Abstract
The objective of this study is to evaluate the conventional dietary recommendations for stone prevention among patients in the National Health and Nutritional Examination Survey (NHANES) and compare dietary components and special diets between stone formers and non-stone formers. We analyzed the NHANES 2011–2018 dietary and kidney condition questionnaires, among 16,939 respondents who were included in this analysis. Dietary variables were selected based on the American Urological Association (AUA) guideline for Medical Management of Kidney Stones and from other studies on kidney stone prevention. Weighted multivariate logistic regression models were used to assess the relationship of dietary food components (categorized into quartiles) and dietary recommendations with kidney stone formation (yes vs no), adjusted for total caloric intake, comorbidities, age, race/ethnicity, and sex. The prevalence of kidney stones was 9.9%. Our results showed association of kidney stones with lower levels of potassium (p for trend = 0.047), which was strongest for < 2000 mg (OR = 1.35; 95% CI 1.01–1.79). Higher vitamin C intake was inversely associated with stone formation (p for trend = 0.012), particularly at daily intake levels between 60 and 110 mg (OR = 0.76; 95% CI 0.60–0.95) and above 110mcg (OR = 0.80; 95% CI 0.66–0.97). There were no associations between other dietary components and kidney stone formation. Higher levels of dietary vitamin C and potassium intake may be indicated for stone prevention and warrants further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney stones are estimated to affect 1 in 11 people in the United States, and total health care costs related to kidney stones exceed $5 billion [1]. With the increasing prevalence of kidney stones despite the adoption of evidence-based guidelines for prevention and treatment, it is important to revisit the efficacy of recommendations set in place, particularly those addressing modifiable risk factors [2].
Both genetic and environmental factors contribute to the development of nephrolithiasis [3]. The risk of stone formation is 2.5 times higher with a family history of stone disease. Diet is a highly variable yet key modifiable risk factor, and as such the American Urological Association (AUA) and other urologic organizations have shared evidence-based guidelines and expert opinions on optimal ranges for stone prevention, which can minimize the need for medical or surgical intervention [4]. For instance, the AUA guidelines emphasize high fluid intake with a urine volume goal of 2.5 L daily, maintaining calcium intake of 1000–1200 mg, reducing salt intake to < 2300 mg, increasing consumption of fruits and vegetables (a major source of potassium and citrate), limiting oxalate-rich foods and limiting non-dairy animal protein [4]. Other studies cited demonstrate the potential anti-lithogenic effects of moderate consumption of caffeine, increased magnesium and vitamin B6 uptake, and reduced vitamin C [5,6,7].
There remains a lack of standardization of an optimal diet regimen for stone prevention. The goal of this study is to compare the dietary components between stone formers and non-stone formers and to evaluate associations of conventional dietary recommendations for stone prevention among participants in the National Health and Nutritional Examination Survey (NHANES).
Methods
Study population
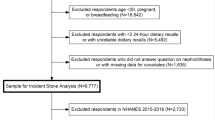
The NHANES is a nationally representative, population-based study in the United States conducted every two years by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) [8]. The NHANES methods for sampling have been well described previously [9]. Respondents to four consecutive, two-year cycles of the “Kidney Conditions—Urology” questionnaire conducted between 2011 and 2018 were combined. A total of 22,617 potential participants were identified. Participants with missing responses (n = 48), incomplete dietary values (n = 3,587), and those with a total caloric intake of < 500 kcal or those with > 5,000 kcal (n = 2,139) were excluded from analyses, leaving n = 16,939 respondents. The NHANES study was granted approval by the NCHS Research Ethics Review Board [10].
Dietary variable selection and outcome data
Participants with a self-reported history of kidney stones were identified from the “Kidney Conditions—Urology” dataset using the question “Have you ever had kidney stones?” Dietary data were collected from all participants using the “Dietary Interview—Individual Foods” questionnaire, which included types and amounts of food and beverages consumed in the 24-h period prior. Values were then estimated from these data to assess intake of energy and individual nutrients [11]. Reponses were recorded from two interview days, with the second occurring 3 to 10 days following the first interview. Subjects not completing dietary information on both days (n = 1978) were excluded.
Dietary variables analyzed were selected based on the AUA dietary guidelines aimed at prevention of kidney stone formation (Table S1) [4]. Continuous dietary values were averaged from the values obtained from the first and second dietary interviews and were then further categorized into quartiles for analysis based on their distribution ranges among non-kidney stone formers. Slight modifications were made to the quartile ranges to better align with existing guideline thresholds. Total caloric intake was used to adjust for inherent differences in subject total intake.
