Abstract
Purpose
Perimesencephalic hemorrhage (PMH) is a benign subtype of nonaneurysmal subarachnoid hemorrhage (SAH). We aimed to investigate if cerebral perfusion in PMH is less affected than in aneurysmal SAH (aSAH).
Methods
From a prospective cohort of 80 patients with spontaneous SAH, we included PMH patients (n = 15) and selected aSAH patients (n = 39) with similar clinical grade at admission (World Federation of Neurosurgeons Scale-WFNS I/II). Computed tomography (CT) perfusion was performed at < 72 h and/or at 8–10 days. Cerebral perfusion parameter values were compared between groups with nonparametric tests. Subgroup analyses compared PMH and aSAH patients stratified according to aneurysmal location (anterior or posterior circulation) and blood burden (Fisher grade).
Results
At < 72 h, no significant differences in perfusion parameters were found between PMH and aSAH patients. At 8–10 days, PMH patients had lower MTT than aSAH patients, and a trend for higher CBF. PMH patients had higher CBF and CBV at < 72 h when compared to posterior circulation aSAH patients. When compared to aSAH patients with similar blood burden, PMH patients had higher CBF and lower MTT at < 72 h, and lower MTT at 8–10 days.
Conclusion
PMH patients had better cerebral perfusion compared to patients with aSAH, particularly during the vasospasm time window. After stratifying for the amount of blood, PMH patients also had better cerebral perfusion in the first 72 h after SAH. These results are in line with the better clinical presentation and prognosis of PMH, and possibly with a different etiology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perimesencephalic subarachnoid hemorrhage (PMH) is a distinct clinical and radiological subtype of subarachnoid hemorrhage (SAH) with blood typically circumscribed to the perimesencephalic cisterns, eventually extending to the ambiens cistern and proximal sylvian fissure [1], comprising around 5–10% of all cases of spontaneous SAH [2]. Patients with PMH tend to have better clinical condition on admission and a relatively benign course, without the severe complications of aneurysmal SAH (aSAH), such as vasospasm and hydrocephalus [1, 2]. Considering the differences above, PMH is considered etiologically distinct from aSAH, with some reports suggesting a venous instead of an arterial origin [3, 4]. However, even with better clinical prognosis, long-term cognitive and mood disorders are described in PMH patients [5,6,7].
The acute rise in intracranial pressure caused by aneurysm rupture may contribute to decrease in cerebral perfusion immediately after aSAH [8]. After the first 3 days post aSAH, vasospasm may ensue, affecting proximal and distal arteries, reducing cerebral perfusion. The pathophysiological mechanisms leading to vasospasm in aSAH are multiple and not fully understood, but they appear to relate to the presence of arterial oxyhemoglobin in the subarachnoid space [8, 9].
It is known that vasospasm is less frequent in PMH patients, and if it occurs, it has less clinical impact [10]. Previous research based on CT perfusion (CTP) suggested that cerebral blood flow (CBF) is higher in PMH patients compared to aSAH in the first 72 h after SAH [11]. However, differences in cerebral perfusion during the vasospasm time window have not been evaluated in PMH patients. We hypothesize that cerebral perfusion in PMH is less affected than in aneurysmal SAH (aSAH).
We therefore aimed to evaluate if cerebral perfusion parameters in the acute phase (< 72 h) or during the vasospasm period are different in PMH patients as compared to aSAH patients, matched by clinical condition at admission.
Methods
Patients
All patients with acute spontaneous SAH admitted at Centro Hospitalar de Lisboa Central between May 2013 and November 2014 were enrolled in a prospective cohort study approved by our institutional review board. Inclusion criteria for the main study were as follows: (1) age > 18 years, (2) acute non-traumatic SAH diagnosed by CT and/or lumbar puncture performed within the first 72 h of SAH onset, and (3) informed consent obtained from patient or legal representative. Patients in a very poor clinical condition (GCS 3), pregnant women, patients with renal insufficiency, and patients with any contraindication to perform MRI or whose time of onset of SAH was unknown were excluded.
