Abstract
Background
Perfusion computed tomography (CT) is a rapid technique that allows the measurement of acute disturbances in local and global cerebral blood flow in patients suffering stroke and spontaneous subarachnoid haemorrhage (SAH). The purpose of this study was to establish the relationship between different measures of brain perfusion made on dynamic-contrast CT reconstructions performed as soon as SAH has been diagnosed and the severity of the bleeding determined by the clinical grade, the extent of the bleeding and the outcome of the patients.
Methods
After the diagnosis of SAH by conventional CT, a perfusion CT was performed before CT angiography. All imaging studies were performed on a six-slice spiral CT scanner. All images were analysed using perfusion software developed by Philips, which produces perfusion CT quantitative data based on temporal changes in signal intensity during the first pass of a bolus of an iodinated contrast agent. Measurements of mean transient time (MTT), time to peak (TTP), cerebral blood volume (CBV) and cerebral blood flow (CBF) in volumes of interest corresponding to territories perfused by the major cerebral arteries were performed. Different data regarding severity of the bleeding—such as level of consciousness, amount of bleeding in conventional CT—were collected. All poor-grade patients received a ventriculostomy catheter so that ICP recordings were obtained. Also, the occurrence of delayed cerebral ischaemia (DCI) was recorded. Outcome was assessed by the Glasgow Outcome Scale 6 months after the bleeding. For statistical analysis, non-parametric correlations between variables were performed.
Findings
Thirty-nine patients have been included in the study since January 2007. In SAH patients there are increasing perfusion abnormalities as the severity of the bleeding increases. The most affected perfusion parameters are TTP and MTT, as they significantly increase with the clinical severity of the bleeding and the total volume of bleeding (P < 0.01, Spearman’s Rho). When average MTT time is increased over 5.9 s there is a 20-fold (95% CI = 2.1-182) risk of poor outcome. All patients presenting this MTT time suffered from DCI. This value has a positive predictive value of 100% for DCI and 90% for a poor outcome.
Conclusions
SAH causes cerebral blood flow abnormalities even in the acute phase of the illness, consisting mainly of an increase in circulation times (TTP and MTT), which are correlated with the severity of the bleeding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subarachnoid haemorrhage (SAH) is still associated with high morbidity and mortality despite technical advances in the treatment of cerebral aneurysms and new discoveries in the treatment of delayed cerebral ischaemia due to vasospasm. In fact the severity of the initial bleeding, expressed by the disturbance of the level of consciousness and the amount of bleeding in the initial computed tomography (CT), and age are still the major determinants of outcome [4, 12]. There are few studies characterising the pathophysiological changes occurring in the initial moments after SAH. It is known that after the initial bleeding there is a global decrease in cerebral blood flow that lasts for many hours—even days—after aneurysm rupture [8]. These disturbances in the cerebral blood flow seem to be caused by an increase in intracranial pressure (ICP), which initially stops the bleeding. Another proposed mechanism is global vessel vasoconstriction in response to the presence of blood in the subarachnoid space and intraluminal obstruction because of vascular micro-thrombi formation [23–25]. All these mechanisms provoke an inadequate brain perfusion that ends in ischaemia, which is mainly responsible for the acute brain injury.
There are many techniques for studying cerebral perfusion deficit, like magnetic resonance perfusion, Xenon computed tomography, positron emission tomography (PET) and single photon emission computed tomography (SPECT), but they are limited by their cost, their availability or patient tolerance. Perfusion CT is a rapid technique that allows qualitative and quantitative evaluation of cerebral perfusion. The aim of this study was to explore the feasibility of acutely performing CT perfusion in these patients and correlate the CT perfusion parameters with the acute bleeding severity and the outcome of patients suffering spontaneous SAH.
