Abstract
Aims
Silent ischemic brain lesions and ischemic stroke are known complications of transcatheter aortic valve replacement (TAVR). We aimed to investigate the occurrence and distribution of TAVR-related silent ischemic brain lesions using diffusion-weighted magnetic resonance imaging (DWI).
Methods
Consecutive patients with severe aortic valve stenosis treated with TAVR underwent cerebral DWI within 5 days after the index procedure. DWI scans were analyzed for the occurrence and distribution of new ischemic lesions post-TAVR.
Results
Forty-two patients were enrolled in this study. After TAVR, a total of 276 new cerebral ischemic lesions were detected in 38 (90 %) patients, with a median of 4.5 (interquartile range 2.0–7.0) lesions per patient. A total of 129 (47 %) lesions were detected in the cortical regions, 97 (35 %) in the subcortical regions, and 50 (18 %) in the cerebellum or brainstem. The median lesion volume was 20.2 µl (10.0, 42.7) and the total ischemic lesion volume was 132.3 µl (42.8, 336.9). The new ischemic brain lesions were clinically silent in 37 (97 %) patients; the other patient had a transient ischemic attack. Age (B = 0.528, p = 0.015), hyperlipidaemia (B = 5.809, p = 0.028) and post-dilatation of the implanted prosthesis (B = 7.196, p = 0.029) were independently associated with the number of post-TAVR cerebral DWI lesions. In addition, peak transaortic gradient was independently associated with post-procedural total infarct volume.
Conclusion
Clinically silent cerebral infarcts occurred in 90 % of patients following TAVR, most of which were small (<20 μl) and located in the cortical regions of the cerebral hemispheres. An independent association was found between age, hyperlipidaemia and balloon post-dilatation and the number of post-TAVR ischemic brain lesions. Only peak transaortic gradient was independently associated with post-procedural total infarct volume.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 10 years after its first introduction in 2002 by Cribier, transcatheter aortic valve replacement (TAVR) has evolved to the new standard-of-care treatment for patients with a severe symptomatic aortic stenosis considered inoperable or at high surgical risk [1, 2]. In spite of many improvements in the technique and the valve prostheses, questions remain related to the safety and durability of this technique. Significant concerns exist with regard to the incidence of cerebral complications after TAVR. Recent large multicenter series and national registries reported a rate of stroke or TIA of 3.3 % in the first 30 days after TAVR, with the majority being major strokes (2.9 %) [3]. These rates are among the highest in the field of interventional cardiology. Furthermore, the average 30-day mortality in TAVR patients with post-procedural stroke was more than 3.5-fold higher than in non-stroke patients (25.5 vs. 6.9) [3]. In addition to the clinically apparent ischemic brain lesions, several cerebral magnetic resonance imaging studies have shown a very high (58–91 %) incidence of new ischemic lesions after TAVR, regardless of the transcatheter valve type and approach (Table 1) [4–9]. These small, usually asymptomatic, DWI lesions are most probably caused by micro-embolic particles arising during the valve implantation procedure. Hypoperfusion is thought to contribute to the occurrence of brain infarcts in TAVR patients only under the circumstances of cardiac arrest or severe systemic underperfusion. Several steps during the TAVR procedure may increase the embolic load towards the cerebral circulation. Previous studies using transcranial Doppler (TCD) have shown that cerebral microemboli are most common at the time of interaction of the prosthesis with the native valve, specifically during positioning and expansion of the prosthesis [10].
Although the consequences of silent ischemic brain lesions are still uncertain, in some studies they have been associated with cognitive decline and an increased risk of dementia and depression [11–13]. Furthermore, they have been shown to indicate an increased risk of subsequent stroke in patients with previous minor stroke and atrial fibrillation [13]. To understand the causal mechanism of silent cerebral ischemic lesions and their consequence for the cerebral function, exploring the characteristics of concurrent lesions and their location in human brain is pivotal. The typically silent aspect of the majority of TAVR-related DWI lesions might be explained by their location in the brain and their size. Furthermore, data on risk factors associated with new silent ischemic brain lesions after TAVR are scarce. In this paper, we aimed to report our findings with regard to cerebral ischemic injury contributable to TAVR in patients undergoing this procedure at our center, as post-procedural cerebral DWI is part of standard patient care at our center. We intended (1) to investigate the topographic pattern of cerebral ischemic lesions on DWI in patients undergoing TAVR, (2) to evaluate the risk factors that may be associated with the number of new lesions after TAVR and (3) to evaluate the risk factors that may be associated with the total infarct volume.
