2004). Effects of methoxychlor, dieldrin and lindane on sea urchin fertilization and early development. Aquatic Toxicology, 66, 225–239.
Pétinay, S., Chataigner, C., & Basuyaux, O. (2009). Standardisation du développement larvaire de l’oursin, Paracentrotus lividus, pour l’évaluation de la qualité d’une eau de mer. Comptes Rendus Biologies, 332, 1104–1114.
Rial, D., Vázquez, J. A., Menduiña, A., García, A. M., González, M. P., Mirón, J., & Murado, M. A. (2013). Toxicity of binary mixtures of oil fractions to sea urchin embryos. Journal of Hazardous Materials, 263, 431–440.
Saco-Alvarez, L., Duran, I., Lorenzo, J. I., & Beiras, R. (2010). Methodological basis for the optimization of a marine sea-urchin embryo test (SET) for the ecological assessment of coastal water quality. Ecotoxicology and Environmental Safety, 73, 491–499.
Toumi, H., Boumaiza, M., Millet, M., Radetski, C. M., Felten, V., Fouque, C., & Férard, J. F. (2013). Effects of deltamethrin (pyrethroid insecticide) on growth, reproduction, embryonic development and sex differentiation in two strains of Daphnia magna (Crustacea, Cladocera). Science of the Total Environment, 458–460, 47–53.
Warnau, M., Iaccarino, M., De Biase, A., & Temara, A. (1996). Spermiotoxicity and embryotoxicity of heavy metals in the echinoid Paracentrotus lividus. Environmental Toxicology and Chemistry, 15(11), 1931–1936.
Weston, D. P., Ding, Y., Zhang, M., & Lydy, M. J. (2013). Identifying the cause of sediment toxicity in agricultural sediments: the role of pyrethroids and nine seldom-measured hydrophobic pesticides. Chemosphere, 90, 958–964.
Wolansky, M. J., & Harrill, J. A. (2008). Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicology and Teratology, 30, 55–78.
Woodworth, J. G., King, C., Miskiewicz, A. G., & Laginestrae, S. J. (1999). Assessment of the comparative toxicity of sewage effluent from 10 sewage treatment plants in the area of Sydney, Australia using an amphipod and two sea urchin bioassays. Marine Pollution Bulletin, 39, 174–178.
Xu, X., Li, Y., Wang, Y., & Wang, Y. (2011). Assessment of toxic interactions of heavy metals in multi-component mixtures using sea urchin embryo-larval bioassay. Toxicology in Vitro, 25, 294–300.
Acknowledgments
This work was supported by a fund from the Ministry of Scientific Research and Technology, University of Monastir, Tunisia (Research Laboratory “Bioresources: Integrative Biology & Valorisation”, High Institute of Biotechnology of Monastir, Tunisia).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gharred, T., Ezzine, I.K., Naija, A. et al. Assessment of toxic interactions between deltamethrin and copper on the fertility and developmental events in the Mediterranean sea urchin, Paracentrotus lividus . Environ Monit Assess 187, 193 (2015). https://doi.org/10.1007/s10661-015-4407-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4407-8