Methods and materials
Patients
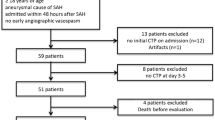
A series of patients consecutively admitted to Hospital 12 de Octubre over a 2-year period (2007-2009) with the diagnosis of SAH, on whom a CT, CT angiography and CT perfusion could be performed in the first 24 h after the onset of the symptoms, were included in the study. The diagnosis of SAH was made by the presence of a typical clinical history of spontaneous SAH and the presence of intraventricular blood or blood in the basal cisterns in the admission CT scan. The onset of the haemorrhage was considered at the point in time where neurological symptoms or clinical deterioration started. In awake and cooperative patients, this information was provided by the patients themselves. In unconscious, disoriented or dysphasic patients, the information was obtained by relatives who had witnessed the moment of deterioration. Exclusion criteria for this study were age below 18 years of age, history of previous SAH or stroke and non-aneurysmal SAH. All procedures were reviewed by our local institutional ethical committee and informed consent was obtained.
Data collection
Data corresponding to clinical characteristics, such as age, sex, history of previous hypertension and medical history, were recorded at admission. Patients were evaluated in the Emergency Department and their clinical situation was assessed by the Glasgow Coma Scale and the World Federation of Neurological Surgeons scale (WFNS). Diagnostic CT was performed and the presence and amount of cisternal blood was evaluated by using the Fisher scale. In addition, the presence of hydrocephalus was recorded as well as the Evan’s ratio in all cases; that is, the ratio of the largest width of the frontal horns to the maximal biparietal diameter. Hydrocephalus was considered as present when Evan’s ratio was over 0.3. The amount of bleeding was also quantitatively assessed by using volumetric software. Diagnostic CT DICOM images were evaluated using ANALYZE 8.1 software running on a personal computer. SAH bleeding and total bleeding volume (subarachnoid, intraventricular and/or intracerebral bleeding) was estimated using region-of-interest (ROI) measurement with semi-automated delineation of the bleeding in each section. This volumetric study was performed by two independent neurosurgeons that were not aware of the situation of the patient. Differences between independent measures were below 5% for the two measures, and therefore bleeding volume measures were averaged between the two observers. ICP was recorded in all poor-grade patients by means of a ventricular catheter. Mean and maximum recordings during the first 2 days of the bleeding were used to determine their relation to perfusion and outcome variables.
The presence and location of the aneurysm responsible for the bleeding was recorded. Delayed cerebral ischaemia (DCI) was defined as clinical deterioration attributable to vasospasm, when other causes of deterioration could be ruled out such as hydrocephalus, rebleeding or electrolyte abnormalities, or by the detection of a new infarct on brain CT related to vasospasm that was not visible on the admission or inmediate post-treatment scan, or both. Outcome was evaluated 6 months after the bleeding by means of the Glasgow Outcome Scale (GOS).
Perfusion CT technique and determinations
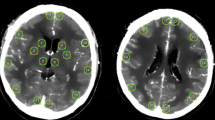
Perfusion CT was performed after diagnostic CT and previously to the performance of helical CT to determine the presence and location of the aneurysm responsible for the bleeding. All imaging studies were performed on a six-slice spiral CT scanner. Perfusion CT consisted of two 45-s series during the intravenous administration of contrast medium. Both series studied a 2.4-cm slice of brain (Fig. 1) obtaining images corresponding to territories of the main cerebral arteries and basal ganglia. These brain slices were serially scanned in cycles, acquiring two consecutive 1.2-cm thickness images in each cycle. A total of 45 cycles for each slice were acquired, at a rate of two images per second, after the intravenous administration of a 40-ml bolus of contrast (Omnipaque 300 mg/ml, General Electric) at a flow rate of 4 ml/s into the cubital vein (18-gauge needle) for each slide, with a total dose of contrast of 80 ml per study. The acquisition parameters were 120 kvp, 80 mAs, using a 512 × 512 matrix. A period of 10 min between slices was used for contrast washout.
All images were analysed using perfusion software developed by Philips, which produces PCT quantitative data based on temporal changes in signal intensity during the first pass of a bolus of an iodinated contrast agent. This software relies on the central deconvolution principle to obtain different flow parameters. The deconvolution operation needs an artery and a vein to be identified by the operator. Different ROIs were drawn corresponding to white matter related to the territory of the major cerebral arteries in both hemispheres as well as deep ROIs corresponding to basal ganglia and measurements of mean transient time (MTT), time to peak (TTP), cerebral blood volume (CBV) and cerebral blood flow (CBF) were performed in each ROI. Each ROI was named according to the cerebral artery responsible for their irrigation. PCT quantitative data were obtained by two independent neuroradiologists, who were not aware of the clinical situation of the patient.