For the current study, we included all PMH patients and aSAH patients matched by clinical grade at admission (World Federation of Neurological Surgeons (WFNS) scale I or II). PMH was defined as SAH limited to the cisterns around the midbrain, or not extending beyond the proximal Sylvian or interhemispheric fissures, with no parenchymal extension, and with no aneurysm documented on conventional angiogram (DSA) [1]. Aneurysmal SAH was considered whenever the presence of an aneurysm relating to the hemorrhage was confirmed by CT angiography or DSA.
Clinical and imaging data
Demographic data and clinical presentation were collected from the patients’ medical records. The neurological status at admission was evaluated using the Glasgow Coma Scale (GCS), WFNS, and Hunt & Hess scale (HH). The amount of blood in brain CT scan was assessed using the modified Fisher scale [12] and the Hijdra scale [13].
Other variables collected included the presence of hydrocephalus (known to reduce cerebral perfusion in SAH [14]), the location of aneurysm for aSAH patients, the occurrence of delayed cerebral ischemia (DCI), and outcome at discharge and at 3 months quantified by the Modified Rankin Scale (mRS).
The presence of hydrocephalus was defined as a bicaudate index above the 95th percentile for age [15], occurring at any time between admission and discharge. Patients were classified as having DCI if (1) presenting with a new focal neurological deficit/decrease in level of consciousness non-attributable to other causes (for example, hydrocephalus, seizures, metabolic derangement, infection, or sedation), (2) there was a new infarct on follow-up CT/MR imaging, or (3) both 1 and 2, after 4 days post ictus [16, 17].
Because similar PMH patterns of blood distribution may occur in posterior circulation aneurysm rupture, we stratified aSAH patients in anterior or posterior circulation aneurysms. Anterior circulation aneurysms were defined as aneurysms from the carotid circulation (anterior communicating artery, middle cerebral artery, and supraclinoid carotid artery including posterior communicating artery). Posterior circulation aneurysms were defined as originating from the vertebro-basilar circulation (PICA, AICA, SCA, and basilar tip).
We also stratified PMH and aSAH patients according to Fisher grade on admission (Fisher grades 2 and 3 versus grade 4), in order to match blood burden.
CT perfusion protocol
CTP studies were performed either at < 72 h (CTP-1), or at 8–10 days (CTP-2) after SAH. All CTP studies were performed on the same 64-multidetector CT scanner (Lightspeed VCT; GE Healthcare, Milwaukee, WI, US). For the CTP scan, a bolus of 40 ml of nonionic contrast agent (Ultravist 300 mg iodine/ml, Bayer Healthcare, Berlin, Germany) was injected intravenously at a rate of 4 ml/s, followed by a 40 ml saline flush at the same rate, using a dual power injector. The following parameters were used: 80kVp, 400mAs, 64 × 0.625 collimation, 520 × 520 matrix, FOV 250 mm, 272 image acquisition in 46 s.
Post processing
CTP post-processing and quantitative evaluation was performed by one neuroradiologist (IF), using OLEA software (La Ciotat, France). The arterial input function (AIF) and the venous output function (VOF) were manually defined at the A2 segment of the anterior cerebral artery and at the superior sagittal sinus, respectively. Hemodynamic maps were calculated using the block circulant singular value decomposition deconvolution method [18]. A total of 19 regions of interest (ROI) were predefined bilaterally and symmetrically covering arterial territories of the anterior, middle, and posterior cerebral artery, and at midpons and subcortical cerebellar hemispheres (Fig. 1). For each ROI, mean quantitative values of CBF, CBV, and MTT were obtained. Global cerebral perfusion was calculated using the mean of all ROIs for each parameter. Measurements avoided areas of parenchymal hematoma and ventricular drainage trajectory.
Statistical analysis
Patients were described using the mean (standard deviation) or median (minimum, maximum) for continuous variables, and the frequencies (percentages) for categorical variables. To compare cerebral perfusion values between the PMH and aSAH patients, CBF, CBV, and MTT mean values were calculated with 95% confidence intervals (CI). Nonparametric Mann-Whitney test, Chi-square test, and Fisher’s exact test were used, as appropriate. Differences between groups were considered significant for p < 0.05. We performed subgroup analysis, comparing PMH and aSAH patients, stratified accordingly to aneurysmal location (anterior or posterior circulation) and blood burden (Fisher grade).
