Abstract
To clarify the association between regional variations in urolithiasis incidence and nutrition intake, we evaluated associated data from Japanese national surveys. The incidence of urolithiasis in 12 regions of Japan was calculated from 2005 patient data obtained from 430 hospitals (n = 92,797). Nutrition intake data were obtained from the National Health and Nutrition Survey. We examined the association between urolithiasis incidence and average intake of various types of food or nutrients by region. Continuing surveys in Japan reveal fixed variations in urolithiasis incidence among geographic regions. The national average of patients with urolithiasis was estimated as 203.1 per 100,000 citizens. Regarding food, intake of fruit correlated negatively with the incidence of urolithiasis (r = −0.721, p = 0.008), while intake of eggs (r = 0.537, p = 0.072) and sugar (r = 0.475, p = 0.119) tended to positively correlate with incidence. Regarding nutrients, intake of potassium (r = −0.500, p = 0.098), vitamin K (r = −0.562, p = 0.057), and pantothenic acid (r = −0.560, p = 0.058) tended to negatively correlate with incidence. The incidence of urolithiasis is higher in geographic areas with populations having low fruit and high sugar intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is a common nephrologic disorder whose prevalence is increasing, likely due to lifestyle factors, including diet and obesity. Because urolithiasis is a complex disease, an understanding of the epidemiology, particularly of the interactions among different factors, may help lead to measures that reduce the risk of stone formation. Diet plays an important role in the pathogenesis of urolithiasis, and changes in dietary habits have likely contributed to the substantial increases in urolithiasis observed over the past several decades [1–3]. Although previous studies focusing on the effect of dietary measures (e.g., low animal protein, low sodium, high fluid, high fiber, or low calcium diets) on risk factors for stone recurrence in idiopathic calcium urolithiasis have been conducted [4, 5], studies on the relationship between the intake of specific foods and incidence of urolithiasis based on large-scale epidemiological information are lacking.
National surveys in Japan conducted in 1955, 1965, 1975 [6], 1985 [7], 1995 [8, 9], and 2005 [10] have demonstrated changes in the profile and incidence of urolithiasis among the Japanese population. We conducted the sixth national survey of urolithiasis in cooperation with the Japanese Society on Urolithiasis Research and the Japanese Urological Association in 2005 to evaluate chronological trends of urolithiasis in Japan [10]. These surveys all revealed that the incidence of urolithiasis differs by region and that regions with high or low incidence are fixed. However, previous studies did not investigate the association between food intake and incidence of urolithiasis by region. In the present study, we evaluated urolithiasis incidence and nutrition data for each of 12 predefined geographic regions using information obtained from the aforementioned Japanese national surveys to clarify the association between regional variations in urolithiasis incidence and nutrition intake.
Methods
Urolithiasis incidence by each region
We calculated the incidence of urolithiasis for each of 12 regions (Hokkaido, Tohoku, North Kanto, South Kanto, Hokuriku, Tokai, North Kinki, South Kinki, Chugoku, Shikoku, North Kyushu, and South Kyushu, Fig. 1a) using data derived from the 2005 Japanese national survey [10]. The methods used in this study have been described previously [10]. Briefly, to estimate the total number of Japanese patients with urolithiasis, the national survey enrolled all 1,218 hospitals approved by the Japanese Urological Association in 2004. Among the 1,218 eligible hospitals, questionnaires were mailed in December 2004 to 702 institutions that had agreed to participate in the investigation. Final responses were obtained by June 2006 from 430 institutions (35.3 % of the 1,218 eligible hospitals). Data obtained from all patients diagnosed as having urolithiasis by urologists in the year 2005 were separately analyzed by each region (n = 92,797 patients at 430 hospitals). The mailed questionnaire asked the respondent hospitals to report on all patient visits during the year 2005 that resulted in a diagnosis of urolithiasis. The diagnostic criteria for urolithiasis consisted of radiographic or ultrasonographic confirmation or clinical diagnosis by urologists. Patients with only a history of stone passage (no stones in 2005) were excluded from the study to arrive at an estimate of incidence.
Hospitals were classified into seven strata according to the number of beds (1–99, 100–199, 200–299, 300–399, 400–499, 500–999, and over 1,000 inpatient beds), and the numbers of patients were separately estimated for each stratum of hospital. The total number of patients was estimated using the following formula [10]:
Incidence of urolithiasis (upper urinary tract stones) was then calculated for each of the 12 geographic regions. Our estimate of the annual incidence did not include patients with stones who visited only private physicians or hospitals that were not approved by the Japanese Urological Association; the 1965–1995 national surveys of urolithiasis in Japan used similar data [6–9].