Statistical analysis
For the purpose of statistical analysis, the patient’s clinical grade was classified into subgroups of good (WFNS I, II and III) and poor grade (WFNS IV and V). Also outcome was dichotomised into good (GOS 4 and 5) and poor outcome (GOS 1, 2 and 3). The existence of differences in the perfusion parameters in each ROI corresponding to a major cerebral artery between cerebral hemispheres was analysed with the paired sample t-test for continuous variables. A mean value for all ROIs in each patient in each hemisphere as well as both hemispheres was calculated for the different perfusion parameters. Simple non-parametric (Spearman’s Rho) correlations were used for determining the relationship between the different clinical variables and perfusion values. Also the mean perfusion parameters were compared between groups of good and poor clinical grade, presence or absence of DCI, and good and poor outcome by means of non-parametric Mann-Whitney U test. Threshold values for MTT obtained in other studies [3] were used to determine the potential prognostic value of these parameters in terms of sensibility, specificity, positive predictive value and area under the ROC curve (AUC) for developing a poor outcome, and compared with poor clinical grade and dichotomised fisher grade.
Results
Thirty-nine patients have been included in the study within the first 24 h after the bleeding. Mean age was 48 and the female-to-male ratio was 1.4:1. Nearly half of the patients presented in poor clinical grade and most of them were classified as having presented a Fisher 3 or 4 SAH. Demographic and clinical data are presented in Table 1. None of them presented secondary adverse effects because of the use of contrast agent and the CT perfusion did not delay the diagnosis process in any case. In all the poor-grade cases, ICP was monitored and no pathological elevation in ICP could be detected after initial ventricular drainage. Mean ICP readings in the first 2 days after the bleeding were below or equal to 20 mmHg (median = 13 mmHg, range 9-20 mmHg) and maximum ICP during the recording was always below 25 mmHg (median = 20 mmHg, range 13-23 mmHg).
Median CBF among the different ROIs calculated among patients with SAH in the first day was 108 ml/100 g/min [interquartile range (IQR) 59-162 ml/100 g/min]; median CVB was 8 ml/100 g (IQR 5-11 ml/100 g); median MTT was 5 s (IQR 3.8-7.3 s) and median TTP 21 s (IQR 17-26 s). There were no significant differences between the different equivalent ROIs between hemispheres or the mean values for each parameter between hemispheres.
Simple correlations revealed a relationship mainly between MTT and TTP to the clinical severity of the initial bleeding (WFNS), amount of initial bleeding and outcome, both in individual ROI values as well as in averaged values (mMTT and mTTP) (see Table 2 correlation matrix). Longer perfusion times correlated to worse clinical grade at admission, higher haemorrhage volume, the occurrence of DCI and poorer outcome. This relationship is not altered if correlations are adjusted for age or the presence of hydrocephalus by the use of partial correlations. The presence of DCI is also related to lower CBF. No correlation was found between perfusion parameters and mean or maximum ICP values in poor-grade patients.
