Abstract
Nanobacteria have been isolated from kidney stones and it has been suggested that they may act as a nucleus for the initiation of the renal stones. In the present study, we examine their role in biocrystallization and their in vivo effects on kidney pathology. Calcium oxalate monohydrate (COM) assay was carried out in the presence of nanobacteria to study biocrystallization. Wistar rats were given an intravenous injection of nanobacteria and the kidneys were examined for pathological changes. The COM assay showed accelerated biocrystallization of 14C-oxalate in the presence of nanobacteria, indicating them to be efficient candidates for biomineralization. Histopathological studies revealed bacteria induced renal tubular calcifications and various manifestations of infection. Our studies confirm that nanobacteria may be involved in the pathogenesis of renal tubular calcification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biocrystallization of calcium is encountered in many diseases such as kidney stone disease, atherosclerosis, chronic pancreatitis, rheumatoid arthritis, and various other tissues in the body [1]. Several theories have been put forward to explain the etiology of renal calcification but none has been able to answer fully the questions concerning the genesis of renal calculi [2]. This is due to the fact that several factors such as hypercalciuria, hyperoxaluria, stone matrix, promoter and inhibitor molecules, and bacteria, etc. may be involved in the etiology of this disease. These molecules may alter or modify crystal formation or may provide preexisting surfaces for biocrystallization [3–7]. Bacteria and other cell types can create nucleation sites or nucleus, which is considered to be central in the genesis of kidney stones [8, 9]. It has been shown that microorganisms could be a factor in dental plaque calcium nucleation and the same may be true for urinary lithiasis [7, 19, 20].
Recently, it was reported that a novel bacteria, nanobacteria, may be involved in the genesis of renal stones. These bacteria have been isolated from renal stones [9, 10]. These are the smallest described bacteria to date and are phylogenetically close relatives of mineral forming bacteria [11, 12]. Nanobacteria have been shown to mineralize calcium and phosphate even under physiological conditions, indicating that these bacteria could have a contributory role in urolithiasis [13, 14]. It has been proposed that nanobacteria may serve as biomineralization centers as they produce carbonate apatite on their cell walls for the initiation of kidney stones.
Cuerpo et al. [15] showed that when these bacteria were injected intravenously, they accumulate in the kidney and produced apatite. These bacteria have been shown to accumulate in the kidney [16]; however, studies on in vivo effects of these bacteria on the kidneys are lacking. In our study we investigated the effects of intravenous administration of nanobacteria on kidney in Wistar rats. We found evidence of their inflammatory infiltration and accumulation in the cortex; in addition, small uniform renal calcification was seen on the surface of renal tubules. It could be that the kidney is a preferred site for mineralization by these tiny bacteria. It is also plausible that other body calcifications could have a nanobacterial element.
Materials and methods
The nanobacterial cultures were produced according to the method of Ciftcioglu et al. [10]. Subcultures were carried out in serum free RPMI-1640 (HiMedia Laboratories, Mumbai, India) after 4 weeks of initial inoculation and kept under tissue culture conditions (37°C, 5% CO2, and 95% air). The cultures were harvested by centrifugation at 20,000 g for 30 min at 4°C, washed with phosphate buffered saline (PBS, pH 7.2), and freshly used for experimental work.
For energy dispersive X-ray microanalysis (EDX), a 30-day old nanobacterial culture was harvested by centrifugation at 20,000 g for 30 min at 4°C. The bacterial pellet (biofilm) was washed in a phosphate buffered solution (pH 7.2). The biofilm was dehydrated in absolute alcohol and air dried overnight. The sample was further processed for Scanning electron micrographs (SEM) with EDX analysis system (S-360 Leiche Cambridge, UK, ISIS-300).
In vitro calcium oxalate monohydrate assay
Preparation of COM seed crystals
Calcium oxalate monohydrate (COM) seed crystals were prepared by the method of Pak et al. [17] by mixing equal volumes of 0.01 M CaCl2.2H2O and 0.01 M sodium oxalate by drop wise addition of sodium oxalate solution to calcium chloride solution with constant stirring for 1 week at 4°C. The solution was centrifuged at 2,000 g for 10 min at room temperature. The crystal pellet was washed with distilled water followed by methanol and these crystals were air dried before their use for further studies.
Preparation of seed crystal slurry
The crystal slurry was prepared by equilibrating seed crystals in 50 mM sodium acetate buffer (pH 5.7) containing 96 mM NaCl at 37°C for 2 h with constant stirring prior to use.
COM crystal growth assay
The crystal growth was measured by the method of Nakagawa et al. [18]. The reaction mixture contained 10 ml of 2 mM CaCl2, seed slurry (1.5 mg/ml), and 10 ml of 0.4 mM sodium oxalate containing 10 μl of 14C-oxalate (0.5 μCi) (BARC, Mumbai, India). The 500 μl (PBS) of purified nanobacterial pellet was inoculated into this mixture. This reaction mixture was incubated at 37°C for 15 min with constant stirring. The rate of decrease of the amount of radiolabelled 14C-oxalate at different time intervals was determined by measuring the counts in the infiltrate, after the reaction mixture had been filtered through 0.2 μm Millipore filter. After filtration, the 100 μl of each reaction mixture were mixed into 7 ml of Bray’s scintillation fluid (POPOP) at different time intervals and the radioactivity counts in the infiltrate were measured with a scintillation counter (LKB1214 Rack beta) at different time intervals for a period of 72 h. The reaction mixture, without bacterial suspension, served as a control. The result was expressed as percent decrease in radioactivity/mg of protein.
