Abstract
The use of herbs as prophylactic and prevent recurrence of urolithiasis was counseled. Musa balbisiana fruits have long been used folk in the treatment of urinary stones. However, there is no clear scientific evidence. This study was designed to investigate the diuretic and prophylactic effects of M. balbisiana fruit extract in ethylene glycol-induced urolithiasis in rats. In this study, crude extracts were extracted by maceration, hot extraction, and decoction methods. The extracts were examined in vitro biological activities of anti-urolithiatic, anti-inflammatory, antibacterial, and antioxidant properties. For in vivo study, the experimental groups of rats were given the extract for 7-day to evaluate the diuretic potential. In prophylactic activity against urolithiasis, rats were given the extract for 28-day on ethylene glycol-induced urolithiasis in male Wistar rats. The results showed that all extracts of M. balbisiana fruits exhibited in vitro anti-urolithiatic, anti-inflammatory, antibacterial, and antioxidant activities with the hydroethanolic extract by hot extraction presented the highest activities. The hydroethanolic extract at the doses of 0.8 and 1.6 g/kg had diuretic effect after 7-day of treatment. The extract at these two doses had significantly prophylactic activity restored the parameters in urine and serum to near-normal level. The histopathological examinations revealed that calcium oxalate crystal deposits in the renal tubules and congestion and dilation of the renal tubules were significantly reverted after the extract treatment for 28-day. Taken together, the hydroethanolic extract of M. balbisiana fruits had diuretic effect and reduced the growth of urinary stones showing its effect as an antiurolithiatic support agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is a worldwide problem that affects many group ages and is one of the major sources of morbidity around the world. As the economic develops, the incident of kidney stones and the prevalence of lifetime risk for urolithiasis has been increasing worldwide (Ingale et al. 2012). Urolithiasis causes pain, loss of work time, medical expenses, and the need for hospitalization and yet it is an infrequent cause of renal failure (Velu et al. 2017). Currently, doctor eliminate kidney stones by several methods such as shockwave lithotripsy, open renal surgery, and pharmacological intervention. Lithotripsy and survey methods remain effective currently but caused high invasion, stroke, and high recurrence rate (70–81% in male and 47–60% in female). Some drugs seem ineffective in some major-urolithiasis patients and hinder with many side effects such as lowering blood pressure, affecting on liver and kidneys function (Coe et al. 2005; Evan et al. 2005). Thus, doctors are now looking for alternatives and traditional medicine are one of those.
Not only in Vietnam, many Asian countries have used medicinal herbs as medicinal herbs are safe, fewer side effect while their effects on disease remain throughout human life (Astutik et al. 2019; Nguyen and Nguyen 2008). In Vietnam, with a diverse herbal resource, scientists are now working hard on screening and utilizing the potential natural medicines. Some popular traditional medicinals were applied in nephrolithiasis treatment such as Primrose, Corn Beard, Dandelion, Psyllium, Turmeric, and Artichoke (Butterweck and Khan 2009; Zhou et al. 2018; Aziz et al. 2005). Therefore, utilizing herbs in preventing and alleviating the symptoms of some clinical diseases, especially urolithiasis is trending in this decade.
Musa balbisiana is a plant species included in the Musaceae family, native to eastern South Asia, northern Southeast Asia, southern China, and some parts of Africa. It has been broadly utilized as an important wild food and herbal medicine. Every parts of M. balbisiana are used to treat some diseases such as bronchitis, dysentery, ulcer, diabetes, epilepsy leprosy, and common fevers (Ponnambalam and Sellappan 2014).
Over the last several decades, the health benefits of M. balbisiana have attracted considerable attention from the scientific research community. Recently study found that oral administration of M. balbisiana fruit pulp powder significantly attenuated isoproterenol-induced cardiac hypertrophy which was associated with myocardial inflammation and oxidative stress (Kumari et al. 2020). Moreover, it can inhibit the synthesis of extracellular protein and bacterial cell wall by damaging the cell membrane, cell wall, enzymes and other genetic material (Deka et al. 2018). Since it contains many active compounds such as chlorogenic acid, epicatechin, catechol kaempferol 3-O-sophoroside, rutin (Kumari et al. 2020), it plays important role in treating various of diseases such as cancer, diabetes, chronic inflammation symptoms, and cardiac vascular diseases (Borah and Das 2017). In addition, some research has shown the strong free radical scavenging property of crude extract of M. balbisiana that revealed the antioxidant activity of this fruit (Kumari et al. 2020; Ly et al. 2020a). Saponins and flavonoids in fruits have been found in our previous study (Ly et al. 2020a) to be potentially useful for the treatment of kidney stone disease and diuretic activity (Patel et al. 2012; Zeng et al. 2019; Junior et al. 2011; Schlickmann et al. 2018). It was suggested that M. balbisiana fruits can have beneficial effects against urolithiasis.
Other close relatives in same Musa species have proven to have anti-urolithiatic activity such as pseudo stem of Musa paradisiaca (Panigrahi et al. 2017), dry pulpy fibrous of Musa acuminate (Zarin et al. 2020) on in vivo and in vitro scale, respectively. However, there were no reported effectiveness of M. balbisiana fruits. Thus, M. balbisiana is expected to have similar activities that can be utilized as another herbal therapy in treating kidney stones.
Furthermore, since diuretics used in edema caused by renal dysfunction by lowering urinary calcium excretion, making them useful in preventing calcium-containing kidney stones. M. balbisiana fruits, one of vital herbal medicine in Asia, is believed to have diuretic effect that increase the rate of urination thereby decreasing the body fluid. Traditionally, drinking decoction from M. balbisiana fruits to treat kidney stones is widely known but the scientific evidence for this information is flawed. Hence, it is necessary to conduct real-life experiment to check dosage, concentration, toxicity, and other parameters to get effective treatment. In this study, several in vitro and in vivo pharmacological effects related to the supportive treatment of urinary stones of M. balbisiana fruits have been studied.
Materials and methods
Reagents and standards
Reagents and reference standards, for example, ethylene glycol, sodium citrate, diclofenac sodium, ascorbic acid, 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), 1,1-diphenyl-2-picrylhydrazyl, and methanol were purchased from Sigma-Aldrich Products (Merck KGaA, Darmstadt, Germany). Other chemicals used were high-purity.
Plant material
M. balbisiana fruits (Fresh green banana including peel, flesh, and seeds) were collected on May 2019 from Tan Thanh District, Long An Province. The plant sample were identified and authenticated by MSc. Le Duc Thanh (Research Center of Ginseng and Medicinal Materials, Ho Chi Minh City) and a voucher specimen (TNDL-QCH-2019) was deposited for M. balbisiana Colla. The dried samples were ground to a fine powder and stored individually in airtight PVE bag at the Research Center of Ginseng and Medicinal Materials in Ho Chi Minh City (Sample code: QCH-TTS-2019).