In addition to the survey, we investigated calculus components of urolithiasis patients by each region (a total of 8,877 stones from 174 hospitals).
Nutrition intake by each region
To obtain nutritional data for each of the 12 regions, we used data from the National Health and Nutrition Survey, an examination of national health and nutrition conducted by the Japanese Ministry of Health, Labour and Welfare in 2002 to improve the health of Japanese citizens [11]. The 2002 survey, which obtained the cooperation of 11,491 people, was conducted by nutritionists and registered dietitians. Briefly, subjects of the survey were members of randomly sampled households. Researchers distributed a questionnaire on nutrition status to each household and explained the purpose of the survey and how to complete it. Subjects gauged the amounts of food consumed during the day using scales and filled out the questionnaire based on this information. The nutrition survey was conducted on one day in November 2002, and the data were separated by the 12 previously mentioned regions of residence.
We examined the average nutrition intake of various types of foods or nutrients by region. Food groups were classified according to the 2002 National Health and Nutrition Survey (animal food, vegetable food, potatoes, sugar, legumes, nuts and seeds, vegetables, fruits, mushrooms, algae, seafood, meat, eggs, milk, fats, confectionery, beverages, and seasoning) [11]. Nutrient intake was classified using the standard tables of food composition in Japan (total energy, protein, lipid, carbohydrate, salt, potassium, calcium, magnesium, phosphorus, iron, zinc, copper, vitamins A, C, D, E, K, B1, B2, B6, and B12, niacin, folic acid, pantothenic acid, cholesterol, and dietary fiber) [12].
Association between urolithiasis incidence and nutrition
We examined the association between urolithiasis incidence and nutrition intake data for each region. The association between stone composition and nutritional data was also examined. We then analyzed the association among the incidence rate of each region with the nutritional intake, including both food and nutrients. Regression lines and correlation coefficients were evaluated using SAS version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Urolithiasis incidence by each region
The coverage ratio of this survey was 36.7 % of inpatient beds among enrolled hospitals (185,042 beds in respondent hospitals/504,752 beds in enrolled hospitals). The estimated number of patients with urolithiasis, including both those with first and recurrent episodes in 2005, was 259,491. In 2005, the Japanese population was 127,767,994; thus, the national average of the number of patients with urolithiasis per 100,000 individuals was 203.1. The distribution of annual incidence per 100,000 individuals in each of the 12 regions as compared to the national average is shown in Table 1. The ratios compared with the total national average (203.1 per 100,000 population) are 112.5, 77.8, 54.6, 85.5, 122.1, 118.6, 106.2, 132.1, 123.7, 96.9, 93.9 and 163.9 % in Hokkaido, Tohoku, North Kanto, South Kanto, Hokuriku, Tokai, North Kinki, South Kinki, Chugoku, Shikoku, North Kyushu and South Kyushu, respectively. The association of incidences for men and women was correlated in all 12 regions (r = 0.942, p = 4.6 × 10−6). The incidence of urolithiasis was higher in South Kyushu, South Kinki, and Chugoku and lower in Tohoku, North Kanto, and South Kanto compared with the national average.
The national trend of urolithiasis incidence from 1975 to 2005 is shown in Fig. 1. Data for 2005 (Fig. 1d) were compared with data for 1975 (Fig. 1b) and 1985 (Fig. 1c), and the relative incidence was shown to be lower in Tohoku and Kanto but higher in Kinki and Chugoku for all reports. The relative incidence in Hokkaido in 1985 and that in Kyushu in 2005 was higher than in 1975.
Association between food type and incidence of urolithiasis
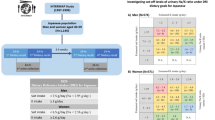
We analyzed the association between urolithiasis incidence and data from the 2002 National Health and Nutrition Survey for each region (as collected by the Japanese Ministry of Health, Labour and Welfare). The intakes of total, animal food, and vegetable food were not correlated by region with the incidence of urolithiasis (Table 2). Intake of fruit correlated negatively with the incidence of urolithiasis (r = −0.72, p = 0.008), while intake of eggs (r = 0.537, p = 0.072) and sugar (r = 0.475, p = 0.119) tended to be positively correlated with incidence. The intake of the other foods, including cereals, rice and wheat, vegetables, seafood (including fish), and meat, showed no correlation with the incidence of urolithiasis. The association of consumption of sugar, fruit, and eggs with the incidence of urolithiasis is shown by scatter diagrams in Fig. 2a–c.