Results
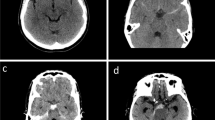
From the full cohort of 80 patients of the main prospective study, we included 15 patients with PMH and 39 aSAH patients matched by clinical status at admission (WFNS I and II). The mean age did not significantly differ between PMH and aSAH patients. PMH patients were more frequently male; they had less blood burden on admission CT and significantly less DCI than aSAH patients. Outcome at discharge and at 3 months was better in PMH patients (Table 1). Fifteen PMH and 36 aSAH patients had CT perfusion at < 72 h, 11 PMH and 25 aSAH patients had CT perfusion at 8–10 days (Fig. 2). Representative non-enhanced CT and CBF color maps of a PMH patient and an aneurysmal SAH patient are depicted in Fig. 3.
CT perfusion studies performed at < 72 h did not show significant differences in any of the perfusion parameters between PMH and aSAH patients. At 8–10 days, PMH patients had lower values for MTT (6.1 s, 95% CI 5.4–6.7 versus 8.1 s, 95%CI 6.7–9.5, p < 0.001), and had a trend for higher CBF values (31.9 ml/100 g/min, 95% CI 24.0–39.8 versus 24.8 ml/100 g/min, 95% CI 21.9–27.6, p = 0.07) (Table 2).
Stratified analysis for aneurysm location showed no differences in CTP parameters between PMH patients and SAH patients with anterior circulation aneurysms (results not shown). However, comparing with posterior circulation aneurysms, PMH patients had higher CBF (25.6 ml/100 g/min, 95% CI 20.2–31.0 versus 18.3 ml/100 g/min, 95% CI 13.7–23.0, p = 0.04) and CBV (3.0 ml/100 g, 95% CI 2.7–3.3 versus 2.3 ml/100 g, 95% CI 1.8–2.8, p = 0.02) at < 72 h, and the same trend was observed at 8–10 days although not reaching statistical significance (Table 3).
Stratified analysis for comparable amounts of blood showed that, in Fisher grade 4 patients, at < 72 h, CBF was significantly higher in PMH patients (29.23 ml/100 g/min, 95% CI 14.85–43.60 versus 22.57 ml/100 g/min, 95% CI 17.74–27.39, p = 0.047), and MTT was significantly lower in PMH patients (6.25 s, 95% CI 5.03–7.47 versus 7.58 s, 95%CI 6.87–8.29, p = 0.005). At 8–10 days, in Fisher grades 2 and 3 patients, MTT was significantly lower in PMH patients (5.95 s, 95% CI 4.21–7.69 versus 6.95 s, 95% CI 5.54–8.35, p = 0.026).
Discussion
In our study, although we could not demonstrate an overall better perfusion in the acute phase (< 72 h) of PMH patients, we have clearly shown that patients with PMH had better cerebral perfusion than patients with aneurysmal SAH during the time window for vasospasm, at 8–10 days. Besides, after stratifying for similar amounts of blood, cerebral perfusion was better in PMH than in aSAH patients in both moments. Patients with SAH after rupture of posterior circulation aneurysms, sharing a similar distribution of subarachnoid blood as PMH, showed a trend for worse cerebral perfusion both early in the course of hemorrhage (< 72 h), as well as later at 8–10 days.
Cerebral perfusion changes have been described both on the acute phase of SAH [19,20,21,22], and during the time window for vasospasm [20, 23,24,25]. Using CTP studies, reduced cerebral perfusion was shown in aneurysmal SAH patients, as opposed to normal cerebral perfusion parameters in nonaneurysmal SAH patients [26]. The mechanisms implied in the reduction of cerebral perfusion after SAH possibly include increased intracranial pressure (ICP) at the moment of hemorrhage [27, 28] and acute vasoconstriction independent of intracranial pressure [29].