Preparation of M. balbisiana fruit extracts
The three prepared M. balbisiana fruit extracts were included hydroethanolic extract by maceration method (E1), hydroethanolic extract by hot extraction (E2), and water extract by decoction method (E3). For maceration method: the raw material was extracted with 45% (v/v) ethanol at room temperature during 24 h, the liquid extract was then filtered and the extraction was repeated two more times to achieve a ratio of 1/20 (w/v). For hot extraction: the raw material was extracted with 45% (v/v) ethanol at 70–80 °C during 60 min, the liquid extract was then filtered and the extraction was repeated two more times to achieve a ratio of 1/20 (w/v). For decoction method: the raw material was extracted with distilled water at 100 °C during 60 min, the liquid extract was then filtered and the extraction was repeated two more times to achieve a ratio of 1/20 (w/v). The above liquid extracts were individually concentrated using a rotary evaporator at 70–80 °C under reduced pressure to get corresponding crude extracts. The extraction yield of E1, E2, and E3 was 29.54%, 30.85%, and 34.20%, respectively.
Animals
Animal experimental was obtained by Institute of Medical Military 103, Hanoi. Healthy Swiss albino mice of both genders, 5 to 6-week-old and with an average body weight of 20 ± 2 g, and and healthy Wistar rat of male, 8 to 12-week-old, weighing from 160 to 200 g were used for the study. Mice and rats were housed in polypropylene cages maintained under standard condition (12-h light/dark cycle in a room temperature controlled at 27 ± 3 °C, 35–50% humidity), allowed free access to water and fed with standard pellet diet provided by National Institute of Hygiene and Epidemiology. Mice and rats were allowed to acclimatize to laboratory conditions for a week before commencing the actual experimental sessions. Studies using these mice and rats were adhered to principles stated in the Guide for Care and Use of Laboratory Animals and Decision 141/QĐ-K2ĐT on October 27th, 2015 of the Ministry of Health.
In vitro anti-urolithiatic activity
Nucleation assay
The nucleation assay on calcium oxalate (CaOx) crystal formation was carried out according to a previously described method with minor modifications (Bawari et al. 2018). Calcium chloride (CaCl2) (5 mmol/L) and sodium oxalate (Na2C2O4) solution (7.5 mmol/L) were prepared in Tris–HCl (0.05 mol/L) and NaCl (0.15 mol/L) buffer (pH = 6.5). Briefly, 250 µL of each extract or sodium citrate (positive control) at different concentrations was mixed with 750 μL CaCl2 solution. Crystallization was started by adding 750 μL of Na2C2O4 solution. Then, final mixtures were incubated for 30 min at 37 °C and the absorbance was measured at 620 nm. In the control group, 250 μL of the buffer was added to the CaCl2 solution. The measurements were performed in triplicate. Microscopic evaluation number, size, and morphology of CaOx crystals formed in absence or presence of extract as well as sodium citrate were overally determined using a Leica DM 2500 LED microscope at 400 × magnification.
Aggregation assay
The aggregation assay of CaOx crystals was conducted as previously described with slight alterations (Devkar et al. 2016). CaCl2 (50 mmol/L) and Na2C2O4 solutions (50 mmol/L) were prepared in Tris–HCl (0.05 mol/L) and NaCl (0.15 mol/L) buffer (pH = 6.5), then mixed together, heated to 60 °C in a water bath for 1 h and then incubated overnight at 37 °C to prepare seed CaOx crystals. After drying, CaOx crystal solution (5 mg/mL) was prepared in buffer (pH = 6.5). 1 mL of extracts or sodium citrate (positive control) at different concentrations were added to 3 mL CaOx solution, the mixture was vortexed and then incubated at 37 °C for 30 min. Optimal density of the final mixtures was then measured at 620 nm wavelength. The tests were done in triplicate.
Percentage inhibition of calcium oxalate crystal formation/aggregation was estimated using the expression:
In which, Abscontrol was the absorbance of the blank without test extract and Abssample was the absorbance of the sample with test extract minus the absorbance of the test blank. The IC50 values were also calculated using GraphPad Prism 8.0.2.
In vitro anti-inflammatory activity
Protein denaturation assay
The protein denaturation assay was performed as described previously (Ly et al. 2020b). Diclofenac sodium was used as a positive control.
Membrane lysis assay
Preparation of red blood cells (RBCs) suspension
The blood was taken from the tails of healthy Swiss albino mice (20–25 g) and RBCs suspension was prepared as described previously (Ly et al. 2021).
Heat-induced hemolysis and hypotonicity-induced hemolysis
Membrane lysis assay by heat-induced hemolysis and hypotonicity-induced hemolysis was achieved as described previously (Ly et al. 2021). Diclofenac sodium was used as a positive control.
In vitro antibacterial activity against E. coli
Determination of zone of inhibition method
The zone of inhibition was determined according to a previously described with test extract at concentrations of 200, 300, 400, and 500 mg/mL (Ly et al. 2020b).
Quantitative antibacterial activity assay by MIC and MBC
The minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were determined according to a previously described method with minor modifications (Ly et al. 2020b). Amoxicillin was used as a positive control. The MIC was the lowest concentration of antimicrobial agents inhibiting the growth of the pathogen and the MBC was the lowest concentration almost killing the initial bacterial population. The MIC and MBC were observed for the presence or absence of bacterial growth after incubation.
In vitro antioxidant activity
DPPH assay
DPPH free radical quenching assay was applied to evaluate antioxidant activity of M. balbisiana fruit extracts based on a previously described method (Ly et al. 2020b). Ascorbic acid was used as a positive control.
ABTS assay
The ABTS antioxidant test was carried out according to the following description: ABTS solution was prepared by adding a 7 mM ABTS solution to a 2.4 mM potassium persulfate solution of equal volume and then incubating the solution (Pellegrini et al. 2003). Fluid in the dark for 16 h at room temperature. The solution is then diluted by mixing 1 mL of ABTS solution with 50 mL of methanol to obtain an absorbance of 0.706 ± 0.01 units at 734 nm using a spectrophotometer. Use this solution for testing. 40 μL of the test sample at various concentrations or ascorbic acid (positive control) was mixed with 1160 μL of ABTS solution (1:29 v/v), measure the absorbance at 734 nm after 6 min at room temperature. All tests were done in triplicate and an average of each sample was calculated. The results were expressed as an IC50 value for each sample from proportion of the radical quenching activity, which was calculated by the formula:
In which, Abscontrol was the absorbance of the blank without test extract and Abssample was the absorbance of the sample with test extract minus the absorbance of the test blank.