Lithogenesis of rats
The experimental model of nephrolithiasis was produced in male Wistar rats as described below. The male Wister rats (n=3) of 150–200 gm body weight were on normal rat diet and water, and were injected intravenously with nanobacteria (0.19 mg of bacterial protein/0.4 ml normal saline). The control group (n=3) were administered normal saline without nanobacteria. After 8 weeks, the animals were killed, and the kidneys were removed and processed for histopathological examination. The kidney sections were also processed for SEM studies.
Results
Energy dispersive X-ray microanalysis of nanobacterial pellet obtained after 30 days of subculture was analyzed for its inorganic chemical constituents using EDX. The nanobacterial pellet showed high content of Na, Ca, P, Mg etc. The predominant components were found to be calcium (32%) and phosphorus (28%) (Fig. 1).
The COM crystallization assay was carried out to examine the effect of nanobacteria on calcium oxalate incorporation in vitro. A decrease in residual radioactivity 14C counts of the filtrate in the presence of nanobacterial pellet was more than that in the control without nanobacteria (Fig. 2).
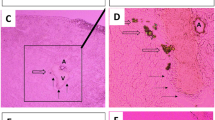
The histological sections of the kidney showed focal areas of chronic inflammatory infiltrate in the cortex and medulla (Fig. 3). Glomeruli were normal but the tubular epithelial cells revealed degenerative changes (Fig. 3). In addition, there were small uniform calcifications on the surface of renal tubular epithelium as evidenced by von Kossa staining (Fig. 4). Scanning electron microscopy revealed nanobacteria adhered to the surface of the epithelium and their penetrance into the renal epithelial cells (Fig. 5).
Longitudinal sections of Wistar rat kidney exhibiting various pathological manifestations. a Interstitial inflammation by lymphomononuclear cells in the medulla; magnification 280×. b Focus of epithelial cell lysis; magnification 280×. c, d Focus of interstitial inflammation and fibrosis in the renal cortex; magnification 140×
Longitudinal sections of Wistar rat kidneys showing renal calcifications. Magnification, 280×. a Photomicrograph shows renal calcification in the inner surface of tubules. b Photomicrograph shows calcifications in the inner surface of tubules obtained by von Kossa staining. c Photomicrograph shows extracellular calcifications in the renal tubules obtained by von Kossa staining. d Control did not reveal any calcification
Scanning electron micrographs. Scanning electron microscopic analysis showing nanobacteria like structures on the renal tubular cell surfaces. Their diameter was between 50 and 200 nm. Bar 1 μm. a Renal epithelial cell showing adhered nanobacteria like structures. Magnification, 11,000×. b SEM showing internalization view of nanobacteria into renal tubular epithelial cells. Magnification, 12,000×. c Control samples did not reveal any nanobacteria. Magnification, 12,000×
Discussion
Recently, nanobacteria have been linked to the etiology of kidney stone disease as they can produce sufficient calcium apatite in their cell walls to initiate pathologic calcifications and stone formation [6, 8]. It has been suggested that nanobacteria may serve as nucleus for mineral deposition and may lead to genesis of renal calculi.
In order to see if nanobacteria could accelerate crystal growth we carried out an in vitro assay, COM assay. We observed a significant increase in 14C-oxalate incorporation into the calcium seed crystals in the presence of nanobacterial pellet. Earlier, we have reported significant calcium (Ca2+) uptake by these bacteria in a growing culture [9]. These bacteria have also been found to mineralize calcium and phosphate at physiological conditions [13, 14]. Thus, it appears that these bacteria are biomineralizing calcium that could lead to apatite formation. The EDX analysis of bacterial pellet also showed the presence of calcium (Ca2+) and phosphorus (P) as main constituents in these bacteria indicating that these bacteria are rich in these minerals. Calcium (Ca2+) and phosphorus (P) are important chemical constituents of hydroxyapatite. These results further confirm the mineral forming activities of nanobacteria and support biocrystallization, a hallmark of these bacteria.