We found only one study in the literature that compared CTP perfusion in PMH and aneurysmal SAH on the first 72 h after ictus [11]. In that study, which included 45 patients with PMH and 45 with aSAH, Cremers and colleagues showed that PMH patients have higher CBF than aSAH patients. However, when adjusting for the location of blood, they did not find statistically significant differences in CBF between PMH and SAH from posterior circulation aneurysm rupture, concluding that future studies should further elaborate on cerebral blood flow in posterior circulation aneurysms. Our results are somewhat different from that study. First, we could not demonstrate differences in CT perfusion parameters at < 72 h between PMH and aneurysmal SAH patients, except when we stratified for the amount of cisternal blood, but a possible explanation could be the small sample of our study. Second, differently to Cremers and colleagues, we found a trend for higher CBF values in PMH compared with aSAH from aneurysms in the posterior circulation. One explanation could be that we included the evaluation of perfusion parameters in the infratentorial compartment that was lacked in the Cremers study. This conceptual possibility of physiologic differences in perfusion between the infratentorial and supratentorial compartments has not however, to our knowledge, been described in the literature.
Regarding the vasospasm time window, there is abundant evidence reporting perfusion changes in aSAH [20, 23,24,25], and that CBF, MTT, and time to drain (TTD) have the highest diagnostic accuracy in predicting vasospasm, DCI, and poor clinical outcomes [20, 30]. However, we did not find other studies in the literature that addressed the cerebral perfusion in PMH in this time window. At 8–10 days, the PMH patients in our cohort showed a significant lower MTT and non-significant trend for higher CBF than aSAH patients, thus suggesting that cerebral perfusion may be globally better in PMH patients, also in a subacute phase. Again, the lack of statistical significance in other perfusion parameters could be related to the small sample of patients.
The amount of blood on CT, that is usually smaller in patients with PMH compared with aneurysmal SAH, could be one of the factors that could explain why perfusion is less affected in PMH patients. However, after adjusting for Fisher grade, we still found increased cerebral perfusion in PMH patients, both in the first 72 h and at 8–10 days, meaning that the differences in perfusion cannot be explained only by differences in blood burden between the two types of hemorrhage. It might be that the proposed venous origin of PMH would lead to a lower rise in intracranial pressure, and have less impact on cerebral perfusion, but this remains to be proven.
The more favorable clinical presentation, prognosis, and the reduced incidence of complications such as DCI in patients with PMH are in agreement with the findings of better cerebral perfusion in these patients.
Our study has some limitations, the most important being the small sample size that may have prevented it from showing a clearer difference between PMH and aneurysmal SAH cerebral perfusion; the subgroup analyses also included few patients in each group, so we should be cautious when interpreting their results. A selection bias may also have existed, as by selecting aSAH patients in good clinical condition to match PMH patients we may have underestimated perfusion differences between the two groups. However, including patients with aneurysmal SAH with worse clinical condition would introduce other confounders that would impair the interpretation of the results of cerebral perfusion.
Quantification of CTP is also a known limitation, since it may vary with the CT equipment and software programs [31]. There are other ways of quantifying perfusion parameters, such as automatic segmentation of white and gray matter, however, we chose to measure perfusion in different vascular territories, as described previously [32]. Likewise, the quantification of perfusion parameters is dependent on the measurement of the arterial input function, making CTP results difficult to generalize between centers. However, we believe reproducibility was maximized as our aim was to highlight perfusion differences between the two different kinds of SAH, and not to quantify thresholds, and all measurements were performed by the same operator, using the same CT scanner. Qualitative evaluation of color CT perfusion maps is one equivalent alternative to quantitative measurements, and it is more applicable in clinical practice [33]. However, quantitative measurements are possibly more sensible to subtle changes in perfusion, as those occurring in PMH patients.
Finally, with the use of CT perfusion, there is a concern on patient radiation exposure. For CTP, the patient is exposed to doses around 1–5 mSv, up to 10 mSv [34]. The advent of new scanners allowing volumetric perfusion CT in only one acquisition might reduce the total radiation exposure [35].
Conclusions
Cerebral perfusion was better in PMH than aSAH patients at 8–10 days, and also at < 72 h after adjusting for location of blood and amount of blood on admission.
Further research is needed to understand if better perfusion in PMH patients could be related to a non-arterial source of hemorrhage and to clarify its impact on clinical prognosis.