Reducing power assay
The sample will reduce Fe3+ ions in K3[Fe(CN)6] to Fe2+ ions in K4[Fe(CN)6]. When FeCl3 is added, Fe3+ will react with ferrocyanide ions to form blue ferrocyanide complex (K4[Fe(CN)6]3) (Oyaizu 1986). Briefly, 0.2 mL of sample at the concentrations examined was added with 0.5 mL of PBS phosphate buffer solution (pH = 6.6) and 0.5 mL of K3[Fe(CN)6] (carried out in the dark), incubated at 50 °C, 30 min. After that, each tube was cooled and then added with 0.5 mL of 10% trichloacetic acid solution TCA and centrifuge 3000 cycles for 10 min. Then, 0.5 mL of the above solution (the supernatant) was taken into a new eppendorf, 0.1 mL of FeCl3 solution and 0.5 mL of distilled water were added. Measuring absorbance at a wavelength of 700 nm, the optical density value OD reflects the reducing ability of the sample. Ascorbic acid was used as a positive control. The antioxidant ability of the test sample was judge via optical density and EC50 values. The lower the optical density value, the weaker the reduction activity of the sample. EC50 value is concentration of effective antioxidant for optical density reaches 0.5, which was calculated through the equation illustrating the correlation between the concentration of the sample and the its optical density using Graphpad Prism software.
Acute toxicity test on mice
In this experiment, ten Swiss albino of both genders weighing between 20 and 22 g will be randomly distributed into groups with equal number. Before the day of the experiment, the animals were deprived of food for 18 h and water was given ad libitum. The volume of dosage is 20 mL/kg (p. o.) mouse body weight (b. w.) and is administered around 8–9 AM. On day one, two mice were received a limited dose of maximum concentration through the needle of the hydroethanolic crude extract orally. The animals were then observed continuously for 72 h, with more attention paid to the first 4 h to observe overt signs of morbidity and mortality. After 72 h, another three mice (from each group) were given the same dose and observed for general sign of toxicity. The animals were kept under observation for up to 14 days thereafter (OECD 2001; Ministry of Health 2015). According to the dose used folk effects and the results of the acute toxicity test on mice, the doses used in experiments on rats were from human and mice to rat converted doses.
Diuretic activity on rats
The animals were divided randomly into four groups, each with six mice. Group I received 0.9% (w/v) sodium chloride (NaCl) and served as control, Groups II and III received 0.8 g/kg and 1.6 g/kg dissolved in 0.9% NaCl solution, respectively, of the test substances (hydroethanolic crude extract), Group IV received 10 mg/kg Furosemide in NaCl 0.9%, the standard diuretic drug. All groups were administered orally with the volume of 10 mL/kg b. w. (p. o.) at 8–9 AM for 7 consecutive days using a cannula needle at the laboratory. At the end of the experiment, rats kept separated and supplied with normal drinking water but not food. They were then being determined the volume of urine, and ionic compound (Na+, K+, Cl−) of urine collected in 24 h after 7 days of oral administration. The ratio of urinary excretion in the test group to urinary excretion in the control group is used as a measure of the diuretic action for the given dose of a drug. As the diuretic action is prone to variability, a parameter known as diuretic activity was calculated. To obtain diuretic activity, the diuretic action of the test substance was compared to that of the standard drug in the test group. The ratio of urinary excretion in the test group and control group was expressed as diuretic action, which was used to be the measure of degree of diuresis. Diuretic index and diuretic activity will be calculated as,
Diuretic index and diuretic activity are calculated as following:
It was decided prior to the start of the experiment that diuretic activity will be considered “nil”, “little”, “moderate”, and “good”, if the values were < 0.72, 0.72–0.99, 1.0–1.5, and > 1.5, respectively. Saliuretic, natriuretic and carbonic anhydrase inhibition: The sum of Na+ and Cl− urinary excretion was calculated as a parameter of saliuretic activity. The ratio Na+/K+ was calculated for natriuretic activity. The ratio Cl−/(Na+ + K+) was calculated to estimate carbonic anhydrase inhibition (CAI). If the Na+/K+ ratio > 1 shows a adequate natriuretic index and if > 2 shows propitious Na+ urinary excretion without exorbitant urinary K+ loss and if > 10 shows a prosperous K+ sparing effect. If the Cl−/Na+ + K+ ratio is from 0.8 to 1.00 excludes CAI activities and if < 0.8, it is considered to have a strong CAI index (Welu et al. 2020; Al-Saikhan and Ansari 2016).
Prophylactic activity against urolithiasis on rats
Animals were divided into five different groups containing six animals each. Ethylene glycol (0.75% v/v) in drinking water was fed to all groups except control for induction of renal calculi till the 28th day. All groups except control received extract (two doses, once daily by oral route) from 1st day till 28th day. Sodium citrate was used as standard drug. During the study animals were allowed free access to food.
-
Group I: Physiological control (distilled water, p. o.).
-
Group II: Pathological control (Ethylene glycol 0.75% in drinking water + water, p. o.).
-
Group III: Pathological 1st test (Ethylene glycol 0.75% in drinking water + extract 0.8 g/kg, p. o.).
-
Group IV: Pathological 2nd test (Ethylene glycol 0.75% in drinking water + extract 1.6 g/kg, p. o.).
-
Group V: Positive control (Ethylene glycol 0.75% in drinking water + sodium citrate 2.5 g/kg, p. o.)
All groups were administered orally with the volume of 10 mL/kg b. w. (p. o.) at 8–9 AM for 28 consecutive days using a cannula needle at the laboratory. After 1 h on day of 28, urine sample and tail blood sample were determined biochemical index. On the day of 29, kidney tissue is collected and studied by Hematoxylin and Eosin staining (rats are kept in steady state after 24 h before kidneys are collected).
Collection and analysis of urine: Urine samples of 24-h were collected on the 28th day. Animals had free access to drinking water during the urine collection period. Urine was analyzed for calcium, phosphate, and magnesium contents using an automated system.
Serum analysis: Tail blood was collected on the 28th day. Serum was separated by centrifugation at 10,000 × g for 10 min and was analyzed for creatinine, urea nitrogen, calcium, and phosphate by semi-automatic biochemical system.
Kidney histopathology: On day 29th, rats from each group are sacrificed and kidneys are identified. A portion of the kidneys is excised and fixed in formalin solution, neutral buffered, 10% and processed for histological studies stained with hematoxylin and eosin for histological evaluation using microscopy (Vyas et al. 2011; Das and Malipeddi 2016).
Statistical analysis
All the measurements were done in triplicate and results are expressed in terms of mean ± standard error of the mean and IC50 values were calculated using Graphpad Prism software (version 8.0.2, Inc., La Jolla, CA, USA). Data were analyzed by Graphpad Prism software using t-test and One-way ANOVA. Differences were considered significant at p < 0.05.