Association analysis of nutritional data with the incidence of urolithiasis and stone composition. Association analysis of food type (a–c) and nutrient (d–f) with the incidence of urolithiasis. a Sugar, b fruits, c egg, d potassium, e vitamin K, and f pantothenic acid. Asterisk indicates the incidence of urolithiasis (per 100,000 population)
Association between nutrients and incidence of urolithiasis
Table 3 shows the association between nutrient intake and incidence of urolithiasis by region. Total energy was not related to the incidence of urolithiasis by region. Intake of protein, lipid, or carbohydrate, as well as proportions of fat, carbohydrate, and animal protein, to total energy input showed no correlation with incidence. Intake of potassium (r = −0.500, p = 0.098), vitamin K (r = 0.562, p = 0.057), and pantothenic acid (r = −0.560, p = 0.058) tended to be negatively correlated with incidence, whereas all nutrients showed no significant correlation. Association analyses between the nutrients (potassium, vitamin K, and pantothenic acid) and the incidence of urolithiasis are shown by scatter diagrams in Fig. 2d–f.
Association between stone components and nutritional data
The ratios of uric acid stone patients from an infrared analysis of the composition of 8,877 upper urinary tract stones is 4.5 % in total for Japan (5.4, 2.7, 3.5, 3.5, 3.8, 3.1, 5.7, 5.2, 4.1, 21.4, 4.2 and 5.1 % in Hokkaido, Tohoku, North Kanto, South Kanto, Hokuriku, Tokai, North Kinki, South Kinki, Chugoku, Shikoku, North Kyushu and South Kyusyu, respectively). The findings revealed that the relative content of uric acid in stones was higher in Hokkaido, Shikoku, and North Kinki, whereas that the relative content of calcium was higher in North Kanto, Hokuriku, Tokai, and North Kyushu. Using this composition data, we then examined the association between stone components and nutritional data. Intake of potatoes, vegetables, and confectionery correlated positively with the content of calcium in stones (r = 0.676, p = 0.016; r = 0.614, p = 0.034; r = 0.692, p = 0.013, respectively) and negatively with that of uric acid in stones (r = −0.637, p = 0.026; r = −0.628, p = 0.029; r = −0.715, p = 0.009, respectively).
Discussion
Geographic differences in urolithiasis occurrence have been previously reported [13–15]. Variations in the frequency of urolithiasis in geographically diverse regions could be caused by various factors such as lifestyle, nutrition, climate [16], or race, but definitive answers remain elusive. The major ethnicity in all areas of Japan is Japanese; therefore, ethnicity was not considered to influence the incidence of urolithiasis in the present study. An epidemiological investigation has shown that the incidence of urolithiasis over the past 40 years has escalated, in Japan, with the increasing adoption of a European-style diet [17]. Our study focused on regional differences in diet and examined the relationship between diet and the incidence of urolithiasis in 12 different regions. We also identified a wide variety of dietary factors that impacted urolithiasis incidence.
We investigated the total population, including both men and women. However, no distinction was made between men and women in the analyses because the incidences of stones in men and women in each region were correlated, and the nutrition survey was according to household, with no breakdown by gender.
Our results show that intake of fruit correlated negatively with the incidence of urolithiasis, while intake of eggs and sugar tended to be positively correlated with incidence (Table 2). However, the total amount of animal food and other foods consumed, such as cereals, vegetable, seafood, and meat, revealed no correlation with the incidence of urolithiasis. Intake of the nutrients (potassium, vitamin K, and pantothenic acid) tended to be negatively correlated with incidence (Table 3). In general, since 91.8 % of urolithiasis consists of calcium oxalate and/or calcium phosphate in Japan [10], the incidence of urolithiasis reflects the incidence of stones composed of calcium. An inverse relationship occurs between renal potassium and calcium excretion, which highlights the role of potassium-rich foods, such as vegetables and fruits, in the prevention of stone formation [11]. Dietary potassium restriction can increase urolithiasis. Hypokalemia stimulates tubular citrate reabsorption, thereby decreasing urinary excretion of citrate, an important inhibitor of calcium oxalate stone formation. Food potassium accompanies organic anions such as citrate, which leads to the metabolism of bicarbonates. Thus, consumption of potassium-containing foods such as fruits and vegetables represents an alkali load that increases the urinary excretion of citrate. The European Association of Urology Guidelines on Urolithiasis [18] recommends that fruit and vegetable intake should be encouraged because of the beneficial effects of fiber. In addition, the alkaline content of a vegetarian diet also gives rise to a desirable increase in urinary pH [19]. Indeed, our survey results support this recommendation: in the present study, vegetable consumption correlated negatively with uric acid ratio and positively with the proportion of calcium stones. For vitamin K, although it has been reported that reduced activity of renal vitamin K-dependent gamma-glutamyl carboxylase was observed in calcium oxalate calculi patients [20], the significance of vitamin K and pantothenic acid intake for prevention of urolithiasis remains unclear based on our findings.
