Abstract
Introduction
The purpose of this study was to evaluate the feasibility of a contrast medium (CM), radiation dose reduction protocol for cerebral bone-subtraction CT angiography (BSCTA) using 80-kVp and sinogram-affirmed iterative reconstruction (SAFIRE).
Methods
Seventy-five patients who had undergone BSCTA under the 120- (n = 37) or the 80-kVp protocol (n = 38) were included. CM was 370 mgI/kg for the 120-kVp and 296 mgI/kg for the 80-kVp protocol; the 120- and the 80-kVp images were reconstructed with filtered back-projection (FBP) and SAFIRE, respectively. We compared effective dose (ED), CT attenuation, image noise, and contrast-to-noise ratio (CNR) of two protocols. We also scored arterial contrast, sharpness, depiction of small arteries, visibility near skull base/clip, and overall image quality on a four-point scale.
Results
ED was 62% lower at 80- than 120-kVp (0.59 ± 0.06 vs 1.56 ± 0.13 mSv, p < 0.01). CT attenuation of the internal carotid artery (ICA) and middle cerebral artery (MCA) was significantly higher on 80- than 120-kVp (ICA: 557.4 ± 105.7 vs 370.0 ± 59.3 Hounsfield units (HU), p < 0.01; MCA: 551.9 ± 107.9 vs 364.6 ± 62.2 HU, p < 0.01). The CNR was also significantly higher on 80- than 120-kVp (ICA: 46.2 ± 10.2 vs 36.9 ± 7.6, p < 0.01; MCA: 45.7 ± 10.0 vs 35.7 ± 9.0, p < 0.01). Visibility near skull base and clip was not significantly different (p = 0.45). The other subjective scores were higher with the 80- than the 120-kVp protocol (p < 0.05).
Conclusion
The 80-kVp acquisition with SAFIRE yields better image quality for BSCTA and substantial reduction in the radiation and CM dose compared to the 120-kVp with FBP protocol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Technical advances have rendered cerebral CT angiography (CTA) an accurate and useful imaging modality for the evaluation of intracranial aneurysms [1]. Although 3D digital subtraction angiography (3D-DSA) remains the reference standard [2], it is time consuming, technically demanding, invasive, and has a 0.12–0.5% rate of permanent neurologic complication [3–5]. As a non-invasive imaging modality, MR angiography (MRA) is more expensive and less available than CTA, requires long scanning times (which could cause motion artifacts, especially in settings of acute subarachnoid hemorrhage (SAH)), and has limitations in the evaluation of clipped aneurysms because of susceptibility artifacts [6, 7]. On the other hand, cerebral bone subtraction CTA (BSCTA) is fast, minimally invasive, widely available, and its diagnostic performance is comparable to 3D-DSA for the detection of intracranial aneurysms, even lesions adjacent to the skull base that can be missed by non-subtraction CTA (NSCTA) [8–10]. In addition, compared to NSCTA, it improves the accuracy for detecting aneurysm remnants after clipping surgery [11]. Consequently, BSCTA might be considered a first-line imaging modality for the diagnosis, treatment planning, and follow-up of intracranial aneurysms, especially in patients with lesions near the skull base and clip.
However, BSCTA involves substantial radiation exposure because it requires two consecutive (pre- and post-contrast enhanced) scans and the injection of iodinated contrast medium (CM). To minimize the biological effect of ionizing radiation, the radiation dose should be as low as possible without impairing diagnostic performance [12–14]. Especially in patients with unruptured aneurysms or SAH, the cumulative radiation exposure from CT is of concern because these patients tend to be relatively young and may need to be followed long term [15–17]. A reduction in the CM dose may be also desirable to decrease the risk of CM-induced nephropathy (CIN) in patients with renal dysfunction [18, 19], because they may have to receive additional intra-arterial CM administrations for subsequent invasive DSA examination and endovascular therapy.
Low tube voltage and iterative reconstruction (IR) techniques effectively reduce the radiation and the CM dose, because the radiation dose is proportional to the square of the tube voltage and the attenuation of iodine CM is increased at lower tube voltages since the mean photon energy of the X-ray is closer to the iodine K-edge (33 keV). Although the image noise increases because the X-ray spectrum is less penetrable, it can be lowered by using IR instead of filtered back-projection (FBP) algorithms. The usefulness of these techniques for various diagnostic tasks has been documented [20, 21].