Results
Effect of extraction methods on biological activities of M. balbisiana fruits
As shown in Fig. 1, the crude extracts of M. balbisiana inhibited the formation and aggregation of CaOx crystals in a concentration dependent manner. The inhibitory activity of E2 on CaOx crytal nucleation and aggregation were relatively higher than the other two extracts.
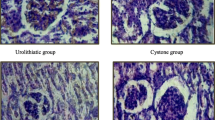
The morphology of CaOx crystals forms observed in the experiments was analyzed (Fig. 2). In the negative control group, crystals were identified as a mixture of abundant COM (Calcium oxalate monohydrate) crystals with monoclinic prismatic shapes or in twin form and only scattered a few COD (Calcium oxalate dihydrate) crystals with typical bipyramidal shapes. Crystals were large with COM showing a roughly orthorhombic structure. Meanwhile, the extracts (E1, E2, E3) and sodium citrate (SC) have impact on the structure, number, and size of crystals produced. The crystals of treatment groups exhibited smaller in size, decreases the number of crystals compared to the negative control, and had more COD crystals forms than COM cystals. This indicated that the crude extracts of M. balbisiana fruits had significant effects on calcium oxalate stones.
The photomicrograph showing activity of M. balbisiana fruit extracts on the calcium oxalate crystallization. The reactions were incubated with sodium citrate (SC, positive control) or 1000 μg/mL of hydroethanolic extract by maceration method (E1), hydroethanolic extract by hot extraction (E2), water extract by decoction method (E3), respectively. Scale bars = 50 µm
Inflammation is a symptom of urinary stone patients. So, anti-inflammatory agents make important contributions to the treatment of urinary stones. The results showed that the crude extracts of M. balbisiana fruits presented anti-inflammatory potential at the tested concentrations via the capacity to inhibit the protein denaturation, heat-induced hemolysis and hypotonicity-induced hemolysis. Similarly, the proportion of inhibition in the assays was concentration dependent and the hydroethanolic extract by hot extraction (E2) exhibited a relatively better activity (Fig. S1).
The E. coli bacterium is known to be a leading cause of urinary tract infections. For patients with urinary stones, an antibiotic regimen is essential. In this study, the hydroethanolic extract by hot extraction (E2) was demonstrated to be more effective against E. coli (Fig. S2).
Besides, cellular oxidative stress is one of the causes or consequences of many pathologies, including urinary stones, especially kidney cell oxidative stress. Thus, the antioxidant activity of crude extracts was also evaluated, showing this activity in three assays of the crude extracts was quite similar (Fig. S3).
More specifically, inhibitory values in experiments were calculated as a basis for the selection of the extract for in vivo testing (Table 1). Synthesizing the results, the hydroethanolic extract by hot extraction (E2) was selected as the potential extract for the follow-up trials in this study.
Acute toxicity test of hydroethanolic extract of M. balbisiana fruits on Swiss-albino mice
After giving the mouse the highest possible concentration (dose equivalent to 43.75 g extract/kg mouse b. w.), no abnormal appearance, activity and excretion were observed. Also, no dead mouse was found in the first 2 days. After 3 days, 05/10 mice died (mortality rate of 50%), other mice behave normally and no dead mice are found after 14 days. As the mortality rate was lower than 100%, LD100 (Lethal dose, 100%) and LD50 (Lethal dose, 50%) cannot be determined. However, the maximum dose which ensures normal activities—LD0 (Lethal dose, 0%) can be determined. Therefore, another tested that reduced dose by 20% was carried out to check the maximum safe dose.
Mice were then given the dose of 35.0 g extract/kg b. w. orally, no abnormal activities nor appearance were observed. After 3 days, 02/10 mice died (mortality rate of 20%) and other mice were normal and healthy. Mice were then given the dose of 28.0 g extract/kg b. w. orally, no abnormal activities nor appearance were observed. After 3-day and 14-day periods, all mice were healthy and behave normally (mortality rate of 0%).
Based on the result of oral acute toxicity, the maximum safe dose of the M. balbisiana fruit extract LD0 is 28.0 g extract/kg mouse weight (relevant absolute dried extract of 22.74 g extract/kg b.w.) but LD100, and LD50 cannot be determined. From the mortality rate, the LD50 was expected to be around 43.75 g extract/kg b. w.. Convert that dose into one for white Wistar rat and determine the safe dose for experiments on diuretic effect and prophylactic effect of urolithiasis (Safe dose for experiment equal 1/5 LD0 or 1/10 LD0). Traditionally, 30–50 g dried medicinal herbs are used to treat urolithiasis so the dose for the experiment on white Wistar rats equal 20 and 40 g of dried herbs (relevant ratio of 1/12 to 1/6 LD0) was 0.8 and 1.6 g/kg b. w., respectively.
Diuretic effect of hydroethanolic extract of M. balbisiana fruits on Wistar rats
In this study, the diuretic effect of hydroethanolic extract from M. balbisiana fruit was evaluated on Wistar rats with two doses 0.8 and 1.6 g/kg b. w. after 7 days on some main parameters such as urinary excretion and urine electrolytes concentration (Na+, K+, Cl−) (Fig. 3). The weight of rats of GII group (0.8 g/kg extract) and GIII group (1.6 g/kg extract) increase slightly but not significantly compared to the negative control group (GI). The rat weight in the positive control group (GIV, Furosemide 10 mg/kg) was significantly different compared to the GI group and not significantly different compared to the GII and GIII groups.
Effect of hydroethanolic extract of M. balbisiana fruits on urinary excretion and electrolytes concentration in rats after 7-day drinking (n = 6). GI: Group I, negative control group, given NaCl 0.9% (w/v); GII: Group II, given extract of 0.8 g/kg b. w./day in NaCl 0.9%; GIII: Group III, given extract of 1.6 g/kg b. w./day in NaCl 0.9%; GIV: Group IV, positive control group, given Furosemide 10 mg/kg b. w./day in NaCl 0.9%; nsp > 0.05: no significant, *p < 0.05; **p < 0.01: significant
The results about the urinary excretion showed that the GII group had no difference compared to the GI group while for GIII and GIV groups had much higher urinary excretion as for GI group. The electrolyte concentration of three type of electrolytes including Na+, K+, Cl− of the GII, GIII, and GIV groups was considerably different compared to the GI group.
From the analysis results in Table 2 showed that the hydroethanolic extract of M. balbisiana fruits at 0.8 g/kg had a medium effect with the diuretic index (DI) value of 1.19 (DI of 1–1.5). While the extract at 1.6 g/kg had a relatively good effect with a DI value of 2.03 (DI > 2) was near to the DI value of Furosemide 10 mg/kg. The diuretic activity (DA) of the hydroethanolic extract at 0.8 and 1.6 g/kg doses were 52% and 89%, respectively which are relatively high as compared to Furosemide 10 mg/kg.