Demographic and comorbidity variable selection
Demographic data were collected from the NHANES “Demographic Variables” dataset, which included age, race/ethnicity, gender, and body mass index (BMI). Race was further categorized into Non-Hispanic White, Non-Hispanic Black, Hispanic, and Other/Multiracial. BMI was categorized according to the National Institute of Health classification as < 18.5, 18.5–24.9, 25.0–29.9, and > 30 kg/m2 [12]. Information on subject comorbidities was gathered from the “Medical Conditions”, “Diabetes”, and “Blood Pressure and Cholesterol” questionnaires. Comorbidities were categorized as “yes” or “no.” Hypertension and cardiac disease were combined into one variable to increase statistical power. Diabetes, hyperlipidemia, gout, and thyroid disease were also included.
Statistical analysis
Our dataset was weighted according to NHANES recommendations due to the complex oversampling of minority populations. Baseline weighted characteristics between stone formers and non-formers were assessed using the Chi-square test for categorical variables, and the Student’s t-test or Mann–Whitey U test for continuous normally or non-normally distributed variables, respectively. All tests were two sided with statistical significance set at p < 0.05.
Weighted multivariate logistic regression models were used to assess the relationship of dietary food components (categorized into quartiles) and dietary recommendations with kidney stone formation (yes vs no), while accounting for established risk factors for kidney stones. Reference categories were set to the recommended range for each dietary component when possible. Logistic regression models were weighted to account for oversampling of various racial populations and initially adjusted for age, and then additionally adjusted for race/ethnicity, sex, and total caloric intake (kcal). The final regression models also included BMI, cardiovascular disease, and diabetes as potential confounders of the diet and kidney stone associations. To evaluate the linear trend of kidney stone risk associated with increasing quartiles of dietary measurements, we performed both the Cochran–Armitage and Wald tests (we present the Wald test p-values from multivariate logistic regression models) [13]. Statistical analysis was conducted using the SPSS version 28.0 (IBM Corp., Armonk, NY).
Results
Baseline characteristics
A total of 16,939 participants were included in the final analysis, of which 1,675 (9.9%) self-reported having a kidney stone, representing a weighted total population estimate of 229.2 million and 23.2 million, respectively (Table 1). Compared to non-stone formers, kidney stone formers were on average older at survey (53.8 vs 47.3 years, p < 0.001) and more likely to be male (54.1% vs 47.2%, p = 0.007), non-Hispanic White (76.1% vs 63.9%, p < 0.001), and obese (47.6% vs 38.4%, p < 0.001). Socioeconomic status as determined by poverty to income ratio (PIR) was similar between the two groups (p = 0.95). Participants with kidney stones had also significantly higher prevalence of diabetes (20.9% vs 9.6%, p < 0.001), cardiovascular disease (48.9% vs 32.4%, p < 0.001), hyperlipidemia (46.2% vs 33.4%, p < 0.001), gout (7.4% vs 3.8%, p < 0.001), and thyroid disease (15.7% vs 11.5%, p = 0.002).
Diet components and dietary guidelines
Among the dietary components analyzed, stone formers had a significantly higher intake of caffeine and lower intake of water, potassium, phosphorus, magnesium, vitamin B6, vitamin C, vitamin K, protein, carbohydrates, and fiber (Table 2). In age-adjusted models (Model 1), there were statistically significant associations between stone formers and lower vitamin B6 (1.75–2.5 mg) (OR = 0.78; 95%CI 0.63–0.97), lower vitamin K (80-150mcg) (OR = 1.24; 95%CI 1.02–1.60) and higher vitamin C (p for trend = 0.003), particularly between 60 and 110 mg (OR = 0.71; 95%CI 0.56–0.90). Higher intake of saturated fat (p for trend = 0.025) was also associated with stone formers, which was strongest above 30 g (OR = 1.34; 95%CI 1.02–1.77).
After further adjustment for sex, race/ethnicity, and total caloric intake (Model 2), there were no significant associations between vitamin K and saturated fat with kidney stones. Lower levels of vitamin B6 remained associated, (p for trend = 0.028), while higher levels of vitamin C were inversely associated with kidney stone formation (p for trend = 0.006), particularly in the 60–110 mg range (OR = 0.73; 95%CI 0.58–0.92). Lower levels of daily intake of potassium (p for trend = 0.019) below 2000 mg were also associated with stones (OR = 1.44; 95%CI 1.09–1.90), as were lower levels of magnesium (p for trend = 0.045) especially less than 200 mg (OR = 1.38; 95%CI 1.05–1.82).