Several studies indicated the usefulness of the low tube voltage technique for cerebral CTA [22–28]. Among these, only one study [27] combined a low tube voltage with IR technique to reduce the radiation dose at NSCTA. However, our search of the literature found no reports that examined the feasibility of combining a low tube voltage with an IR to obtain a reduction in not only the radiation but also the CM dose. In addition, the image quality of subtraction images acquired at low tube voltage with the use of IR also remains to be assessed. Therefore, the purpose of this study was to evaluate the image quality yielded by a radiation and CM dose reduction protocol using 80 kVp and sinogram-affirmed iterative reconstruction (SAFIRE; Siemens Healthineers, Forchheim, Germany), a second-generation IR algorithm, for cerebral bone subtraction CTA.
Materials and methods
Patients
This retrospective study received institutional review board approval; patient informed consent was waived. All patients consented to the use of their medical records for research purposes. We identified 90 consecutive patients who had undergone BSCTA under the 120- or 80-kVp protocols. Each examination was performed for the suspicion, ruling out, or follow-up of intracranial aneurysms as the standard of care in our institution between November 2014 and October 2015. The standard 120-kVp protocol (n = 44) was performed until April 2015, and the 80-kVp protocol (n = 46) was started from May 2015 for the purpose of radiation dose reduction, because national diagnostic reference levels were enacted in 2015 in our country. We excluded patients with severe motion artifacts (n = 3) and patients scanned using CM injection protocols or image reconstruction that differed from our routine 120- or 80-kVp protocols (n = 12). Consequently, a total of 75 patients (120 kVp, n = 37; 80 kVp, n = 38) were included in this study.
Image acquisition and reconstruction for each protocol
All scans were performed on a 128-slice single-source MDCT scanner (Definition AS+; Siemens Healthineers, Forchheim, Germany) and under both protocols; the tube current was modulated by automated exposure control (CARE Dose 4D; Siemens Healthineers, Forchheim, Germany). The 120-kVp scans were performed with 350 quality reference mAs, and the acquired images were reconstructed with FBP (H30f). The 80-kVp scans were performed using automated tube voltage selection (CARE kV; Siemens Healthineers, Germany), which employs reference value of 120 kV and 350 mAs for CTA setting (slider bar 11). That system automatically selected 80 kVp and 469 quality reference mAs for all patients. The acquired 80-kVp images were reconstructed with SAFIRE at strength level 3. We selected this medium IR strength level according to the vendor’s recommendation.
Under the 120-kVp protocol, the patients received a CM dose of 370 mgI/kg body weight (BW) over 12 s. We delivered a CM dose of 296 mgI/kg BW over 10 s for the 80-kVp protocol to minimize venous enhancement [24]. This was followed by a 30 ml saline flush performed at the CM injection rate. In all examinations, iopamidol 370 mgI/ml (Iopamiron 370; Nihon Bayer, Tokyo, Japan) was injected via a 20-gauge catheter inserted into an antecubital vein with a power injector (Dual Shot GX-7; Nemoto Kyorindo, Tokyo, Japan). A bolus-tracking technique was used to time the start of scanning after CM injection. A region of interest (ROI) was placed in the right internal carotid artery (ICA). Scanning began 4 s after attenuation reached the pre-defined threshold of 100 Hounsfield units (HU).
BSCTA image data were obtained by subtracting non-enhanced image data from the CTA image data using a 3D workstation (Synapse Vincent, Fuji Film Medicals) equipped with bone subtraction software. The scanning parameters and image reconstruction algorithms for non-enhanced CT scans were the same as for enhanced CT scans to reduce potential misregistration artifacts [29]. The detailed scanning and reconstruction parameters for each protocol are shown in Table 1.
Radiation dose measurement
The volume CT dose index (CTDIvol) and dose length product (DLP) were recorded for each protocol. The estimated effective dose (ED) was calculated by multiplying the DLP by the conversion factor 0.0021 mSv/(mGy×cm) [30].