The incidence of urolithiasis in Hokkaido, a colder, Northern region of Japan, has been shown to be consistently higher than the national average. Nutritionally, fruit and potassium (114.9 g/day and 2,303 mg/day, respectively) consumption in this region is lower than the national average (124.3 g/day and 2,389 mg/day). These nutritional factors may influence the incidence of urolithiasis. Egg and sugar consumption were also suggested to be associated with urolithiasis, confirming that a high protein diet is crucial for stone formation. The impact of carbohydrate on stone formation was previously shown in Japan, with the over-consumption of carbohydrates possibly leading to urolithiasis [21].
Epidemiological and metabolic studies have implicated the intake of various nutrients in the etiology of urolithiasis, including high consumption of animal protein [22, 23] and fructose [24]. Evidence exists that consumption of animal protein is closely related to the prevalence of urolithiasis in a given population. High consumption of animal protein has been consistently associated with an increase in urinary calcium excretion, with a reduction in both urinary pH and citrate excretion, and with metabolic changes that are considered risk factors for calcium oxalate stone formation [25–27].
This study also compared the incidence of urolithiasis with the regional average consumption of nutrients and found that while the variation in nutrient intake per person is large, the variation in average consumption between regions is small. For example, the average and standard deviation of protein, lipid, and carbohydrate intake in individuals was 72.2 ± 24.8, 54.4 ± 24.8, and 271.2 ± 88.4 g, respectively, whereas the average and standard deviation of protein, lipid, and carbohydrate intake among regions was 72.4 ± 2.2, 53.6 ± 2.6, and 273.2 ± 7.2 g, respectively. Although this study examined the relationship between diet and incidence by region, the variation of these factors in individual patients has not been sufficiently considered. Thus, we are unable to conclusively demonstrate the variation in nutrient consumption that actually causes urolithiasis in individuals.
This study does not consider changes in urolithiasis incidence over time or compare incidences between countries. National surveys in Japan have shown increases in the incidence of urolithiasis over time, and these increases are assumed to be caused by nutritional factors, diagnostic progress, or aging of the overall population [10]. The average national nutritional status in Japan has also changed from 1965 to 2002. However, nutritional differences among regions cannot explain the increase in urolithiasis. One of the limitations of the present survey is the inability to detect regional changes in nutritional status over time.
The composition of urine is influenced by dietary intake, and several dietary factors have been proposed for modifying the risk of urolithiasis. Taylor et al. [28] reported that consumption of a dietary approaches to stop hypertension (DASH)-style diet, which is high in fruits and vegetables, moderate in low-fat dairy products, and low in animal protein, was associated with a marked decrease in the risk of kidney stone incidence in three distinct cohorts. A DASH-style diet may reduce stone risk by increasing urinary volume and citrate content [29]. Furthermore, urolithiasis may be associated with a diagnosis of subclinical carotid atherosclerosis [30].
Food characteristics may also influence local variations in urolithiasis incidence. Since dietary factors play an important role in kidney stone formation, dietary modification can also influence the risk of stone recurrence. Thus, results of epidemiologic studies implicating a relationship among various nutrients and stone incidence can be applied in the clinical setting with the goal of reducing the likelihood of stone formation. Nevertheless, randomized trial data are lacking for most dietary interventions. Dietary interventions and subsequent evaluations of therapeutic efficacy should be based on results of multiple 24 h urine collection and a prospective study for stone recurrence; therefore, further research is necessary to make definitive recommendations for dietary modification.
Conclusions
The incidence of urolithiasis is higher in geographic areas where populations have a low fruit and high sugar intake. These data are useful as a basis for investigating risk and nutritional factors associated with urolithiasis.