After additional adjustment for BMI, diabetes, and CVD (Model 3), we saw similar patterns of associations between lower levels of potassium and kidney stones (p for trend = 0.047; OR = 1.35; 95%CI 1.01–1.79), and between higher levels of vitamin C and kidney stones (p for trend = 0.012) particularly at daily intake levels between 60 and 110 mg (OR = 0.76; 95%CI 0.60–0.95) and above 110mcg (OR = 0.80; 95%CI 0.66–0.97). Lower levels of vitamin B6 (p for trend = 0.100) and the lowest quartile of magnesium (OR = 1.25; 95%CI 0.94–1.67) were no longer significant in the final model. Notably, fiber intake below 10 g had a weak association with stones (OR = 1.42; 95%CI 0.97–2.07). Total intake of water, caffeine, sodium, phosphorus, protein, carbohydrates, cholesterol, sugar, total fat, and other fat subtypes were not significantly associated (Fig. 1).
Special diet recommendations
The types of special dietary recommendations were also assessed for associations with kidney stones (Table 3). There were significantly more kidney stone formers among those on a diabetic diet (4.1% vs 2.0%, p < 0.001) and those on a low carbohydrate diet (3.4% vs 1.5%, p = 0.003). In age-adjusted models, only diabetic diets (OR = 1.07; 95%CI 1.01–1.13) and low carbohydrate diets (OR = 1.10; 95%CI 1.03–1.17) were associated with stones (Table 4, Model 1). On multivariate analysis (Model 3), only low carbohydrate diet remained significantly associated with kidney stones (OR = 1.95; 95%CI 1.07–3.55).
Discussion
As the prevalence of kidney stones continues to rise worldwide, there is a need to reassess the current recommendation guidelines for stone prevention. Using a nationally representative sample, we analyzed these components and found significant results that are both in-line with and can potentially challenge current recommendations.
Fluid and electrolyte intake
Fluid intake has been established as a key modifiable risk factor for kidney stone prevention. The AUA dietary recommendations guidelines suggest a fluid intake to produce a urine volume of 2.5 L daily (grade B standard). While the protective effect can be attributed to an elevated urine output regardless of the type of fluid intake, specifically drinking more water has been shown to reduce stone risk in previous meta-analyses [14]. Although our study did not find an association of kidney stones with water intake, it may be reflective of the categorization of water intake into quartiles, or possibility of misclassification or recall bias inherent in this study design. Furthermore, less than 10% of our population consumed over 2.5 L of water per day and we could not quantify other types of fluid intake. Interestingly, while previous studies have found moderate consumption of coffee and caffeine to be associated with a lower stone risk, we did not find a relationship [5].
The AUA also recommends a daily sodium intake of < 2300 mg (grade B standard), particularly for calcium stone formers in the setting of hypercalciuria [4]. We initially observed a weak association between stone formers and higher levels of sodium intake that was no longer significant after controlling for known risk factors, suggesting the protective effect of reducing intake may be secondary to its benefit with known comorbidities. Similarly, calcium stone formers are advised to consume between 1000 and 1200 mg daily, as this range has been shown to maintain normal levels of urinary calcium while sufficiently preventing oxalate gut absorption [15]. Although not statistically significant, we observed an association in stone formers at all levels of calcium outside the recommended range. Interestingly we noted that regular consumption of milk, which is high in calcium and phosphorus content, was also not associated.
Although recommended in the form of potassium citrate, the AUA recommends potassium supplementation for the prevention of calcium, uric acid, and cysteine stone recurrence. No range or threshold has been proposed for dietary potassium; however, stone formers are advised to keep intake high and supplement as needed [16]. Our findings showed a significant association with stone formers at lower levels of potassium, particularly below 2000 mg. Diets rich in fruits and vegetables provide ample potassium and have been shown to alkalinize the urine and prevent calcium oxalate crystallization [17, 18]. While magnesium has also been associated with urine pH and oxalate absorption in the gut, we only found a weak association with stone formers.
Vitamins
While vitamin C has previously been shown to contribute to higher levels of urinary oxalate and subsequent stone formation, our findings showed that higher levels of vitamin C were inversely associated with kidney stone formation with daily intakes above 60 mg. Vitamin C can either be supplemented or consumed in the form of ascorbic acid, which is later converted into oxalate in humans and excreted in the urine [19]. While numerous studies have demonstrated that even minor increases in supplementary Vitamin C intake can increase stone risk, we suspect that the alkalinizing effect of its major dietary source, fruits and vegetables, may be the reason why it is significant in the non-stone-forming population in our study [6, 20].