References
van Gijn J, van Dongen KJ, Vermeulen M, Hijdra a. (1985) Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology 35:493–493. https://doi.org/10.1212/WNL.35.4.493
Flaherty ML, Haverbusch M, Kissela B, Kleindorfer D, Schneider A, Sekar P, Moomaw CJ, Sauerbeck L, Broderick JP, Woo D (2005) Perimesencephalic subarachnoid hemorrhage: incidence, risk factors, and outcome. J Stroke Cerebrovasc Dis 14:267–271. https://doi.org/10.1016/j.jstrokecerebrovasdis.2005.07.004
Yamakawa H, Ohe N, Yano H, Yoshimura S, Iwama T (2008) Venous drainage patterns in perimesencephalic nonaneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 110:587–591. https://doi.org/10.1016/j.clineuro.2008.03.001
Watanabe A, Hirano K, Kamada M, Imamura K, Ishii N, Sekihara Y, Suzuki Y, Ishii R (2002) Perimesencephalic nonaneurysmal subarachnoid haemorrhage and variations in the veins. Neuroradiology 44:319–325. https://doi.org/10.1007/s00234-001-0741-3
Madureira S, Canhão P, Guerreiro M, Ferro JM (2000) Cognitive and emotional consequences of perimesencephalic subarachnoid hemorrhage. J Neurol 247:862–867
Kapadia A, Schweizer TA, Spears J, Cusimano M, Macdonald RL (2014) Nonaneurysmal perimesencephalic subarachnoid hemorrhage: diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurg 82:1131–1143
Krajewski K, Dombek S, Martens T, Köppen J, Westphal M, Regelsberger J (2014) Neuropsychological assessments in patients with aneurysmal subarachnoid hemorrhage, perimesencephalic SAH, and incidental aneurysms. Neurosurg Rev 37:55–62. https://doi.org/10.1007/s10143-013-0489-3
Sehba FA, Pluta RM, Zhang JH (2011) Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol 43:27–40. https://doi.org/10.1007/s12035-010-8155-z
Khurana VG, Besser M (1997) Pathophysiological basis of cerebral vasospasm following aneurysmal subarachnoid haemorrhage. J Clin Neurosci Off J Neurosurg Soc Australas 4:122–131
Prat D, Goren O, Bruk B, Bakon M, Hadani M, Harnof S (2013) Description of the vasospasm phenomena following perimesencephalic nonaneurysmal subarachnoid hemorrhage. Biomed Res Int 2013:1–8. https://doi.org/10.1155/2013/371063
Cremers CHP, van der Schaaf IC, Dankbaar JW, Velthuis BK, Rinkel GJE (2014) Cerebral CT perfusion in patients with perimesencephalic and those with aneurysmal subarachnoid hemorrhage. Int J Stroke 9:183–187. https://doi.org/10.1111/ijs.12021
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, MacDonald RL, Mayer SA (2006) Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 59:21–27. https://doi.org/10.1227/01.NEU.0000218821.34014.1B
Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J (1990) Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke 21:1156–1161. https://doi.org/10.1161/01.STR.21.8.1156
Van Asch CJJ, Van Der Schaaf IC, Rinkel GJE (2010) Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. Am J Neuroradiol 31:67–70. https://doi.org/10.3174/ajnr.A1748
van Gijn J, Hijdra A, Wijdicks EF et al (1985) Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg 63:355–362. https://doi.org/10.3171/jns.1985.63.3.0355
Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, Connolly ES, Mayer SA (2009) Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40:1963–1968. https://doi.org/10.1161/STROKEAHA.108.544700
Vergouwen MDI, Vermeulen M, Muizelaar JP et al (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/STROKEAHA.110.589275
Kudo K, Sasaki M, Ogasawara K, Terae S, Ehara S, Shirato H (2009) Difference in tracer delay-induced effect among deconvolution algorithms in CT perfusion analysis: quantitative evaluation with digital phantoms. Radiology 251:241–249. https://doi.org/10.1148/radiol.2511080983
Murphy A, de Oliveira Manoel AL, Burgers K, Kouzmina E, Lee T, Macdonald RL, Bharatha A (2015) Early CT perfusion changes and blood-brain barrier permeability after aneurysmal subarachnoid hemorrhage. Neuroradiology 57:767–773. https://doi.org/10.1007/s00234-015-1529-1
