Abstract
Introduction
Cerebellar cortical infarct cavities are a newly recognised entity associated with atherothromboembolic cerebrovascular disease and worse physical functioning. We aimed to investigate the relationship of cerebellar cortical infarct cavities with symptomatic vertebrobasilar ischaemia and with vascular risk factors.
Methods
We evaluated the MR images of 46 patients with a recent vertebrobasilar TIA or stroke and a symptomatic vertebral artery stenosis ≥50 % from the Vertebral Artery Stenting Trial (VAST) for the presence of cerebellar cortical infarct cavities ≤1.5 cm. At inclusion in VAST, data were obtained on age, sex, history of vertebrobasilar TIA or stroke, and vascular risk factors. Adjusted risk ratios were calculated with Poisson regression analyses for the relation between cerebellar cortical infarct cavities and vascular risk factors.
Results
Sixteen out of 46 (35 %) patients showed cerebellar cortical infarct cavities on the initial MRI, and only one of these 16 patients was known with a previous vertebrobasilar TIA or stroke. In patients with symptomatic vertebrobasilar ischaemia, risk factor profiles of patients with cerebellar cortical infarct cavities were not different from patients without these cavities.
Conclusion
Cerebellar cortical infarct cavities are seen on MRI in as much as one third of patients with recently symptomatic vertebral artery stenosis. Since patients usually have no prior history of vertebrobasilar TIA or stroke, cerebellar cortical infarct cavities should be added to the spectrum of common incidental brain infarcts visible on routine MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although brain infarcts are typically associated with clinical stroke syndromes, they may remain clinically silent or present with nonspecific clinical findings [1–3]. In addition, recent infarction may be missed on CT, and, if performed beyond the stage of diffusion restriction, even on MRI [4]. Afterwards, central liquefaction and cavitation of infarcted brain tissue ensues, and therefore, many infarcts only present later in life as an incidental infarct cavity on CT or MRI [5]. In the cerebrum, subcortical infarct cavities of small size (lacunes of presumed vascular origin) have been associated with an increased risk of future stroke and cognitive decline [5, 6]. In the cerebellum, small cortical infarct cavities (≤1.5 cm) have been recently found to be far more common than subcortical lacunes [7–10]. Such cerebellar cortical infarct cavities are easily distinguishable on routine MRI because of the characteristic sparing of adjacent subcortical white matter as well as their typical location along cerebellar fissures [9, 10]. They have been found on 1.5-T MRI in almost 10 % of patients with arterial disease (including cardiovascular disease, peripheral arterial disease, aortic aneurysms, and cerebrovascular disease), and have been associated with atherothromboembolic disease [7, 11, 12] and worse physical functioning [12]. Nevertheless, the relationship of cerebellar cortical cavities with symptomatic vertebrobasilar ischaemia (TIA or stroke) and with prior history of vertebrobasilar TIA or stroke has not yet been established.
The present study aimed to evaluate the relationship between cerebellar cortical infarct cavities, symptomatic vertebrobasilar ischaemia and vascular risk factors. Therefore, we evaluated MRI scan(s) of patients with a recent vertebrobasilar TIA or stroke and a symptomatic stenosis of the vertebral artery ≥50 % from the randomized Vertebral Artery Stenting Trial (VAST) [13, 14].
Materials and methods
Patients
The present study is a post hoc analysis of the VAST, a prospective multicentre randomised clinical trial in which patients with a vertebrobasilar TIA or stroke in the last 6 months and a vertebral artery stenosis ≥50 % were randomised to stenting and best medical treatment or best medical treatment alone. The methods of VAST have been described previously [13, 14]. The VAST was approved by the ethics committee of the University Medical Center Utrecht in The Netherlands and all patients provided written informed consent. At inclusion in VAST, all patients data were obtained on age, sex, history of vertebrobasilar TIA or stroke, vascular risk factors (hypertension, hyperlipidaemia, current smoking, diabetes mellitus, and history of heart disease or peripheral arterial disease), cerebellar symptoms at initial vertebrobasilar TIA or stroke (vertigo, diplopia, dysarthria, or loss of coordination of extremities or trunk), and cerebellar signs on physical examination (dysarthria, nystagmus, or ataxia).
A total of 115 patients have been included in VAST from June 2008 to April 2013. For the present study, we evaluated the 46 patients included in the University Medical Centre Utrecht, The Netherlands, in whom a MRI scan of the brain had been performed at presentation before inclusion in the VAST and before digital subtraction angiography (with or without vertebral artery stenting).
Image analysis
MRI scans of the brain were re-evaluated for cerebellar cortical infarct cavities (<1.5 cm) by a European Board Certified neuroradiologist with special expertise in the cerebellum and stroke imaging (LDC). Cavitation was defined as the intralaesional presence of fluid intensity on T1- and T2-weighted images. Signal nulling within a lesion on FLAIR was interpreted as cavitation, while its absence was not necessarily indicative of absent cavitation due to the low sensitivity of FLAIR-weighted images in the posterior fossa [10]. Acute (diffusion-weighted imaging (DWI) positive) brain infarcts were re-evaluated by the same observer (LDC) on the MRI of 44 out of 46 patients in which the protocol included a DWI sequence. An isolated infarction in one of the temporal or occipital lobes was considered an anterior circulation infarction if this had occurred in the presence of a dominant (‘foetal type’) ipsilateral posterior communicating artery. Acute infarcts in the posterior circulation were specified according to location in- or outside the cerebellum. Whenever follow-up MRI exams of acute cerebellar infarcts were available, evidence of cavitation over time was evaluated.