Akerman et al. [16] reported that radiolabeled, viable nanobacteria accumulated in the kidney and appeared in urine within 15 min of their intravenous injection into rabbits. This could be due to the fact that kidneys are the preferred sites for these bacteria, and the presence of injured epithelium or a nucleus in the kidney/urinary tract provides a preferable niche for these bacteria to adhere and grow, resulting in biocrystallization. We too observed that nanobacteria, when injected intravenously into Wistar rats, were localized in the kidneys and produced renal tubular calcifications as evidenced by von Kossa staining. These results are similar to the early reports of Cuerpo et al. [15] who have demonstrated genesis of renal calculi in Brown Norway rats after administration of nanobacteria, thereby indicating their contributory role in the urolithiasis. In addition, we observed regions of chronic inflammatory infiltrate in the cortex and medulla, which could be due to damage induced by these bacteria. Glomeruli were normal but the tubular epithelial cells showed degenerative changes, which is again indicative of bacterial infiltration. The SEM showed these bacteria adhering to the surface of the epithelium and their penetrance into the renal epithelial cells, which again confirms increased bacterial affinity for the kidney epithelial surfaces, which then serve as nucleus on which these bacteria grow. Thus, our study shows that nanobacteria may induce renal tubular extracellullar calcifications and histopathological manifestations.
References
Carson DA (1998) An infectious origin of extraskeletal calcification. Proc Natl Acad Sci USA 95:7846–4747
Resick MI, Boyce WH (1979) Aetiological theories of renal lithiasis: a historical review. In: Wickham JEA (ed) Urinary calculous disease, chap 1. Churchill Livingstone, New York, pp 1–20
Fleisch H (1978) Inhibitors and promoters of stone formation. Kidney Int 13:361–371
Lanzalaco AC, Singh RP, Smesko SA (1988) The influence of urinary macromolecules on calcium oxalate monohydrate crystal growth. J Urol 139:190–195
Asplin J, DeGannelli S, Nakagawa Y, Coe FL (1991) Evidence that nephrocalcin and urine inhibit nucleation of calcium oxalate nephrolithiasis. Am J Physiol 260:569–578
Sharma SK, Khuller M, Singh SK, Sharma M, Sheikh F, Pratibha, Sherawat B, Nath R (2001) Role of nanobacteria in renal stone diseases. 19th World Congress on Endourology and SWL 17th Basic Research Symposium, November 14–17. Bangkok, Thailand
Khan SR, Patricia A, Glenton, Backov R, Daniel R, Talham (2002) Presence of lipids in urine, crystals and stones: implications for the formation of kidney stones. Kidney Int 62:2062–2072
Kajander EO, Ciftcioglu N (1998) Nanobacteria: an alternative mechanism for pathogenic intra and extra cellular calcification and stone formation. Proc Natl Sci USA 95:8274–8279
Khullar M, Sharma SK, Singh SK, Bajwa P, Sheikh FA, Sharma M (2004) Morphological and immunological characteristics of nanobacteria from human renal stones of north Indian population. Urol Res 32:190–195
Ciftcioglu N, Bjorklund M, Kuorikoski K, Bergstrom K, Kajander EO (1999) Nanobacteria: an infectious cause of Kidney stone formation. Kidney Int 56:1893–1898
Drancourt M, Jacomo V, Lepidi H, Lechevallier E, Grisoni V, Coulange C, Ragni E, Alasia C, Dussol B, Berland Y, Raoult D (2003) Attempted isolation of Nanobacterium Sp microorganisms from upper urinary tract Stones. J Clin Microbiol 41:368–372
Wood AP, Kelly DP (1985) Int J Syst Bacteriol 35:434–437
Kajander EO, Bjorklund M, Cifcioglu N (1998) Mineralization by nanobacteria. Proc SPIE Int Soc Opt Eng 3441:86–94
Cifcioglu N, Bjorklund M, Kajander EO (1998) Stone formation and calcification by nanobacteria in human body. Proc SPIE Int Soc Opt Eng 3441:105–111
Cuerpo EG, Kajander EO, Ciftcioglu N, Lovaco CF, Correa C, Gonzalez J, Mampaso F, Liano F, Garcia E, Escudero BA (2000) Nanobacteria: an experimental neo-lithogenesis model. Arch Esp Urol 53:291–303
Akerman KK, Kuikka JT, Ciftcioglu N (1997) Radiolabeling and in vivo distribution of nanobacteria in rabbit. Proc SPIE Int Soc Opt Eng 3111:436–442
Pak CYC, Ohata M, Holt K (1975) Effect of diphosphate on crystallization of calcium oxalate in vitro. Kidney Int 7:154
Nakagawa Y, Ahmed MA, Hall SL (1987) Isolation from human calcium oxalate renal stones nephrocalcin, a glycoprotein inhibition of calcium oxalate crystal growth. J Clin Invest 79:1782
Slomiany BL, Murty VLN, Aono M (1982) Lipid composition of the matrix of human submandibular salivary gland stones. Arch Oral Biol 27:673–677
Boskey AL, Boyan-Salyers BD, Burnstein LS, Mandel ID (1981) Lipids associated with mineralization of human submandibular gland sialoliths. Arch Oral Biol 26:779–785
Acknowledgement
We acknowledge Professor K. Joshi for histopathological work and scientific interpretation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiekh, F.A., Khullar, M. & Singh, S.K. Lithogenesis: induction of renal calcifications by nanobacteria. Urol Res 34, 53–57 (2006). https://doi.org/10.1007/s00240-005-0034-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-005-0034-0