Quantitative image analysis
A board-certified radiologist with 9 years of neuroimaging experience performed quantitative image analysis. On axial source images, we placed circular ROIs in the bilateral ICA at the cavernous and in the middle cerebral arteries (MCA) at the M1 segments and in the thalamus. Attempts were made to select ROIs measuring approximately 3.0 and 0.5 mm2 in the ICA and the MCA, respectively, carefully avoiding the vessel wall or partial volume effects. An ROI of approximately 30 mm2 was placed in the thalamus. Visible blood vessels and focal lesions were carefully excluded from the ROI measurements. To minimize bias from single measurements and to ensure data consistency, the average of the two values was calculated. Image noise was defined as the standard deviation (SD) of attenuation in the thalamus. The contrast-to-noise ratio (CNR) was calculated with the formula
Qualitative image analysis
Two board-certified radiologists with 10 and 8 years of neuroimaging experience, respectively, independently graded the image quality yielded by each protocol. They used multi-planar reconstruction (MPR), slab maximum intensity projection (slab-MIP), and volume-rendered (VR) images. The CT data sets were randomized, and the readers were blinded to the acquisition parameters. On a four-point subjective scale, they independently graded arterial contrast (1 = unacceptable for diagnosis, 2 = suboptimal but still diagnostic, 3 = average, 4 = excellent). Vascular sharpness was assessed by evaluating vessel wall sharpness (1 = blurry, 2 = poorer than average, 3 = average, 4 = sharpest). Visualization of arteries adjacent to the skull base—and, in patients who had undergone clipping surgery, visualization around the clip—was also graded (1 = unacceptable because of severe venous enhancement or artifacts, 2 = venous enhancement or artifacts present and partially interfering with depiction, 3 = venous enhancement or artifacts present without interfering with depiction, 4 = no venous enhancement or artifact). In addition, they scored the depiction of small arteries (1 = no depiction, 2 = faint depiction, 3 = average depiction, 4 = excellent depiction). Lastly, the overall image quality was graded (1 = unacceptable for diagnosis, 2 = suboptimal but still diagnostic, 3 = average, 4 = excellent). A training session was provided to both readers before image review to familiarize them with the scoring system. Interobserver disagreement was resolved by the two readers’ consensus during joint reading to determine the final score.
Statistical analysis
All numeric values are reported as the mean ± SD. To compare the patient age, BW, the CM amount and injection rate, the ED, CT attenuation, image noise, and CNR of the two protocols, we used the unpaired Student’s t test after normal distribution was confirmed with Kolmogorov–Smirnov test. We compared the male/female ratio and the ratio of patients with and without clipped aneurysm using the chi-squared test. The visual scores assigned to the 120- and the 80-kVp images were compared with the Mann–Whitney U test. Differences of p < 0.05 were considered statistically significant. The scale for the kappa coefficients was less than 0.20 = poor, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, and 0.81–1.00 = near perfect. Statistical analyses were performed with statistical software (R, version 2.6.1; www.r-project.org/).
Results
Patient characteristics and CM and radiation doses
As shown in Table 2, there was no significant difference in the characteristics of the patients scanned with the two protocols (p = 0.32–0.56). The indications for CTA in the 120-kVp group were suspicion of aneurysmal rupture due to non-traumatic SAH confirmed by non-enhanced CT (n = 12), suspicion of aneurysms on MRA (n = 7), oculomotor paralysis (n = 1), and follow up of known untreated aneurysm (n = 8) and clipped aneurysm (n = 9). The indications in the 80-kVp group were suspicion of aneurysmal rupture due to non-traumatic SAH confirmed by non-enhanced CT (n = 11), suspicion of aneurysms on MRA (n = 5), and follow up of known untreated aneurysm (n = 9) and clipped aneurysm (n = 13). The 120-kVp protocol identified a total of nine clipped aneurysms in 9 patients (ICA, n = 4; MCA, n = 3; anterior communicating artery (AcomA), n = 2); the 80-kVp protocol identified a total of 14 (ICA, n = 5; MCA, n = 4; AcomA, n = 5) in 13 patients. By referring to the surgery records, we confirmed that all clips were made from titanium (Sugita clip, Mizuho, Tokyo, Japan). The radiation dose and the total iodine load of the 80-kVp protocol were significantly lower than those of the 120-kVp protocol (ED: 0.59 ± 0.06 vs 1.56 ± 0.13 mSv, p < 0.01; CM volume: 42.5 ± 7.7 vs 54.9 ± 11.5 ml, p < 0.01) (Fig. 1). The CM injection rate was not significantly different between the two protocols (4.2 ± 0.8 ml/s for 80 kVp vs 4.6 ± 1.0 ml/s for 120 kVp, p = 0.11).