Methods
Patient population and TAVR procedure
From September 2012 to September 2013, 65 consecutive patients with severe symptomatic aortic stenosis who underwent TAVR at our center were screened for inclusion in this prospective registry. The TAVR procedures were performed with the two currently commercially available bioprostheses, the Edwards SAPIEN XT™ valve prosthesis (Edwards Lifesciences, Irvine, CA, USA) and the Medtronic CoreValve system (CoreValve Revalving Technology, Medtronic, Minneapolis, MN, USA). Patients were excluded when contraindications for MRI were present (such as a pacemaker). Between May and July 2013, a cerebral embolic protection device was used during TAVR in 15 consecutive patients and these patients were also excluded from this registry. Before the TAVR procedure, all patients were evaluated carefully for possible cardiac sources of embolism (atrial fibrillation, left ventricular thrombus, etc.) with the use of an electrocardiogram and echocardiography, which were mandatory in all patients as part of the pre-procedural TAVR evaluation protocol. After the TAVR procedures, all patients were evaluated, inter alia, for clinical signs of a new cerebrovascular event. The study was conformed to the guiding principles of the Declaration of Helsinki and patients consented to clinical evaluation. Because during the study period cerebral DWI was part of standard post-procedural care at our center, ethics approval was waived.
Diffusion-weighted magnetic resonance imaging
Magnetic resonance imaging was performed within 4 days after TAVR, using a 3 Tesla system (Philips Medical Systems, the Netherlands). The imaging protocol included a diffusion-weighted single-shot spin echo echo-planar sequence (diffusion gradient b values of 0 and 1,000 s/mm2, repetition time (TR): 3,307 ms, echo time (TE): 68 ms, 26 slices with a slice thickness of 4 mm, field of view: 230 mm, matrix: 256 × 205) and a turbo fluid attenuated inversion recovery (FLAIR; TR/TE 11,000/125 ms). The acquisition time for the diffusion-weighted sequences was 69 s.
All DWI images were assessed by two skilled observers blinded to neurological status and procedure. Number, volume, location and vascular territories of all focal diffusion abnormalities (bright lesions on DWI), signifying acute cerebral ischemia, were documented. All supratentorial infarcts were divided into subcortical infarcts and cortical infarcts. Subcortical infarcts were further divided into deep gray matter infarcts, internal border zone infarcts and true subcortical infarcts. Deep gray matter infarcts were defined as lesions within the region of basal ganglia or thalamus. Internal border zone infarcts were defined as lesions located in the white matter of the centrum semiovale or corona radiate, at the border zone between lenticulostriate perforators and the deep penetrating cortical branches of the middle cerebral artery (MCA) or at the border zone of deep white matter branches of the MCA and the anterior cerebral artery. Cortical infarcts were subclassified into cortical border zone infarcts and cortical territorial infarcts. The distinction between cortical border zone and territorial locations was based on templates of arterial flow territories [14] (Fig. 1). A cortical border zone territory was defined as the area between major cerebral arteries. Finally, infratentorial infarcts were divided into brainstem infarcts and cerebellar infarcts.
Cerebral arterial territory. ACA anterior cerebral artery, ACHA anterior choroidal artery, AICA anterior inferior cerebellar artery, bVA branches from vertebral arteries, LSA lenticulostriate arteries, MCA middle cerebral artery, PCA posterior cerebral artery, PICA posterior inferior cerebellar artery, SCA superior cerebellar artery
Preoperative assessment of calcification on contrast-enhanced MSCT
All patients underwent a contrast-enhanced, electrocardiogram-gated MSCT scan within 3 months before TAVR, as part of the pre-procedural work-up. All MSCT scans were analyzed using a software package and a dedicated 3D aortic valve analysis workflow (3mensio Valves TM, 3mensio Medical Imaging BV, The Netherlands, http://www.3mensio.com). Images of the aortic root reconstructed in systole, at 37.5 % of the R–R interval were selected for analysis of the aortic plane and measurements of aortic valve calcification. Next, a centerline was automatically placed along the ascending aorta and mark points were placed at the anchor points of the three aortic leaflets, marking the aortic annulus. Subsequently, aortic leaflet calcification was measured in a region starting from the aortic annulus to 20 mm above that, marking the most cranial aspect of the aortic leaflets. Because of the inter-patient difference in the amount of contrast used for contrast-enhanced MSCT, the threshold for detecting calcification in the aortic valve apparatus was chosen on an individual basis, using the method described by Mylonas et al. [15] for quantification of coronary artery calcification: for each patient, using axial images, a region of interest was placed in the ascending aorta 25 mm above the level of the aortic annulus. The mean aortic attenuation value (HU aorta) and standard deviation (SD) were measured at this level. Using these measures, the threshold for calcium detection was calculated as 2 SD above the mean attenuation in the aorta (HU Aorta + 2 SD).
Statistical Analysis
Categorical data are presented as frequencies and percentages and compared between groups with Pearson Chi-squared test or Fisher’s exact test. Continuous variables are presented as mean ± SD or medians and interquartile range (IQR) and compared between groups with the t test or Mann–Whitney U test.
Linear regression analysis was used to identify the relationship between patient and procedural factors and the number and the total volume of new ischemic brain lesions following TAVR. Univariate analysis was used to identify individual predictors. Variables were reviewed to exclude collinearity in the multivariate model before testing. Variables with a univariate significance of p < 0.2 were entered into a multivariate regression analysis to determine the independence of these predictors. A p value of <0.05 was considered statistically significant.