Vitamin B6 and vitamin K have garnered interest as potential stone inhibitors [21]. Though we observed increased stone prevalence at lower B6 levels, these relationships were not significant in the multivariable model, similar to prior studies challenging this hypothesis [22]. Similarly, our study did not find a link to vitamin K even though prior research has linked its consumption to known stone risk factors, potentially through its effects on blood vessel calcification [23].
Macronutrients and special diets
We also investigated the recommendations regarding macronutrients and special diets. The AUA recommends consuming more vegetables/fruits and less non-dairy animal protein for all calcium stone-forming patients with hypocitraturia. While fruits and vegetables are great sources of fiber, our study only demonstrated a weak association between low fiber and stone formation, suggesting that higher potassium or lower oxalate are driving its protective effects [24]. We did not observe any association with overall protein intake, although we were not able to specify its source. While dietary fat is not part of formal recommendations, we found a weak association with greater stone risk and higher levels of saturated fat. Though it has been hypothesized that fat intake positively correlates with oxalate excretion, our results indicate further research is needed [7].
Lastly, carbohydrate and sugar intake were not associated with stone formation in our study, despite conflicting previous studies [25]. Interestingly, participants following a low carbohydrate diet in particular were among the stone formers even when adjusting for known comorbidities, with weak associations additionally observed between diabetic and low fat/cholesterol diets. This suggest a potential reverse causation in which stone formers were modifying their diets because of their kidney stone diagnosis. Weight loss/low calorie, high lactose and modified ketogenic diets conferred no additional associations. Further research and a larger sample size are needed to clarify the relationship with these diets to known comorbidities and stone risk.
Strengths and limitations
Our study has strengths and limitations that should be carefully considered when interpreting the results. The strengths of this study include a large nationally representative sample size, detailed information on dietary components and regimens, and ability to determine associations of multiple exposures. Although NHANES aimed at obtaining a comprehensive understanding of the usual dietary intake of the US population, a major limitation is that the survey did not specify the sources for macronutrients; specific diet components including fruit, vegetable, and nut intake; or the portion size and cooking method, which are important factors in nephrolithiasis.
Given the cross-sectional study design, other limitations include non-response and recall bias, misclassification of dietary, as well as patient self-report of kidney stone diagnosis. Another potential limitation is the possibility of reverse causation, as patients with a history of kidney stones are more likely to change their dietary intakes after diagnosis, and thus, the dietary patterns they report are more likely to be due to modification of their lifestyles. Other limitations include lack of kidney stone details such as composition, number of stone events, and date of diagnosis that were not collected in the NHANES questionnaire. Lastly, while we categorized dietary variables into quartiles to dampen the effect of outliers, we noticed that our models might have been sensitive to various cutoff points for quartile categorization.
Conclusion
We assessed the AUA guidelines for kidney stone prevention and found that higher levels of dietary vitamin C and potassium intake may be indicated for stone prevention and warrants further investigation. Recommended targets for total fluid, sodium, calcium, and protein intake may need further studies, while magnesium, vitamin B6, vitamin K, and saturated fat are potential dietary components of interest.
Data availability
Data supporting this work are publicly available.
References
Saigal CS, Joyce G, Timilsina AR, Urologic Diseases in America P (2005) Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 68(4):1808–1814. https://doi.org/10.1111/j.1523-1755.2005.00599.x
Chewcharat A, Curhan G (2021) Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis 49(1):27–39. https://doi.org/10.1007/s00240-020-01210-w
Yamout H, Goldberg S (2019) Genetic and Environmental Risk Factors for Kidney Stones. In: Han H, Mutter WP, Nasser S (eds) Nutritional and Medical Management of Kidney Stones, 1st edn Springer International Publishing, Berlin,pp 43–52
Pearle MS, Goldfarb DS, Assimos DG et al (2014) Medical management of kidney stones: AUA guideline. J Urol 192(2):316–324. https://doi.org/10.1016/j.juro.2014.05.006
Yuan S, Larsson SC (2022) Coffee and caffeine consumption and risk of kidney stones: a mendelian randomization study. Am J Kidney Dis 79:9–14. https://doi.org/10.1053/j.ajkd.2021.04.018