Quantitative image analysis
Table 3 summarizes the result of quantitative analysis. Attenuation of the ICA and MCA was significantly higher under the 80- than the 120-kVp protocol (ICA: 557.4 ± 105.7 vs 370.0 ± 59.3 HU, p < 0.01; MCA: 551.9 ± 107.9 vs 364.6 ± 62.2 HU, p < 0.01). While image noise was significantly higher on the 80- than the 120-kVp images (11.1 ± 1.2 vs 9.3 ± 1.1 HU, p < 0.01), the CNR of the ICA and the MCA was significantly higher on the 80- than the 120-kVp images (ICA: 46.2 ± 10.2 vs 36.9 ± 7.6, p < 0.01; MCA: 45.7 ± 10.0 vs 35.7 ± 9.0, p < 0.01).
Qualitative image analysis
The visibility of arteries adjacent to the skull base and clips was not significantly different between the two protocols (p = 0.45). Higher subjective scores were assigned to the 80- than the 120-kVp images (p < 0.05). There was moderate to substantial interobserver agreement for arterial contrast, vascular sharpness, visibility of arteries near the skull base and clip, depiction of small arteries, and overall image quality (kappa = 0.70, 0.58, 0.61, 0.64, and 0.63, respectively) (Table 4).
Representative cases are shown in Figs. 2, 3, and 4. Figure 2 shows the case of a 71-year-old woman with a left MCA aneurysm who underwent BSCTA under both the 120-kVp and the 80-kVp protocols after 6 months. Although the radiation and the CM doses of the 80-kVp scans were substantially lower than those in the 120-kVp protocol (CTDIvol: 14.8 vs 41.8 mGy; CM dose: 51 vs 65 ml), the image quality of the 80-kVp scan was better than that of the 120-kVp scan. Figure 3 shows the 80-kVp images of a 73-year-old woman who had undergone clipping of a right ICA aneurysm (CTDIvol: 16.4 mGy; CM: 38 ml). Arteries around the skull base and clip were obscured on the non-subtraction images. They were clearly depicted on subtraction images without severe clip-induced artifacts or confounding contrast enhancement of the cavernous sinus. Figure 4 shows the 80-kVp images of an 81-year-old woman with a clipped left MCA aneurysm (CTDIvol: 16.2 mGy; CM: 32 ml). The ICA at the level of the skull base and the aneurysm remnant at the left MCA, obscured on the non-subtraction image, were clearly depicted on the subtraction image.
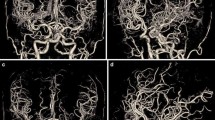
A 71-year-old woman underwent bone subtraction CTA under the 120-kVp (CTDIvol: 41.8 mGy, Iopamiron 370; 65 ml) and the 80-kVp protocols (CTDIvol: 14.8 mGy, Iopamiron 370; 51 ml) at the 6-month interval. Subtraction MIP (a) and volume rendering (b) images of the 120-kVp (left) and the 80-kVp (right) protocols. Compared to the 120-kVp imaging, the 80-kVp protocol allowed for a 64.6% reduction in the radiation exposure and a 20% reduction in the amount of contrast medium while improving the arterial enhancement and overall image quality
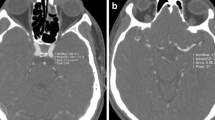
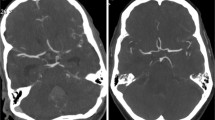
A 73-year-old woman who had undergone clipping of a right ICA aneurysm was scanned with the 80-kVp protocol (iopamidol-370, 38 ml; CTDIvol: 16.4 mGy). The upper panel shows the non-subtraction slab-MIP (left) and subtraction MIP with clip (middle) and without clip (right). The lower panel shows volume-rendered images of non-subtraction (left) and subtraction with clip (middle) and without clip (right). Arteries around the skull base and clip were obscure on the non-subtraction image. They were clearly depicted on subtraction images without severe clip-induced artifacts or confounding contrast enhancement of the cavernous sinus
Volume-rendered (VR) images of an 81-year-old woman with a clipped left MCA aneurysm. Scanning was with the 80-kVp protocol (iopamidol-370, 32 ml; CTDIvol, 16.2 mGy). The upper and lower rows show the right anterior oblique and inferior views, respectively, of the non-subtraction CTA (left) and subtraction CTA with clip (middle) and without clip (right). The ICA at the level of the skull base and the aneurysm remnant at the left MCA, obscure on the non-subtraction image, was clearly depicted on subtraction images
Discussion
Our study demonstrates that under the 80-kVp with SAFIRE protocol, both the radiation and the CM dose were substantially reduced at cerebral BSCTA and that the image quality was better than on scans obtained with the standard 120-kVp with FBP protocol.