The mMTT is significantly different in patients with good and poor grade at admission (Fig. 2). Longer perfusion times are related to poor grade and also to the presence of DCI and poorer outcome. Considering as a threshold for ischaemic damage a MTT of 5.9, as suggested by Dankbaar et al. [3], the percentage of patients suffering a poor outcome is significantly larger if mMTT is 5.9 s or longer (90% poor outcome in patients with mMTT ≥5.9; 30% poor outcome in patients with mMTT <5.9) (Fig. 3). Thus, having an mMTT of 5.9 or more increases the risk of suffering a poor outcome by 20 (OR for poor outcome = 20, 95% confidence interval = 2.1-182). Consequently, if any ROI presents an MTT of 5.9 or higher the probability of having a poor outcome increases by nearly seven times [odds ratio (OR) for poor outcome 6.7, 95% confidence interval (CI) 1.5-30]. This threshold has a high positive predictive value (PPV) for poor outcome {for mMTT ≥5.9 and poor outcome [values (95% CI)], sensitivity = 50% (24-76%), specificity = 95% (83-100%), PPV = 90% (66-100%); for any ROI with MTT ≥5.9 and poor outcome, sensitivity = 83% (63-100%), specificity = 57% (34-81%), PPV = 63% (41-84%)}. The same happens in relation to DCI (Fig. 4). All patients having an mMTT of 5.9 presented with DCI, and the risk of DCI is also increased if any ROI shows an MTT of 5.9 or higher (OR of suffering DCI = 12, 95% confidence interval = 2.5-57). When comparing these parameters with conventionally used prognostic factors, such as poor grade or presenting a Fisher grade 3 or 4, mMTT of 5.9 s or higher shows the highest positive predictive value, achieving a similar AUC (Table 3). If mMTT is combined with presenting a poor grade, the diagnostic capability is further increased.
Discussion
It is well known that in patients suffering from SAH, bleeding severity, expressed by the initial neurological status of the patient, is a major determinant of the final outcome [4, 12, 13]. The majority of patients suffering from SAH present with impaired consciousness and this presentation is related to the severity of the neurological injury caused by the initial bleeding, which at the end profoundly determines their outcome. The primary cause of this neurological global neurological dysfunction after SAH has been studied by different techniques, including positron emission tomography (PET) [6, 9, 20, 21], single photon emission tomography (SPECT) [5], transcraneal Doppler ultrasound [18] or Xenon contrast-enhanced CT [10]. The main finding of the different studies has been that the impairment of the level of consciousness is related to a global decrease in cerebral blood flow, which lasts longer than the initial moments of the bleeding. This hypoperfusion occurs symmetrically, affecting both hemispheres equally. The finding of widespread ischaemic lesions in brain areas not associated with the site of the ruptured aneurysms in diffusion-weighted magnetic resonance imaging (MRI) and SPECT studies [7], as well as watershed hypoperfusion in perfusion CT studies [14] support the idea that a global vascular dysfunction occurring after SAH is likely responsible for the poor clinical condition of the patients. There are two major possible mechanisms for this global initial hypoperfusion after SAH. The first one is related to high ICP after aneurysmal rupture. At this moment blood is extravasated in the subarachnoid space, with subsequent rise in the ICP. This raise in ICP would be maintained for longer than the initial moments of the bleeding and would be responsible for the maintenance of a global low cerebral perfusion state during the initial days after SAH. The other possible mechanism implicated is microvascular or peripheral vasoconstriction. All patients with SAH have abnormalities in cerebral blood flow, including those in good clinical grade and normal angiographic findings. Cerebral vascular autorregulation is affected by the formation of free radicals from the haemoglobin degradation and the consumption of nitric oxide (NO) that produces focal and global vasoconstriction of cerebral vessels [23]. In addition, changes in sympathetic nerve activity, as well as microvascular thrombosis or embolism, could collaborate to this microcirculatory dysfunction [24]. The different functional studies performed in the acute stage of SAH bleeding for the moment have not clarified the possible mechanism responsible for the low perfusion state. In none of them could a maintained raised ICP be recorded relative to the low perfusion state [18, 22]. However, other parameters—such as circulatory times—or global resistance parameters—such as pulsatility index in TCD—have been found to be raised [18], possibly supporting the idea of a raised ICP. However, these findings can also be found in circumstances in which there is a global dysfunction of the cerebral microcirculation.