Taylor EN, Stampfer MJ, Curhan GC (2004) Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol 15(12):3225–3232. https://doi.org/10.1097/01.ASN.0000146012.44570.20
Nouvenne A, Meschi T, Guerra A, Allegri F, Prati B, Borghi L (2008) Dietary treatment of nephrolithiasis. Clin Cases Miner Bone Metab 5(2):135–141
Center for Disease Control (2022) National Health and Nutrition Examination Survey. National Center for Health Statistics. Updated December 29 2022. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed January 4 2023
Levy RV, Brathwaite KE, Sarathy H, Reidy K, Kaskel FJ, Melamed ML (2021) Analysis of active and passive tobacco exposures and blood pressure in US children and adolescents. JAMA Netw Open 4(2):e2037936. https://doi.org/10.1001/jamanetworkopen.2020.37936
Center for Disease Control (2017) Ethics Review Board Approval. National Center for Health Statistics. https://www.cdc.gov/nchs/nhanes/irba98.htm Accessed January 4 2023
Center for Disease Control (2017) MEC In-Person Dietary Interviewers Procedures Manual. National Center for Health Statistics. https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2017_MEC_In-Person_Dietary_Interviewers_Manual.pdf. Accessed January 4 2023
National Institute of Health (1998) Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res 6 (Suppl 2):51S-209S. https://doi.org/10.1002/j.1550-8528.1998.tb00690.x
Klienbaum DG, Nizam A, Kupper L, Muller KE (2007) Applied regression analysis and multivariate methods, 4th edn. Duxbury Press, Pacific Grove
Xu C, Zhang C, Wang XL et al (2015) Self-fluid management in prevention of kidney stones: a PRISMA-compliant systematic review and dose-response meta-analysis of observational studies. Medicine (Baltimore) 94(27):e1042. https://doi.org/10.1097/MD.0000000000001042
von Unruh GE, Voss S, Sauerbruch T, Hesse A (2004) Dependence of oxalate absorption on the daily calcium intake. J Am Soc Nephrol 15(6):1567–1573. https://doi.org/10.1097/01.asn.0000127864.26968.7f
Lemann J Jr, Pleuss JA, Gray RW (1993) Potassium causes calcium retention in healthy adults. J Nutr 123(9):1623–1626. https://doi.org/10.1093/jn/123.9.1623
Ferraro PM, Mandel EI, Curhan GC, Gambaro G, Taylor EN (2016) Dietary protein and potassium, diet-dependent net acid load, and risk of incident kidney stones. Clin J Am Soc Nephrol 11(10):1834–1844. https://doi.org/10.2215/CJN.01520216
Taylor EN, Fung TT, Curhan GC (2009) DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 20(10):2253–2259. https://doi.org/10.1681/ASN.2009030276
Hellman L, Burns JJ (1958) Metabolism of L-ascorbic acid-1-C14 in man. J Biol Chem 230(2):923–930
Baxmann AC, De OGMC, Heilberg IP (2003) Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int 63(3):1066–1071. https://doi.org/10.1046/j.1523-1755.2003.00815.x
Ortiz-Alvarado O, Miyaoka R, Kriedberg C, Moeding A, Stessman M, Monga M (2011) Pyridoxine and dietary counseling for the management of idiopathic hyperoxaluria in stone-forming patients. Urology 77(5):1054–1058. https://doi.org/10.1016/j.urology.2010.08.002
Ferraro PM, Taylor EN, Gambaro G, Curhan GC (2018) Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis 46(3):265–270. https://doi.org/10.1007/s00240-017-0999-5
Li Y, Lu X, Yang B et al (2019) Vitamin K1 inhibition of renal crystal formation through matrix Gla protein in the kidney. Kidney Blood Press Res 44(6):1392–1403. https://doi.org/10.1159/000503300
Sorensen MD, Hsi RS, Chi T et al (2014) Dietary intake of fiber, fruit and vegetables decreases the risk of incident kidney stones in women: a Women’s Health Initiative report. J Urol 192(6):1694–1699. https://doi.org/10.1016/j.juro.2014.05.086
Taylor EN, Curhan GC (2008) Fructose consumption and the risk of kidney stones. Kidney Int 73(2):207–212. https://doi.org/10.1038/sj.ki.5002588
Acknowledgements
Kevin Liu Kot and Kevin Labagnara contributed equally.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material, data collection and analysis were performed by Kevin Liu Kot and Kevin Labagnara. The first draft of the manuscript was written by Kevin Liu Kot and Kevin Labagnara and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: KLK. Methodology: KLK, KL. Formal analysis and investigation: KLK, KL. Writing—original draft preparation: KLK, KL. Writing—review and editing: KLK, KL, JK, JL, KG, IA, AS. Supervision: IA, AS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This research study was conducted retrospectively from data obtained by NHANES. This study was approved by the NHANES ethical review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu Kot, K., Labagnara, K., Kim, J.I. et al. Evaluating the American Urologic Association (AUA) dietary recommendations for kidney stone management using the National Health And Nutritional Examination Survey (NHANES). Urolithiasis 51, 60 (2023). https://doi.org/10.1007/s00240-023-01423-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-023-01423-9