Several prior studies [22–28] applied low tube voltage techniques and investigated strategies to reduce the radiation or the CM dose at NSCTA. Luo et al. who used 80 kVp obtained a substantial radiation and CM dose reduction compared to a 120-kVp protocol [26]. They reported that at 80 kVp, the CNR of arteries was significantly lower than at 120 kVp because of the higher image noise (15 ± 2 vs 8 ± 1 HU). On the other hand, Chen et al. [27] applied IR to NSCTA and reported that compared to a 120 kVp with FBP protocol, at 70 kVp with SAFIRE protocol using the same amount of CM (60 ml), they obtained a decrease in the radiation dose while maintaining the CNR. Despite these suggestive findings, there has been no available literature on the reduction of both the radiation and the CM dose obtained by combining 80 kVp and SAFIRE for cerebral BSCTA.
Our quantitative image analysis showed that the CNR was better at 80 kVp with SAFIRE than at 120 kVp with FBP and that arterial attenuation was markedly higher on the 80- than the 120-kVp images despite a 23% CM dose reduction. Although the image noise on the 80 kVp with SAFIRE scans was higher than on the 120 kVp with FBP images (11.1 vs 9.3 HU), the difference was smaller than in earlier studies that compared lower tube voltage scans without IR and the 120-kVp cerebral CTA protocols [23–27]. The relatively low image noise and substantially higher arterial attenuation on the 80-kVp images resulted in a higher CNR on the 80- than the 120-kVp images. Similarly, in qualitative image analysis, the arterial contrast, vascular sharpness, depiction of small arteries, and overall image quality were significantly better on the 80- than the 120-kVp images (p < 0.05). These findings suggest that a further reduction in CM and radiation dose may be possible at 80 kVp with SAFIRE protocol.
In addition, we evaluated the image quality of the subtraction CTA images, which is considered clinically important because BSCTA has higher diagnostic performance than NSCTA and could replace invasive 3D-DSA for evaluation of aneurysms [8–11]. The visibility of arteries at the skull base on the 80- and the 120-kVp images was rated as equivalent. While higher venous enhancement in the cavernous sinus at low tube voltage may obfuscate the cavernous part of the ICA, we encountered no venous contamination preventing the visibility of the ICA, probably because we used compact CM injection in our 80-kVp protocol [24]. Although clip-induced artifacts are generally stronger at lower than higher tube voltage settings [31], there was no significant difference in visibility around the clips between protocols. All clips in our series were made of titanium; they produce fewer artifacts than cobalt alloy clips [31], and the subtraction technique and the SAFIRE algorithm seem to further decrease these artifacts [31, 32]. As the number of clipped aneurysms was limited in both protocols, further studies are needed to determine the difference in clip-related artifacts between protocols.