The findings obtained in this study are in line with previous works on cerebral perfusion in the acute stage of aneurysmal SAH. We found an increase in circulatory times, which is related to the clinical condition of the patients. As the neurological dysfunction worsens there is an increase in the perfusion times. These perfusion abnormalities equally affect both hemispheres and are widespread over different regions in the supratentorial compartment. In none of the poor-grade patients was there a detectable raise in ICP over normal limits during the examinations (ICP always below 25 mmHg, also mean and maximum ICP recordings during the first 2 days were normal) and hydrocephalus (defined as Evan’s ratio over 0.30) was not associated with increase in MTT or TTP. These findings suggest that most probably the mechanism related to the perfusion abnormalities found are caused by a global dysfunction of cerebral microcirculation and not by a maintained increment in ICP. These perfusion abnormalities are related to the total amount of bleeding recorded in the diagnostic CT and measured by means of volumetry, therefore suggesting an association between the amount of blood extravasated, the presence of perfusion abnormalities and the resultant global neurological dysfunction.
Perfusion CT has proven to be useful in determining the presence of cerebral ischaemia in patients suffering from SAH [1, 2, 11, 15, 17]. It is a non-invasive technique, which is rapid, easy and well tolerated by the patients. It can be performed in any standard spiral CT scanner and its perfusion maps can be simply generated with the appropriate software. The majority of studies using this technique in patients with SAH have centred their objective on establishing its utility in the detection of delayed cerebral ischaemia (DCI), proving that it is a useful technique in its identification and that the most sensitive parameter in order to detect ischaemic events in these patients is the prolongation of circulation times, specifically MTT [3, 16]. This parameter has also been identified as the most sensitive and specific to define ischaemic events in stroke patients [19]. Using this parameter (MTT) and the threshold suggested for detecting ischaemia in these patients, perfusion CT used in the acute stage can detect patients in high risk of poor outcome. Although the small number of patients included in this study precludes obtaining any conclusion regarding the equivalence or superiority of this tool to previously used prognostic factors, it seems that perfusion CT could be indeed another useful instrument to detect patients at risk of both presenting DCI and having a poor outcome. However, conventional prognostic markers, such as clinical grade, age or Fisher grade, are far more readily available than this imaging development and it is our opinion that this test will not be superior to them but complementary.
However, the use of CT perfusion has also several limitations that should be considered in analysing these results. First of all, CT perfusion is very dependent on the technique used in its performance, as perfusion parameters can be affected by the contrast injection velocity, timing of the CT regarding contrast injection and reconstruction of the perfusion maps. In this study all these parameters were taken into consideration and a strict protocol for the performance of the perfusion studies was used. Also, the reconstruction of the perfusion maps was performed by two neuroradiologists, who were not aware of the actual situation of the patient and ROI placement, and measurements were consensuated between both observers. However, all quantitative data extracted from perfusion CT can be affected by these biases. Also, many factors can influence cerebral perfusion in the early stage of SAH, such as medications, seizures, induced hypertension, previous history of smoking, hypertension or other cerebrovascular diseases. Therefore, additional studies are recommended for determining the relation between perfusion parameters and other prognostic factors like age, the amount of blood on CT at admission, the development of delayed cerebral ischaemia and the relation to other perfusion studies in these patients such as TCD, SPECT or PET.
Conclusions
Perfusion CT can be a rapid and easy technique for studying the physiopathology of the cerebral blood flow abnormalities during SAH. Perfusion abnormalities in these patients are related to the clinical severity of the bleeding as well as to the total amount of bleeding caused by aneurysm rupture. The main perfusion parameter affected in SAH is MTT which could detect patients at risk of suffering a poor outcome.