Table 3 illustrated the results for natriuretic, saliuretic activity and carbonic anhydrase inhibition. The Furosemide (10 mg/kg) and hydroethanolic extract of M. balbisiana fruits at 0.8 and 1.6 g/kg doses showed potent saliuretic activity as compared to normal control. The hydroethanolic extract of M. balbisiana fruits at two investigated doses did not display natriuretic effect and carbonic anhydrase inhibition in this study.
In conclusion, hydroethanolic extract of M. balbisiana fruits exhibited a potential diuretic effect on the two tested doses 0.8 and 1.6 g/kg/day on white Wistar rat model after giving extract orally. Moreover, the 1.6 g/kg dose presented a better diuretic potential than the 0.8 g/kg dose and posed a nearly similar effect to the Furosemide 10 mg/kg dose.
Prophylactic effect of hydroethanolic extract of M. balbisiana fruits on urolithiasis on Wistar rats
Change in the bodyweight of Wistar rats of different groups was monitored in 3 periods including day 0, day 14 and day 28 (Fig. S4). These changes of bodyweight were not significant in all the experimental groups and at the chosen period. Thus, it guarantees the fact that drinking the crude extract at the two doses will not affect the bodyweight of the rat during the experiment.
In this study, Wistar rats were given the water with ethylene glycol 0.75% in 28 days to form urinary stones. The results showed that the pathological group (GII) depicted a significant decline in urinary excretion and urine magnesium concentration but a tremendous increment in urine calcium and phosphate concentration. Thus, drinking ethylene glycol 0.75% after 28 days could modify some parameters in the urinary biochemical index in evaluating urolithiasis according to Fig. 4.
Effect of hydroethanolic extract of M. balbisiana fruits on urinary excretion, calcium, phosphate, magnesium in urine and calcium, phosphate, urea nitrogen, creatinine in blood after 28 experimental days on ethylene–glycol-induced-urolithiasis rats (n = 6). GI: physiological group, given normal distilled water; GII: pathological group, given the ethylene glycol with normal distilled water; GIII: test group, given the ethylene glycol with extract 0.8 g/kg body weight/day; GIV: test group, given the ethylene glycol with extract 1.6 g/kg body weight/day; GV: positive control group, given the ethylene glycol with sodium citrate 2.5 g/kg body weight/day. nsp > 0.05: no significant, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001: significant
In terms of urinary excretion, rats in tested groups (GIII and GIV) and sodium citrate group released significant amount of water compared to physiological and pathological groups. There were no differences between the two tested groups and sodium citrate group. Thus, the hydroethanolic crude extract of M. balbisiana fruits of two doses exhibited the diuretic potential.
Regarding the urine calcium concentration, although GIII group (0.8 g/kg extract) was smaller than those in the pathological group, it was not significant. While urine calcium concentration in the GIV group (1.6 g/kg extract) and positive control group was lower than the pathological group. The dose of 1.6 g/kg of crude extract affected the reduction of calcium concentration on ethylene–glycol-induced-urolithiasis rats better than the dose of 0.8 g/kg of crude extract.
In terms of urine phosphate concentration, the rats in the tested groups and sodium citrate group did not reduce the phosphate concentration. Thus, the crude extract from M. balbisiana fruits at two investigating doses and sodium citrate group did not extend the effect on phosphate concentration.
Regarding the urine magnesium concentration, rats in tested groups and sodium citrate group increased significantly as compared to the pathological group. In which, the difference in magnesium concentration between GIII and the pathological group is the highest and followed by GIV and sodium citrate, respectively. Thus, the crude extract at two doses impacted on the magnesium concentration in urine on rats.
The concentration of blood phosphate, urea nitrogen, and creatinine of ethylene–glycol-induced-urolithiasis rats of the pathological group had significantly increased after drinking for 28 days compared to the physiological group. The blood calcium concentration of the pathological group witnesses a reverse trend. Thus, drinking ethylene glycol 0.75% for 28 days affected some of the serum biochemical indexes such as phosphate, urea, creatinine in evaluating urolithiasis.
The blood calcium concentration was not affected by drinking ethylene glycol 0.75% for 28 days. There were not tremendous differences among experimental groups in this parameter.
In terms of the serum phosphate and urea levels, although group GIII (0.8 g/kg) was smaller than those in the pathological group, it was not significant. Whereas group GIV (1.6 g/kg) and sodium citrate (2.5 g/kg) were significantly smaller than the pathological group. Thus, crude extract of M. balbisiana fruits reduced the phosphate concentration of ethylene–glycol-induced-urolithiasis rats.
Regarding the serum creatinine, the difference in rats in group GIII and sodium citrate compared to the pathological group was not significant. While the rats in group GIV had much lower serum urea level compared to the pathological group. Thus, the crude extract had the potential to reduce creatinine levels in the blood in these rats.
The kidneys of the rats were observed before processing to observe histopathology (Fig. S5). The rat kidneys of the physiological group had normal color and appearance, while the kidneys of the pathological group had relatively pale color with some white spots after 28 experimenting days. Rat kidneys in group GIII (0.8 g/kg) and sodium citrate (2.5 g/kg) were physiologically normal but the darker color compared to one from the pathological group but paler than physiological group, the white spot is not found. Rat kidneys in group GIV (1.6 g/kg) were not different from other groups.
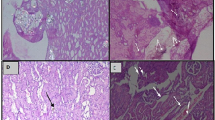
Kidney tissue of rat in the physiological group resembled normal structure in glomerular and renal tubules, and did not create any CaOx deposit or abnormalities. While for the kidney tissue of the pathological group, much CaOx deposition inside the renal tubules, obstructions, and dilatation of parenchymal blood vessels and of renal tubules, and inflammation in kidney slit were detected. The structures of the kidney tissue of rats in group GIII and GIV (0.8 and 1.6 g/kg) remained normal and no CaOx deposit, but with small inflammation at the kidney slit. Also, vacuole degenerations at some renal tubules were seen in the kidney sample of group GIII. The positive control (sodium citrate 2.5 g/kg) showed very optimistic results such as no abnormalities in renal tubules (Fig. 5). Therefore, the hydroethanolic extract of M. balbisiana fruits of 0.8 and 1.6 g/kg dosage had the effect of improving kidney histopathology in rat model causing urinary stone with ethylene glycol 0.75%.