Statistical analyses
The frequency of baseline characteristics was compared between patients with and without cerebellar cortical infarct cavities with Poisson regression analysis and described as prevalence ratios with corresponding 95 % confidence intervals (CIs). In multivariable analysis, adjustments were made for age and sex. Statistical analyses were performed with SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
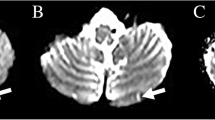
Forty-six patients could be included. Sixteen of them (35 %) showed evidence of cerebellar cortical infarct cavities (Fig. 1) performed at the initial MRI scan. No significant association was found between cerebellar cortical infarct cavities and vascular risk factors, nor with a history of vertebrobasilar TIA or stroke, nor with cerebellar symptoms or signs (Table 1). Only one of 16 patients with cerebellar cortical infarct cavities had suffered from a previous vertebrobasilar stroke (Table 1). None of these patients had a history of vertebrobasilar TIA (Table 1).
Incidental cerebellar cortical infarct cavity in a patient with acute pontine infarction: 56-year-old female with a cerebellar cortical infarct cavity in the cerebellum on the left side (a T2WI) at the time of presentation with acute left pontine infarction (b DWI), the former indicative of prior (‘incidental’) infarction in the posterior circulation. Notice this cavity has a linear configuration since it is cut in the plane of its long axis
On DWI, 32 (73 %) of 44 patients showed acute infarction in the posterior circulation (Figs. 1 and 2), notably ten (23 %) patients with acute infarction in the cerebellum, six (14 %) with acute infarction outside the cerebellum, and another 16 (36 %) patients showed an acute infarction both in- and outside the cerebellum. Of all 26 patients with acute cerebellar infarctions, 24 (92 %) showed evidence of cerebellar symptoms at initial vertebrobasilar TIA or stroke, and 14 (54 %) showed evidence of cerebellar signs on physical examination at inclusion.
Acute cerebellar cortical infarct evolving into an infarct cavity: Top row: 56-year-old female (other patient than in Fig. 1) with acute infarction (<1 cm) in the left anterior lobe of the cerebellum on T2WI (a), FLAIR (b), and DWI (c). Bottom row: The same MRI sequences 2 years later (d–f) show a cerebellar cortical infarct cavity replacing the acute infarct on imaging. This cerebellar infarct was detected in the acute stage together with concomitant infarctions elsewhere in the posterior circulation (left midbrain and thalamus, right occipital and deep temporal lobe, not shown). Notice the cavity has a rounded configuration since it is cut orthogonally to its long axis
Three patients with evidence of acute cerebellar infarction underwent follow-up MRI-scanning after an interval exceeding 6 months, and developed cerebellar cortical infarct cavities replacing the initial DWI-positive acute cerebellar infarctions (Fig. 2). Of these three patients, one showed evidence of two acute cerebellar infarcts and, half a year (186 days) later, had developed cavitation of the smaller infarct (0.9 × 0.5 cm in the acute stage) and gliosis without evidence of cavitation of the larger one (1.4 × 1.2 cm in the acute stage).
Discussion
Our study indicates that cerebellar cortical infarct cavities are present in as much as one third of patients with recently symptomatic vertebral artery stenosis ≥50 %. Only one out of 16 patients with cerebellar cortical infarct cavities showed a positive prior history of TIA or stroke. Thus, many cerebellar infarctions remain initially occult and only present as an incidental finding on MRI after infarct involution. In order not to miss recent cerebellar infarctions, a high clinical index of suspicion and a low threshold for performing MRI studies is required, preferentially within the shortest delay as possible, thus within the stage of restricted diffusion on MRI.
In contrast to the present study, cerebellar cortical infarct cavities were found in only 9.6 % of a large cohort of patients of similar age with various arterial diseases [10, 12]. The higher number of cerebellar cortical infarct cavities observed in patients with vertebral artery stenosis ≥50 % and recent vertebrobasilar TIA or stroke suggests that cerebellar cortical infarct cavities and vertebrobasilar TIA or stroke are interrelated. Since all patients included in the present study had symptomatic vertebrobasilar TIA or stroke, this may also explain why no differential risk factor profiles were found between patients with and without cerebellar cortical infarct cavities. To further support this hypothesis, we followed the longitudinal evolution of acute cerebellar infarcts on MRI in patients of whom follow-up MRI scans were available. In all three patients with follow-up MRI scans after an interval exceeding 6 months, cerebellar infarcts were seen to evolve into cerebellar cortical infarct cavities, adding imaging evidence to the atherothromboembolic origin of cerebellar cortical infarct cavities [12].