Our study has some limitations. First, the number of enrolled patients and of identified clips was relatively small. Additional large-scale studies are needed to confirm this study’s results. Second, we focused on the image quality and did not evaluate diagnostic performance with respect to specific intracranial diseases such as intracranial aneurysms or aneurysm remnants after clipping surgery. 3D-DSA as reference standard is not routinely performed at our institution; almost all patients were diagnosed and followed up on the basis of BSCTA findings. In the most recently reported study, Ni et al. demonstrated that the CTA protocol using 80 kVp with FBP detects aneurysms with the same diagnostic accuracy as 120 kVp while reducing the radiation and CM doses [28]. As they used FBP algorithm at the 80-kVp protocol, additional investigations are needed to confirm whether the BSCTA protocol combining 80 kVp with SAFIRE improves aneurysm detection (especially at the skull base or near the clip) while reducing both the radiation and CM doses. Third, we acquired unenhanced mask CT images using the same scanning parameters used for contrast-enhanced CT. When mask image acquisition is performed at lower radiation dose [9, 33], the quality of BSCTA scans may be affected by misregistration artifacts [29]. Although the total radiation dose increased due to mask image acquisition, this disadvantage was overcome by applying a lower tube voltage and the IR algorithm. The radiation dose of the 80-kVp protocol in this study was substantially smaller than that of previous reported BSCTA protocols [8, 9, 24, 34], and further dose reduction might be possible. Lastly, in our 80-kVp protocol, we evaluated the image quality with only one IR strength level (S3) and additional studies are needed to evaluate the image quality at different IR strengths.
In conclusion, our comparison of an 80 kVp with SAFIRE and a 120 kVp with FBP protocol for cerebral bone subtraction CTA showed that use of the 80 kVp resulted in a substantial decrease in both the radiation and the CM dose and yielded better image quality.
References
Lell MM, Anders K, Uder M et al (2006) New techniques in CT angiography. Radiographics 26(Suppl 1):S45–S62
van Rooij WJ, Sprengers ME, de Gast AN, Peluso JP, Sluzewski M (2008) 3D rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 29:976–979
Dawkins AA, Evans AL, Wattam J et al (2007) Complications of cerebral angiography: a prospective analysis of 2,924 consecutive procedures. Neuroradiology 49:753–759
Fifi JT, Meyers PM, Lavine SD et al (2009) Complications of modern diagnostic cerebral angiography in an academic medical center. J Vasc Interv Radiol 20:442–447
Moran CJ (2011) Aneurysmal subarachnoid hemorrhage: DSA versus CT angiography—is the answer available? Radiology 258:15–17
Gonner F, Lovblad KO, Heid O et al (2002) Magnetic resonance angiography with ultrashort echo times reduces the artefact of aneurysm clips. Neuroradiology 44:755–758
Olsrud J, Latt J, Brockstedt S, Romner B, Bjorkman-Burtscher IM (2005) Magnetic resonance imaging artifacts caused by aneurysm clips and shunt valves: dependence on field strength (1.5 and 3 T) and imaging parameters. J Magn Reson Imaging 22:433–437
Chen W, Xing W, Peng Y, He Z, Wang C, Wang Q (2013) Cerebral aneurysms: accuracy of 320-detector row nonsubtracted and subtracted volumetric CT angiography for diagnosis. Radiology 269:841–849
Aulbach P, Mucha D, Engellandt K, Hadrich K, Kuhn M, von Kummer R (2016) Diagnostic impact of bone-subtraction CT angiography for patients with acute subarachnoid hemorrhage. AJNR Am J Neuroradiol 37:236–243
Hwang SB, Kwak HS, Han YM, Chung GH (2011) Detection of intracranial aneurysms using three-dimensional multidetector-row CT angiography: is bone subtraction necessary? Eur J Radiol 79:e18–e23
Tomura N, Sakuma I, Otani T et al (2011) Evaluation of postoperative status after clipping surgery in patients with cerebral aneurysm on 3-dimensional-CT angiography with elimination of clips. J Neuroimaging 21:10–15
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Mathews JD, Forsythe AV, Brady Z et al (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346:f2360
Yuan MK, Tsai DC, Chang SC et al (2013) The risk of cataract associated with repeated head and neck CT studies: a nationwide population-based study. AJR Am J Roentgenol 201:626–630