Kidney histology of each experimental group after 28 days (×40). GI: physiological group, given normal distilled water; GII: pathological group, given the ethylene glycol with normal distilled water; GIII: test group, given the ethylene glycol with extract 0.8 g/kg b. w./day; GIV: test group, given the ethylene glycol with extract 1.6 g/kg b. w./day; GV: positive control group, given the ethylene glycol with sodium citrate 2.5 g/kg b. w./day
Discussion
Up to date, scientists have not found enough evidence about the acute toxicity of M. balbisiana fruits. The study results showed that the maximum dose of hydroethanolic extract of M. balbisiana through the needle of 43.75 g extract/kg mouse body weight (relevant to 35.53 g absolute dried extract/kg) is equal to 134.49 g dried fruit/kg (146.66 g absolute dried fruit/kg body weight) and is three-time higher than the used dose in treating urolithiasis, have the mortality rate of 50%. According to Globally Harmonized Classification System for Chemical Substances and Mixture (GHS), the LD50 of around 43.75 g extract/kg of the hydroethanolic extract of M. balbisiana belongs to group 5 (higher than 5 g/kg) and is nearly non-toxic. Moreover, with the yield of 29.83%, 29.83 g dried extract can be obtained from 100 g absolute dried material. Thus, to get 35.53 g absolute dried extract, 119.11 g absolute dried material is needed. Also, to produce 119.11 g absolute dried material, 384 g fresh material as 1 kg of fresh material gives 0.31 kg dried material. Knowing that 1 banana weight around 110 g and the maximum dose through the needle is 35.53 g extract/kg body weight (3.5 fresh banana/kg body weight of Swiss-albino mouse). For maximum safe dose LD0 we used in the experiment (28.0 g extract/kg) is relevant to 22.74 g/kg dried extract and 2.2 fresh banana/kg body weight. If one human weighed 50 kg uses 14.5 fresh bananas can be dangerous to his/her health but if use maximum of 9 fresh bananas would be good.
Diuretic agents are known for their tremendous effect on the increment of urinary volume and are used in treating edemata such as heart failure, kidney failure, and more. However, many diuretic agents nowadays have side effects such as under regulation of electrolytes (decline in serum K+, Na+ increment of acid uric), unbalance acid–base, and abnormal metabolic (increment of blood sugar and serum lipid) (Vazir and Cowie 2013). In this study on the Wistar rat, the crude extract of M. balbisiana at 1.6 g/kg dosage has shown similar diuretic effect to the loop diuretic Furosemide 10 mg/kg. Importantly, 2 the tested group (0.8 and 1.6 g/kg extract) and Furosemide have influenced significantly on the electrolytes’ concentration (Na+, K+, Cl−), those are three parameters that play an critical role in regulating urine excretion.
The Na+/K+ ratio illustrates the nature of diuretic activity and mechanism; if this ratio is greater than 1, the urine retains K+. The studies have shown that K+ concentration is higher than Na+ so all ratios are lower than 1. Thus, the crude extract exhibits diuretic activity in a non-saving potassium way. Since the Na+/K+ ratio of crude extract has a relatively similar effect compared to Furosemide, they may have a similar working mechanism. Based on saliuretic index, diuretic index, and diuretic activity, the diuretic activity depends on the dosage, in which dose of 0.8 g/kg has a medium effect (DI value range from 1 to 1.5) and dose of 1.6 has a good diuretic effect (DI value higher than 1.5). A previous study in Vietnam on M. balbisiana fruit extract has shown the dose of 35 mg/kg of the crude extract in non-polar medium results in a non-diuretic effect (DI 0.93 < 1); kerosene ether fractionation of 30 mg/kg dosage has DI values of 1.1 while 20% ethanol of 80 mg/kg has highest DI value of 1.48 (Bui 2006). The higher concentration of the crude extract, the higher the DI value. Besides, the diuretic activity of M. balbisiana fruit extract of 0.8 and 1.6 g/kg dosage is 52% and 89%, respectively compared to loop diuretic Furosemide 10 mg/kg. Therefore, hydroethanolic extract from M. balbisiana fruits belongs to the most potential medicinal herb having diuretic effects since they contain some of the most important phytochemicals such as polyphenols, terpenoids, saponins, and flavonoids (Junior et al. 2011; Schlickmann et al. 2018; Diniz et al. 2009).
In this study, the effect of hydroethanolic extract of M. balbisiana on urolithiasis disease was investigated in a model of Wistar rats with urinary stone induced by ethylene glycol. Ethylene glycol-induced calcium oxalate (CaOx) crystal deposition in the kidneys is a suitable animal model commonly used to simulate stone formation in humans. Many studies have proven that supplying ethylene glycol 0.75% with normal water to rats for 28 days induces kidney stones formation (Atmani et al. 2003; Divakar et al. 2010). Ethylene glycol is easily absorbed in the intestine and transfer into oxalate in the liver. As oxalate precipitate in urine in form of CaOx deposition in the nephron, it damages other tissues and initiates the crystal formation and aggregation processes (Thamilselvan et al. 2003; Scheid et al. 2004).
The decline in urinary excretion of the pathological group compared to the physiological group is due to the formation of some crystal stones in the kidney. Inducing the urinary excretion containing stones and crystal can reduce the situation (Khan et al. 1982). The crude extract from M. balbisiana fruit helps increase the urinary excretion in both pathological and physiological groups, diuretic activity is expected to have prophylactic effects on urolithiasis in this plant.
Since ethylene glycol changes into acidic substances such as hippuric acid, oxalic acid, formic acid, and benzoic acid that negatively affect the reabsorption of HCO3−. Reabsorption of calcium in the intestine and the release of calcium in our bone occur and ultimately led to the rise of calcium concentration in urine and serum. Besides, the increment of calcium in urine provides suitable room for crystal formation, especially in form of CaOx deposition. As the ethylene glycol used in my research did not cause the change in serum calcium concentration, the stones formation induced by it did not peak. Thus, the optimal ethylene glycol concentration which results in the highest increment in serum calcium should be examined. Furthermore, increasing serum and urine phosphate provide a suitable environment for the formation of calcium phosphate stones (Ngo and Assimos 2007). The results revealed that the 1.6 g/kg dose of crude extract reduced the urine calcium, phosphate, and serum phosphate concentration tremendously, it may therefore also contribute to a reduced risk of stone formation.
Hyperoxia is a more significant risk factor in the pathogenesis of kidney stones rather than hypercalciuria. Changes in oxalate concentration in urine are relatively much more significant and have an effect about 15 times greater than calcium. Studies have also shown that there is a significant increase in the oxalate concentration in the urine, an increase in the deposition of calcium and oxalate in the kidneys of the mouse caused by ethylene glycol (Robertson and Peacock 1980; Soundararajan et al. 2006). However, within the limits of this study, the oxalate concentration has not been determined, so later research it is necessary to determine the oxalate index on the model of the Wistar rats caused by ethylene glycol to have a comprehensive assessment effect of research subjects.