References
Taylor EN, Curhan GC (2006) Diet and fluid prescription in stone disease. Kidney Int 70:835–839
Curhan GC, Willett WC, Rimm EB, Stampfer MJ (1993) A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 328:833–838
Robertson WG, Peacock M, Hodgkinson A (1979) Dietary changes and the incidence of urinary calculi in the UK between 1958 and 1976. J Chronic Dis 32:469–476
Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A (2002) Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 346:77–84
Siener R, Schade N, Nicolay C, von Unruh GE, Hesse A (2005) The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol 173:1601–1605
Yoshida O (1979) Epidemiology of urolithiasis in Japan. Nippon Hinyokika Gakkai Zasshi 70:975–983
Yoshida O, Okada Y (1990) Epidemiology of urolithiasis in Japan: a chronological and geographical study. Urol Int 45:104–111
Yoshida O, Terai A, Ohkawa T, Okada Y (1999) National trend of the incidence of urolithiasis in Japan from 1965 to 1995. Kidney Int 56:1899–1904
Terai A, Yoshida O (2001) Epidemiology of urolithiasis in Japan. In: Akimoto M, Higashihara E, Orikasa S (eds) Recent advances in endourology, vol 3. Springer, Tokyo, pp 23–36
Yasui T, Iguchi M, Suzuki S, Kohri K (2008) Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965 and 2005. Urology 71:209–213
Ministry of Health, Labour and Welfare of Japan (2002) The National Health and Nutrition Survey in Japan. Daiichi Shuppan Publishing, Tokyo, pp 121–130
Ministry of Education, Culture, Sports, Science and Technology (2005) Standard tables of food composition in Japan, 5th revised and enlarged ed. National Printing Bureau, Tokyo, pp 10–14
Curhan GC, Rimm EB, Willett WC, Stampfer MJ (1994) Regional variation in nephrolithiasis incidence and prevalence among United States men. J Urol 151:838–841
Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H (1994) Demographic and geographic variability of kidney stones in the United States. Kidney Int 46:893–899
Trinhieri A (2006) Epidemiological trends in urolithiasis: impact on our health care systems. Urol Res 34:151–156
Brikowski TH, Lotan Y, Pearle MS (2008) Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci USA 105:9841–9846
Iguchi M, Kataoka K, Kohri K, Yachiku S, Kurita T (1984) Nutritional risk factors in calcium stone disease in Japan. Urol Int 39:32–35
Türk C, Knoll T, Petrik A, Sarica K, Straub M, Seitz C (2012) Guidelines on urolithiasis. European Association of Urology, pp 82–84. http://www.uroweb.org/gls/pdf/20_Urolithiasis_LR%20March%2013%202012.pdf
Hess B, Mauron H, Ackermann D, Jaeger P (1999) Effects of a ‘common sense diet’ on urinary composition and supersaturation in patients with idiopathic calcium urolithiasis. Eur Urol 36:136–143
Chen J, Liu J, Zhang Y, Ye Z, Wang S (2003) Decreased renal vitamin K-dependent gamma-glutamyl carboxylase activity in calcium oxalate calculi patients. Chin Med J (Engl) 116:569–572
Iguchi M, Umekawa T, Takamura C, Sugihara I, Nakamura K, Kohri K, Kurita T (1993) Glucose metabolism in renal stone patients. Urol Int 51:185–190
Robertson WG, Peacock M (1982) The pattern of urinary stone disease in Leeds and in the United Kingdom in relation to animal protein intake during the period 1960–1980. Urol Int 37:394–399
Taylor EN, Stamper MJ, Curhan GC (2004) Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol 15:3225–3232
Taylor EN, Curhan GC (2008) Fructose consumption and the risk of kidney stones. Kidney Int 73:207–212
Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY (2002) Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 40:265–274
Breslau NA, Brinkley L, Hill KD, Pak CY (1998) Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 66:140–146
Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR (2010) The association of increasing body mass index and kidney stone disease. J Urol 183:571–575
Taylor EN, Fung TT, Curhan GC (2009) DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 20:2253–2259
Taylor EN, Stampfer MJ, Mount DB, Curhan GC (2010) DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol 5:2315–2322
Reiner AP, Kahn A, Eisner BH, Pletcher MJ, Sadetsky N, Williams OD, Polak JF, Jacobs DR Jr, Stoller ML (2011) Kidney stones and subclinical atherosclerosis in young adults: the CARDIA study. J Urol 185:920–925
Acknowledgments
This study was supported by the Japanese Society for Urolithiasis Research and the Japanese Urological Association.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yasui, T., Okada, A., Hamamoto, S. et al. The association between the incidence of urolithiasis and nutrition based on Japanese National Health and Nutrition Surveys. Urolithiasis 41, 217–224 (2013). https://doi.org/10.1007/s00240-013-0567-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-013-0567-6