Villablanca JP, Duckwiler GR, Jahan R et al (2013) Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology 269:258–265
Wong JM, Ho AL, Lin N et al (2013) Radiation exposure in patients with subarachnoid hemorrhage: a quality improvement target. J Neurosurg 119:215–220
Gelfand AA, Josephson SA (2011) Substantial radiation exposure for patients with subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 20:131–133
McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW (1997) Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 103:368–375
Gruberg L, Mintz GS, Mehran R et al (2000) The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 36:1542–1548
Nakaura T, Kidoh M, Sakaino N et al (2013) Low contrast- and low radiation dose protocol for cardiac CT of thin adults at 256-row CT: usefulness of low tube voltage scans and the hybrid iterative reconstruction algorithm. Int J Cardiovasc Imaging 29:913–923
Oda S, Utsunomiya D, Yuki H et al (2015) Low contrast and radiation dose coronary CT angiography using a 320-row system and a refined contrast injection and timing method. J Cardiovasc Comput Tomogr 9:19–27
Waaijer A, Prokop M, Velthuis BK, Bakker CJ, de Kort GA, van Leeuwen MS (2007) Circle of Willis at CT angiography: dose reduction and image quality—reducing tube voltage and increasing tube current settings. Radiology 242:832–839
Cho ES, Chung TS, DK O et al (2012) Cerebral computed tomography angiography using a low tube voltage (80 kVp) and a moderate concentration of iodine contrast material: a quantitative and qualitative comparison with conventional computed tomography angiography. Investig Radiol 47:142–147
Kidoh M, Nakaura T, Ogata T et al (2013) Subtracted 3D CT angiography for the evaluation of intracranial aneurysms in 256-slice multidetector CT: usefulness of the 80-kVp plus compact contrast medium bolus protocol. Eur Radiol 23:3012–3019
Cho ES, Chung TS, Ahn SJ, Chong K, Baek JH, Suh SH (2015) Cerebral computed tomography angiography using a 70 kVp protocol: improved vascular enhancement with a reduced volume of contrast medium and radiation dose. Eur Radiol 25:1421–1430
Luo S, Zhang LJ, Meinel FG et al (2014) Low tube voltage and low contrast material volume cerebral CT angiography. Eur Radiol 24:1677–1685
Chen GZ, Zhang LJ, Schoepf UJ et al (2015) Radiation dose and image quality of 70 kVp cerebral CT angiography with optimized sinogram-affirmed iterative reconstruction: comparison with 120 kVp cerebral CT angiography. Eur Radiol 25:1453–1463
Ni QQ, Chen GZ, Schoepf UJ et al (2016) Cerebral CTA with low tube voltage and low contrast material volume for detection of intracranial aneurysms. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A4803
Watanabe Y, Kashiwagi N, Yamada N et al (2008) Subtraction 3D CT angiography with the orbital synchronized helical scan technique for the evaluation of postoperative cerebral aneurysms treated with cobalt-alloy clips. AJNR Am J Neuroradiol 29:1071–1075
ICRP (2007) Managing patient dose in multi-detector computed tomography (MDCT). ICRP Publication 102 Ann ICRP 37:1–79
van der Schaaf I, van Leeuwen M, Vlassenbroek A, Velthuis B (2006) Minimizing clip artifacts in multi CT angiography of clipped patients. AJNR Am J Neuroradiol 27:60–66
Ebersberger U, Tricarico F, Schoepf UJ et al (2013) CT evaluation of coronary artery stents with iterative image reconstruction: improvements in image quality and potential for radiation dose reduction. Eur Radiol 23:125–132
Venema HW, Hulsmans FJ, den Heeten GJ (2001) CT angiography of the circle of Willis and intracranial internal carotid arteries: maximum intensity projection with matched mask bone elimination-feasibility study. Radiology 218:893–898
Tomandl BF, Hammen T, Klotz E, Ditt H, Stemper B, Lell M (2006) Bone-subtraction CT angiography for the evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 27:55–59
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human studies have been approved by the institutional review board of Kumamoto City Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nagayama, Y., Nakaura, T., Tsuji, A. et al. Cerebral bone subtraction CT angiography using 80 kVp and sinogram-affirmed iterative reconstruction: contrast medium and radiation dose reduction with improvement of image quality. Neuroradiology 59, 127–134 (2017). https://doi.org/10.1007/s00234-016-1776-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1776-9