Strengths of our study include the well-defined inclusion criteria of patients with recently symptomatic vertebral artery stenosis ≥50 % and a careful documentation of vascular risk factors and medical history. Limitations of our study include the post hoc analysis of only a limited number of patients, which precluded the correlation of cerebellar cortical infarct cavities in the posterior inferior cerebellar artery territory with the side (ipsilateral or contralateral) of vertebral artery stenosis [15]; the anterior and superior cerebellar arteries both arise from the basilar artery so that emboli from the vertebral artery may embolise to both sides. Moreover, cerebellar cortical infarct cavities could not be correlated with atrial fibrillation and cardioembolism, as patients with atrial fibrillation were excluded from VAST. Also, since symptomatic vertebral artery stenosis and cerebellar cortical infarct cavities appear to be interrelated, potential cause-effect relationships of individual vascular risk factors may have been obscured due to external validation issues. In addition, gradient echo- and/or susceptibility-weighted imaging (SWI) sequences were not routinely performed in VAST, which precluded the systematic investigation of cerebellar cortical microbleeds. Finally, the number of patients who underwent follow-up MRI was limited.
In conclusion, this study shows that cerebellar cortical infarct cavities are seen on MRI in as much as one third of patients with recently symptomatic vertebral artery stenosis. They are indicative of prior infarction in the posterior circulation, and should be added to the spectrum of common incidental brain infarcts visible on routine MRI.
References
Corea F, Hénon H, Milia P, Amici S, Leys D (2000) Silent infarcts and outcome. Cerebrovasc Dis 10(Suppl 4):42–44
Compter A, Kappelle LJ, Algra A, van der Worp HB (2013) Nonfocal symptoms are more frequent in patients with vertebral artery than carotid artery stenosis. Cerebrovasc Dis 35:378–384
Samim M, Hendrikse J, van der Worp HB, Agostoni P, Nijhoff F, Doevendans PA, Stella PR (2015) Silent ischemic brain lesions after transcatheter aortic valve replacement: lesion distribution and predictors. Clin Res Cardiol 104:430–438
Jauch EC, Saver JL, Adams HP et al (2013) Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44:870–947
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM (2003) Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348:1215–1222
Park K-Y, Chung P-W, Kim YB, Moon HS, Suh BC, Won YS (2009) Association between small deep cerebellar ischemic lesion and small-vessel disease. Cerebrovasc Dis 28:314–320
De Cocker LJ, van Veluw SJ, Fowkes M, Luijten PR, Mali WP, Hendrikse J (2013) Very small cerebellar infarcts: integration of recent insights into a functional topographic classification. Cerebrovasc Dis 36:81–87
De Cocker LJ, van Veluw SJ, Biessels GJ, Spliet WG, Thunnissen IE, Luijten PR, Hendrikse J, Zwanenburg JJ (2014) Ischaemic cavities in the cerebellum: an ex vivo 7 Tesla MRI study with pathologic correlation. Cerebrovasc Dis 38:17–23
De Cocker LJ, Geerlings MI, Hartkamp NS, Grool AM, Mali WP, Van der Graaf Y, Kloppenborg RP, Hendrikse J (2015) Cerebellar infarct patterns: the SMART-Medea study. NeuroImage Clin 8:314–321
De Reuck JL, Deramecourt V, Auger F et al (2015) The significance of cortical cerebellar microbleeds and microinfarcts in neurodegenerative and cerebrovascular diseases. A post-mortem 7.0-tesla magnetic resonance study with neuropathological correlates. Cerebrovasc Dis 39:138–143
De Cocker LJ, Kloppenborg RP, van der Graaf Y, van der Graaf Y, Luijten PR, Hendrikse J, Geerlings MI (2015) Cerebellar cortical infarct cavities: correlation with risk factors and MRI markers of cerebrovascular disease. Stroke 46:3154–3160
Compter A, van der Worp HB, Schonewille WJ, Vos JA, Algra A, Lo TH, Mali WP, Moll FL, Kappelle LJ (2008) VAST: Vertebral Artery Stenting Trial. Protocol for a randomised safety and feasibility trial. Trials 9:65
Compter A, van der Worp HB, Schonewille WJ, Vos JA, Boiten J, Nederkoorn PJ, Uyttenboogaart M, Lo RT, Algra A, Kappelle LJ (2015) Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: a randomised open-label phase 2 trial. Lancet Neurol 14:606–614
Hartkamp NS, De Cocker LJ, Helle M, van Osch MJ, Kappelle LJ, Bokkers RP, Hendrikse J (2013) In vivo visualization of the PICA perfusion territory with super-selective pseudo-continuous arterial spin labeling MRI. Neuroimage 83:58–65
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human and animal studies have been approved by the ethics committee of the University Medical Center Utrecht in The Netherlands and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
JH has received research support from European Research Council (ERC) grant number: ERC-2014-StG – 637024_HEARTOFSTROKE.
Rights and permissions
About this article
Cite this article
De Cocker, L.J.L., Compter, A., Kappelle, L.J. et al. Cerebellar cortical infarct cavities and vertebral artery disease. Neuroradiology 58, 853–857 (2016). https://doi.org/10.1007/s00234-016-1707-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1707-9