References
Aralasmak A, Akyuz M, Ozkaynak C, Sindel T, Tuncer R (2009) CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology 51:85–93
Burns JD, Jacob JT, Luetmer PH, Wijdicks EF (2010) CT perfusion evidence of early global cerebral hypoperfusion after aneurysmal subarachnoid hemorrhage with cardiac arrest. Neurocrit Care 12:261–264
Dankbaar JW, de Rooij NK, Rijsdijk M, Velthuis BK, Frijns CJ, Rinkel GJ, van der Schaaf IC (2010) Diagnostic threshold values of cerebral perfusion measured with computed tomography for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 41:1927–1932
de Toledo P, Rios PM, Ledezma A, Sanchis A, Alen JF, Lagares A (2009) Predicting the outcome of patients with subarachnoid hemorrhage using machine learning techniques. IEEE Trans Inf Technol Biomed 13:794–801
Gambardella G, De Blasi F, Caruso G, Zema A, Turiano F, Collufio D (1998) Intracranial pressure, cerebral perfusion pressure, and SPECT in the management of patients with SAH Hunt and Hess grades I-II. Acta Neurochir Suppl 71:215–218
Grubb RL Jr, Raichle ME, Eichling JO, Gado MH (1977) Effects of subarachnoid hemorrhage on cerebral blood volume, blood flow, and oxygen utilization in humans. J Neurosurg 46:446–453
Hadeishi H, Suzuki A, Yasui N, Hatazawa J, Shimosegawa E (2002) Diffusion-weighted magnetic resonance imaging in patients with subarachnoid hemorrhage. Neurosurgery 50:741–747
Hayashi T, Suzuki A, Hatazawa J, Kanno I, Shirane R, Yoshimoto T, Yasui N (2000) Cerebral circulation and metabolism in the acute stage of subarachnoid hemorrhage. J Neurosurg 93:1014–1018
Hayashi T, Suzuki A, Hatazawa J, Hadeishi H, Shirane R, Tominaga T, Yasui N (2008) Post-operative changes of cerebral circulation and metabolism in the acute stage of low-grade aneurysmal subarachnoid hemorrhage. Neurol Res 30:678–683
Jakobsen M, Enevoldsen E, Bjerre P (1990) Cerebral blood flow and metabolism following subarachnoid haemorrhage: cerebral oxygen uptake and global blood flow during the acute period in patients with SAH. Acta Neurol Scand 82:174–182
Kanazawa R, Kato M, Ishikawa K, Eguchi T, Teramoto A (2007) Convenience of the computed tomography perfusion method for cerebral vasospasm detection after subarachnoid hemorrhage. Surg Neurol 67:604–611
Lagares A, Gomez PA, Lobato RD, Alen JF, Alday R, Campollo J (2001) Prognostic factors on hospital admission after spontaneous subarachnoid haemorrhage. Acta Neurochir (Wien ) 143:665–672
Lagares A, de Toledo P, Fernandez-Alen JA, Ibanez J, Arikan F, Sarabia R, Ballenilla F, Gabarros A, Horcajadas A, Rodriguez-Boto G, de la LA, Maillo A, Delgado P, Yacer JL, Dominguez J, Arrese I (2008) Spontaneous Subarachnoid Haemorrhage multicenter database from the Group for the Study of Vascular Pathology of the Spanish Society for Neurosurgery: presentation, inclusion criteria and development of an internet-based registry. Neurocirugia (Astur ) 19:405–415
Lanterna LA, Lunghi A, Martchenko S, Gritti P, Bonaldi G, Biroli F (2011) Cerebral watershed hypoperfusion in subarachnoid hemorrhage: computed tomography perfusion analysis. J Neurosurg 114:961–968
Laslo AM, Eastwood JD, Chen FX, Lee TY (2006) Dynamic CT perfusion imaging in subarachnoid hemorrhage-related vasospasm. AJNR Am J Neuroradiol 27:624–631
Laslo AM, Eastwood JD, Pakkiri P, Chen F, Lee TY (2008) CT perfusion-derived mean transit time predicts early mortality and delayed vasospasm after experimental subarachnoid hemorrhage. AJNR Am J Neuroradiol 29:79–85
Lefournier V, Krainik A, Gory B, Derderian F, Bessou P, Fauvage B, Le Bas JF, Payen JF (2010) Perfusion CT to quantify the cerebral vasospasm following subarachnoid hemorrhage. J Neuroradiol 37:284–291
Miranda P, Lagares A, Alen J, Perez-Nunez A, Arrese I, Lobato RD (2006) Early transcranial Doppler after subarachnoid hemorrhage: clinical and radiological correlations. Surg Neurol 65:247–252
Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Chan RK, Lee TY (2008) White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology 247:818–825
Sarrafzadeh AS, Haux D, Ludemann L, Amthauer H, Plotkin M, Kuchler I, Unterberg AW (2004) Cerebral ischemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis-PET study. Stroke 35:638–643
Sarrafzadeh AS, Nagel A, Czabanka M, Denecke T, Vajkoczy P, Plotkin M (2010) Imaging of hypoxic-ischemic penumbra with (18)F-fluoromisonidazole PET/CT and measurement of related cerebral metabolism in aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 30:36–45