Besides, magnesium strongly inhibits the formation of CaOx deposition as it reduces over-saturation (Rushton and Spector 1982). Thus, the pathological group exhibits such decline in its concentration compared to the physiological group. The crude extract of 0.8 and 1.6 g/kg doses recover the urinary magnesium concentration. In urolithiasis, the glomerular filtration rate (GFR) decreases because stones in the urinary system interfere with the flow of urine. This results in a buildup of waste products in the blood, especially nitrogen-containing substances such as urea, uric acid and creatinine (Karadi et al. 2006). In this study, the crude extract at dose of 1.6 g/kg reduced urea and creatinine concentrations to reach statistical significance compared with the pathological group and give similar values to the physiological group.
Ethylene glycol helps creating many depositions of calcium and oxalate in kidney tissue by increasing the biological activities of oxide nitric and activating the cGMP (3′,5′-cyclic guanosine monophosphate). Moreover, ethylene glycol catalyzes the synthesis of different types of oxalates such as glycolic acid oxidase and lactate dehydrogenase, catalyzing the oxidation reaction of glyoxylate. Thus, creating a very suitable environment for the hyperoxia and formation and crystallization of CaOx deposition in nephron (Soundararajan et al. 2006). The appearance of many CaOx depositions on the kidney tissue of the pathological group leads to the renal tubules, obstructions, and dilatation of parenchymal blood vessels and renal tubules, and inflammation in kidney slit. While for rats in the extract-administered group, these situations reduced and structures remained similar to the physiological group.
The findings in this study showed that there is need for further fractionation and isolation of secondary phytochemicals responsible for the diuretic and anti-urolithiatic activities. Furthermore, although this research on M. balbisiana has proven the anti-urolithiatic activity, the potential is relatively low. Thus, scientists might investigate the combination of different medicinal sources together on treating urolithiasis.
Conclusion
The hydroethanolic extract of M. balbisiana fruits exhibited great potential in diuretic activity and prophylactic effect on urolithiasis. M. balbisiana fruits had several in vitro biological activities such as anti-urolithiatic, anti-inflammatory, antibacterial, and antioxidant activities.
References
Al-Saikhan FI, Ansari MN (2016) Evaluation of the diuretic and urinary electrolyte effects of methanolic extract of Peganum harmala L. in Wistar albino rats. Saudi J Biol Sci 23(6):749–753. https://doi.org/10.1016/j.sjbs.2016.01.025
Astutik S, Pretzsch J, Kimengsi JN (2019) Asian medicinal plants’ production and utilization potentials: a review. Sustainability 11(19):5483. https://doi.org/10.3390/su11195483
Atmani F, Slimani Y, Mimouni M, Hacht B (2003) Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int 92(1):137–140. https://doi.org/10.1046/j.1464-410x.2003.04289.x
Aziz SA, See TL, Khuay LY, Osman K, Bakar MAA (2005) In vitro effects of plantago major extract on urolithiasis. Malays J Med Sci 12(2):22–26
Bawari S, Sah AN, Tewari D (2018) Antiurolithiatic activity of Daucus carota: an in vitro study. Pharmacogn J 10(5):880–884. https://doi.org/10.5530/pj.2018.5.148
Borah M, Das S (2017) Antidiabetic, antihyperlipidemic, and antioxidant activities of Musa balbisiana Colla. in Type 1 diabetic rats. Indian J Pharmacol 49(1):71–76. https://doi.org/10.4103/0253-7613.201030
Bui ML (2006) Study on three medicinal herbs towards the treatment of kidney stones: Chuoi hot-Kim tien thao-Rau om. Dissertation, University of Medicine and Pharmacy of Ho Chi Minh City (Vietnamese document)
Butterweck V, Khan SR (2009) Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med 75(10):1095. https://doi.org/10.1055/s-0029-1185719
Coe FL, Evan A, Worcester E (2005) Kidney stone disease. J Clin Invest 115(10):2598–2608. https://doi.org/10.1172/JCI26662
Das M, Malipeddi H (2016) Antiurolithiatic activity of ethanol leaf extract of Ipomoea eriocarpa against ethylene glycol-induced urolithiasis in male Wistar rats. Indian J Pharmacol 48(3):270–274. https://doi.org/10.4103/0253-7613.182886
Deka P, Kashyap A, Sharma D, Baruah C (2018) A review on Musa balbisiana Colla. Int J Pharm Sci Invent 7(7):14–17
Devkar RA, Chaudhary S, Adepu S, Xavier SK, Chandrashekar KS, Setty MM (2016) Evaluation of antiurolithiatic and antioxidant potential of Lepidagathis prostrata: a Pashanbhed plant. Pharm Biol 54:1237–1245. https://doi.org/10.3109/13880209.2015.1066397
Diniz LRL, Santana PC, Ribeiro APAF, Portella VG, Pacheco LF, Meyer NB (2009) Effect of triterpene saponins from roots of Ampelozizyphus amazonicus Ducke on diuresis in rats. J Ethnopharmacol 123(2):275–279. https://doi.org/10.1016/j.jep.2009.03.006
Divakar K, Pawar A, Chandrasekhar S, Dighe S, Divakar G (2010) Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol 48(4):1013–1018. https://doi.org/10.1016/j.fct.2010.01.011
Evan AP, Coe FL, Lingeman JE, Worcester E (2005) Insights on the pathology of kidney stone formation. Urol Res 33(5):383–389. https://doi.org/10.1007/s00240-005-0488-0
Ingale KG, Thakurdesai PA, Vyawahare NS (2012) Effect of Hygrophila spinosa in ethylene glycol induced nephrolithiasis in rats. Indian J Pharmacol 44(5):639–642. https://doi.org/10.4103/0253-7613.100402
Junior AG, Gasparotto FM, Boffo MA, Lourenço ELB, Stefanello MÉA, Salvador MJ, da Silva-Santos JE, Marques MC, Kassuya CA (2011) Diuretic and potassium-sparing effect of isoquercitrin—an active flavonoid of Tropaeolum majus L. J Ethnopharmacol 134(2):210–215. https://doi.org/10.1016/j.jep.2010.12.009
Karadi RV, Gadge NB, Alagawadi KR, Savadi RV (2006) Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 105:306–611. https://doi.org/10.1016/j.jep.2005.11.004
Khan SR, Finlayson B, Hackett R (1982) Experimental calcium oxalate nephrolithiasis in the rat. Role of the renal papilla. Am J Pathol 107(1):59–69
Kumari S, Katare PB, Elancheran R, Nizami HL, Paramesha B, Arava S, Sarma PP, Kumar R, Mahajan D, Kumar Y, Devi R, Banerjee SK (2020) Musa balbisiana fruit rich in polyphenols attenuates Isoproterenol-induced cardiac hypertrophy in rats via inhibition of inflammation and oxidative stress. Oxidative Med Cell Longevity 2020:7147498. https://doi.org/10.1155/2020/7147498