Sanelli PC, Anumula N, Johnson CE, Comunale JP, Tsiouris AJ, Riina H, Segal AZ, Stieg PE, Zimmerman RD, Mushlin AI (2013) Evaluating CT perfusion using outcome measures of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 34:292–298. https://doi.org/10.3174/ajnr.A3225
Dankbaar JW, de Rooij NK, Rijsdijk M, Velthuis BK, Frijns CJM, Rinkel GJE, van der Schaaf IC (2010) Diagnostic threshold values of cerebral perfusion measured with computed tomography for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 41:1927–1932. https://doi.org/10.1161/STROKEAHA.109.574392
Lagares A, Cicuendez M, Ramos A, Salvador E, Alén JF, Kaen A, Jiménez-Roldán L, Millán JM (2012) Acute perfusion changes after spontaneous SAH: a perfusion CT study. Acta Neurochir 154:402–405. https://doi.org/10.1007/s00701-011-1267-z
Weidauer S, Lanfermann H, Raabe A, Zanella F, Seifert V, Beck J (2007) Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: a prospective MRI and DSA study. Stroke 38:1831–1836. https://doi.org/10.1161/STROKEAHA.106.477976
Mir DI, Gupta A, Dunning A et al (2014) CT perfusion for detection of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. AJNR Am J Neuroradiol 35:866–871. https://doi.org/10.3174/ajnr.A3787
Cremers CHP, van der Schaaf IC, Wensink E, Greving JP, Rinkel GJE, Velthuis BK, Vergouwen MDI (2014) CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab 34:200–207. https://doi.org/10.1038/jcbfm.2013.208
Griffiths PD, Wilkinson ID, Mitchell P, Patel MC, Paley MN, Romanowski CA, Powell T, Hodgson TJ, Hoggard N, Jellinek D (2001) Multimodality MR imaging depiction of hemodynamic changes and cerebral ischemia in subarachnoid hemorrhage. AJNR Am J Neuroradiol 22:1690–1697
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4:432–446
Grote E, Hassler W (1988) The critical first minutes after subarachnoid hemorrhage. Neurosurgery 22:654–661
Bederson JB, Levy AL, Ding WH et al (1998) Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery 42:352–362
Othman AE, Afat S, Nikoubashman O, Müller M, Schubert GA, Bier G, Brockmann MA, Wiesmann M, Brockmann C (2016) Volume perfusion CT imaging of cerebral vasospasm: diagnostic performance of different perfusion maps. Neuroradiology 58:1–6. https://doi.org/10.1007/s00234-016-1695-9
Sanelli PC, Pandya A, Segal AZ, Gupta A, Hurtado-Rua S, Ivanidze J, Kesavabhotla K, Mir D, Mushlin AI, Hunink MGM (2014) Cost-effectiveness of CT angiography and perfusion imaging for delayed cerebral ischemia and vasospasm in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 35:1714–1720. https://doi.org/10.3174/ajnr.A3947
Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE (2009) Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology 51:813–819. https://doi.org/10.1007/s00234-009-0575-y
Wintermark M, Dillon WP, Smith WS, Lau BC, Chaudhary S, Liu S, Yu M, Fitch M, Chien JD, Higashida RT, Ko NU (2008) Visual grading system for vasospasm based on perfusion CT imaging: comparisons with conventional angiography and quantitative perfusion CT. Cerebrovasc Dis 26:163–170. https://doi.org/10.1159/000139664
Fieselmann A, Kowarschik M, Ganguly A, Hornegger J, Fahrig R (2011) Deconvolution-based CT and MR brain perfusion measurement: theoretical model revisited and practical implementation details. Int J Biomed Imaging 2011:467563. https://doi.org/10.1155/2011/467563
Turowski B, Schramm N (2016) An appeal to standardize CT and MR perfusion. Clin Neuroradiol:1–6. https://doi.org/10.1007/s00062
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
IF was funded by a scholarship from Tecnifar/Sociedade Portuguesa de AVC.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fragata, I., Canto-Moreira, N. & Canhão, P. Comparison of cerebral perfusion in perimesencephalic subarachnoid hemorrhage and aneurysmal subarachnoid hemorrhage. Neuroradiology 60, 609–616 (2018). https://doi.org/10.1007/s00234-018-1997-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-1997-1