Schubert GA, Seiz M, Hegewald AA, Manville J, Thome C (2009) Acute hypoperfusion immediately after subarachnoid hemorrhage: a xenon contrast-enhanced CT study. J Neurotrauma 26:2225–2231
Sehba FA, Bederson JB (2006) Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res 28:381–398
Sehba FA, Mostafa G, Friedrich V Jr, Bederson JB (2005) Acute microvascular platelet aggregation after subarachnoid hemorrhage. J Neurosurg 102:1094–1100
Uhl E, Lehmberg J, Steiger HJ, Messmer K (2003) Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery 52:1307–1315
Acknowledgements
This work has been supported by the Spanish Ministries of Health under grants FIS PI 07/0152 and FIS PI 10/01939 and by Fundacion Mutua Madrileña grant 2007/082.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
In the paper titled “Acute perfusion changes after spontaneous SAH: a perfusion CT study”, the authors look to demonstrate the use of CT perfusion in predicting the severity of bleeding in SAH patients and to correlate it with the clinical grade at presentation as well as outcome. In doing so, they looked at a group of 39 patients from a single institution over a 2-year time period. They collected information about these patients, including basic demographic information as well as other information such as GCS, Fisher score on CT scan and other important characteristics on presentation. They also performed a perfusion CT on these patients and recorded mean transit time (MTT), time to peak (TTP), cerebral blood volume (CBV) and cerebral blood flow (CBF) from these studies. They then analysed these variables to see if there was some correlation between these CT perfusion characteristics and severity of clinical presentation and outcome.
In analysing this paper, it appears that the authors sought to demonstrate that the changes in CT perfusion imaging can provide the physician with vital information regarding the patient’s clinical severity as well as outcome. One must commend these authors for looking into a relatively simple and easily accessible technique in order to provide critical information early on after SAH. Their results do in fact illustrate that there was a positive correlation between a prolonged MTT and poor grade at admission as well as a poor outcome. What these authors do not provide is some detail with regards to how they measure outcome. How long was the follow-up? They do make mention the Glasgow Outcome Score, but were there other parameters that they used? I believe that these are important questions that need to be addressed to give this paper more validation. One can predict that a patient will have changes in their CT perfusion scan after SAH, but the true question is how can we use CT perfusion imaging to predict long-term outcome? For example, can we correlate CT perfusion parameters with likelihood of developing clinically significant vasospasm after SAH? Answers to these questions will help determine the feasibility of obtaining routine CT perfusion scans on patients upon admission with SAH.
All in all, I believe that this paper provides us with a unique perspective on a commonly utilised test, of which we have the capability to expand its usage. I do believe, however, that the authors leave us with some unanswered questions and expanding their scope can help give this paper more validation.
Siraj Gibani
Christopher M. Loftus
Philadelphia, USA
This is an interesting paper that describes a new diagnostic test, and provides calculations, based on data from the original cohort of 39 patients, for PPV, sensitivity and specificity. The possibility that this test, based on perfusion CT, might be clinically beneficial, is enticing. However, this paper is hopefully only the beginning. Now that the authors have defined this new methodology, the authors need to apply this method prospectively to a validation cohort, to determine whether the criteria that they have derived truly have the predictive value, sensitivity and specificity that they have suggested. Also, it is hoped that the authors will pursue the mechanisms that underlie the changes in perfusion CT.
Michael Tymianski
Toronto, Canada
Rights and permissions
About this article
Cite this article
Lagares, A., Cicuendez, M., Ramos, A. et al. Acute perfusion changes after spontaneous SAH: a perfusion CT study. Acta Neurochir 154, 405–412 (2012). https://doi.org/10.1007/s00701-011-1267-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1267-z