Ly HT, Le MH, Lam BT, Le DT, Pham TH, Nguyen MK, Le VM (2020a) Pharmacognostical standardization, phytochemical analysis, and antioxidant activity of Musa balbisiana colla fruits. Int J Res Pharm Sci 11(4):1–12. https://doi.org/10.26452/ijrps.v10i3
Ly HT, Nguyen PMT, Nguyen TKO, Bui TPQ, Ke X, Le VM (2020b) Phytochemical analysis and wound-healing activity of Noni (Morinda citrifolia) leaf extract. J Herbs Spices Med Plants 26(4):379–393. https://doi.org/10.1080/10496475.2020.1748159
Ly HT, Le VKT, Nguyen TM, Phan TAD (2021) Effect of different polarity solvents on the anti-inflammatory activity of Symplocos cochinchinensis leaves and correlation with total polyphenol content. Vietnam J Chem 59(1):106–114. https://doi.org/10.1002/vjch.202000136
Ministry of Health (2015) Guidance on pre-clinical and clinical trials of oriental medicines and herbal medicines. Issued under Decision No. 141/QĐ-K2ĐT dated October 27, 13–17 (Vietnamese document)
Ngo TC, Assimos DG (2007) Uric acid nephrolithiasis: recent progress and future directions. Rev Urol 9(1):17–27
Nguyen DNV, Nguyen T (2008) An overview of the use of plants and animals in traditional medicine systems in Viet Nam. Traffic Southeast Asia, Greater Mekong Programme, Ha Noi, Viet Nam
Organization for Economic Co-Operation and Development (2001) Guideline for testing of chemicals, acute oral toxicity—acute toxic class method. Guideance, no. 425
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
Panigrahi PN, Dey S, Sahoo M, Dan A (2017) Antiurolithiatic and antioxidant efficacy of Musa paradisiaca pseudostem on ethylene glycol-induced nephrolithiasis in rat. Indian J Pharmacol 49(1):77–83. https://doi.org/10.4103/0253-7613.201026
Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR (2012) Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. & Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 144(1):160–170. https://doi.org/10.1016/j.jep.2012.08.043
Pellegrini N, Serafini M, Colombi B, Rio DD, Salvatore S, Bianchi M, Brighenti F (2003) Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr 133(9):2812–2819. https://doi.org/10.1093/jn/133.9.2812
Ponnambalam H, Sellappan M (2014) ICP-MS technique for quantification of potassium and sodium in spray-dried extract of shoot juice of banana plant (Musa balbisiana) responsible for anti-urolithiatic and diuretic activity. Int J Med Chem Anal 4(3):170–174
Robertson WG, Peacock M (1980) The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron 26:105–110. https://doi.org/10.1159/000181963
Rushton HG, Spector M (1982) Effects of magnesium deficiency on intratubular calcium oxalate formation and crystalluria in hyperoxaluric rats. J Urol 127:598–604. https://doi.org/10.1016/s0022-5347(17)53920-8
Scheid CR, Cao LC, Honeyman T, Jonassen JA (2004) How elevated oxalate can promote kidney stone disease: changes at the surface and in the cytosol of renal cells that promote crystal adherence and growth. Front Biosci 1(9):797–808. https://doi.org/10.2741/1265
Schlickmann F, Boeing T, Mariano LNB, da Silva LM, de Andrade SF, de Souza P, Cechinel-Filho V (2018) Gallic acid, a phenolic compound isolated from Mimosa bimucronata (DC.) Kuntze leaves, induces diuresis and saluresis in rats. Naunyn-Schmiedeberg’s Arch Pharmacol 391(6):649–655. https://doi.org/10.1007/s00210-018-1502-8
Soundararajan P, Mahesh R, Ramesh T, Begum VH (2006) Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol 44:981–986
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31(1):3–9. https://doi.org/10.1007/s00240-002-0286-x
Vazir A, Cowie M (2013) The use of diuretics in acute heart failure: evidence based therapy? World J Cardiovasc Dis 3:25–34. https://doi.org/10.4236/wjcd.2013.32A004
Velu V, Das M, Raj NA, Dua K, Malipeddi H (2017) Evaluation of in vitro and in vivo anti-urolithiatic activity of silver nanoparticles containing aqueous leaf extract of Tragia involucrata. Drug Deliv Transl Res 7(3):439–449. https://doi.org/10.1007/s13346-017-0363-x
Vyas B, Vyas R, Joshi S, Santani D (2011) Antiurolithiatic activity of whole-plant hydroalcoholic extract of Pergularia daemia in rats. J Young Pharm 3(1):36–40. https://doi.org/10.4103/0975-1483.76417
Welu GG, Yimer EM, Hailu HG, Bhoumik D, Lema MM (2020) In Vivo diuretic activity of hydromethanolic extract and solvent fractions of the root Bark of Clerodendrum myricoides Hochst. (Lamiaceae). Evid-Based Complement Altern Med 2020:1–9. https://doi.org/10.1155/2020/1718708
Zarin M, Tan J, Ahmad R, Jin N, Aziz N (2020) Determination of nucleation assay for anti-urolithiasis activity from bagasse Musa acuminate x balbisiana Colla cv. Pisang Awak Legor methanolic extracts using uv-spectrometer and size measurement. IOP Conf Ser Mater Sci Eng 716:012018. https://doi.org/10.1088/1757-899X/716/1/012018
Zeng X, Xi Y, Jiang W (2019) Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: a review. Crit Rev Food Sci Nutr 59(13):2125–2135. https://doi.org/10.1080/10408398.2018.1439880
Zhou J, Jin J, Li X, Zhao Z, Zhang L, Wang Q, Li J, Zhang Q, Xiang S (2018) Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-L-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis 46(3):231–241. https://doi.org/10.1007/s00240-017-0985-y
Acknowledgements
The study was supported by The Youth Incubator for Science and Technology Programe, managed by Youth Development Science and Technology Center—Ho Chi Minh Communist Youth Union and Department of Science and Technology of Ho Chi Minh City, the contract number is “01/2019/HĐ-KHCN-VƯ”; and the Ministry of Science and Technology for the financial support under Grant Number NVQG-2017/23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All animal experimental procedures were performed according to the Guide for the Care and Use of Laboratory Animals of Organization for Economic Cooperation and Development-OECD, as well as the guidelines of the Animal Welfare Act.
Conflict of interest
Ly Hai Trieu has no confict of interest. Le Thi Kim Oanh has no confict of interest. Nguyen Minh Khoi has no confict of interest. Le Van Minh has no confict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ly, H.T., Le, T.K.O., Nguyen, M.K. et al. Diuretic efficacy and prophylactic effects of hydroethanolic extract from Musa balbisiana fruits against urolithiasis. ADV TRADIT MED (ADTM) 22, 823–836 (2022). https://doi.org/10.1007/s13596-022-00629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-022-00629-3