Results
Patient and technical characteristics
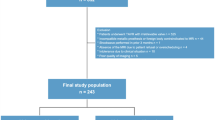
The flowchart of this study is shown in Fig. 2. Of all screened patients, twenty-three were excluded (one due to claustrophobia and hence no MRI, 6 due to pre-TAVR permanent pacemaker therapy, 15 due to the use of a cerebral protection device during TAVR and one due to post-operative hemodynamic instability). Forty-two patients (65 %) were included in this study, of whom 26 received the balloon-expandable Edwards SAPIEN XT™ valve prosthesis and 16 the self-expandable Medtronic CoreValve system. Clinical and procedural characteristics are presented in Table 2. Pre-procedural atrial fibrillation was observed in 15 patients (36 %). Transthoracic echocardiography showed no signs of ventricular thrombus in all cases.
Device success as defined by VARC-2 definitions [16] was achieved in 36 patients (86 %). Reasons for not fulfilling the device success criteria were: additional valve implantation in one patient due to too low placement of a Medtronic CoreValve prosthesis and hence severe paravalvular regurgitation, and residual moderate paravalvular regurgitation after valve implantation in five other patients (3 Edwards SAPIEN XT™ and 2 Medtronic CoreValve prostheses).
Cerebral ischemic brain lesions on DWI
Post-procedural DWI findings are shown in Tables 3, 4. Representative DWI examinations are depicted in Fig. 3. After TAVR, a total of 276 new ischemic lesions were detected in 38 patients (90 %), with a median of 4.5 [IQR 2.0–7.0] lesions per patient. These foci were typically multiple and dispersed in both hemispheres, suggesting a cardio-embolic or aortic origin of these lesions. Of all lesions, 226 (82 %) were supratentorial and 50 (18 %) infratentorial (Table 4). Moreover, 129 (57 %) of the supratentorial lesions were located in the left cerebral hemisphere and 97 (43 %) in the right hemisphere (p = 0.03). The majority (129; 57 %) of these supratentorial lesions was located in the cortical regions: 24 (19 %) in the territory of the anterior cerebral artery, 49 (38 %) in that of the middle cerebral artery, 24 (19 %) in that of the posterior cerebral artery, and 32 (25 %) in the cortical border zones. Of all infratentorial lesions, 46 (92 %) was located in the cerebellum and 4 (8 %) in the brainstem (pons/midbrain).
DWI images of three different patients 4 days after TAVR showing multiple small acute ischemic lesions in different territories. a Two micro-infarcts located in the territory of the right temporal lobe (arrow head) and the right cerebellar hemisphere (arrow). b Small DWI lesion located in the putamen (arrow). c Multiple cortical (arrow) and subcortical (arrow head) micro-infarcts
The median lesion volume was 20.2 µl [10.0, 42.7] and the median total ischemic volume was 132.3 µl [42.8, 336.9]. Half of the lesions (138) had a volume of 20 µl or smaller (Fig. 4). New DWI lesions were found more frequently in patients treated with the Medtronic CoreValve than in patients treated with the Edwards SAPIEN XT™ (6.0 [2.0, 13.5] vs. 4.0 [1.0, 6.3], p = 0.05; Table 3).
Demographic and procedural risk factor assessment
Risk factors for a higher number and greater volume of new ischemic brain lesions are reported in Tables 5, 6, respectively. In the univariate analysis, there were no associations between the presence of diabetes mellitus, hypertension, hyperlipidaemia, coronary artery disease, atrial fibrillation, carotid disease, peripheral vascular disease and prior stroke or TIA, and the number or the total volume of new ischemic brain lesions after TAVR. Pre-procedural transaortic gradients were associated with both the number and the volume of cerebral ischemic lesions after TAVR [peak gradient; (R = 0.412, p = 0.008) and (R = 0.415, p = 0.008), respectively]. The volume of aortic valve calcification was not related to the number or the total volume of new ischemic brain lesions (p = 0.142 and p = 0.443, respectively). Also none of the procedural characteristics were associated with either the number or the total volume of cerebral ischemic lesions (Tables 5, 6).
The univariate variables (age, hyperlipidemia, coronary artery disease, peak transaortic gradient, aortic valve calcification and post-dilatation) were then entered into a multivariate regression model, where age, hyperlipidemia and post-dilatation of the implanted valve prosthesis remained independent predictors of the number of new ischemic brain lesions (p = 0.015, p = 0.028, p = 0.029, respectively) (Table 5). Peak transaortic gradient was the only variable independently associated with the total volume of new ischemic brain lesions (p = 0.039) (Table 6).
Neurological performance
During in-hospital follow-up, no patient developed a new focal neurological deficit suggestive of stroke, except for one patient with temporary dysphasia, which appeared 30 min after the procedure, and was diagnosed as TIA. Cerebral MRI performed after TAVR confirmed a new ischemic lesion (40 µl) in the site corresponding to the symptoms in this patient (Wernicke’s area).
Discussion
The present study adds valuable information to the existing knowledge about silent cerebral ischemic lesions after TAVR procedures. We found new ischemic lesions on post-procedural DWI in 90 % of all cases. In this series, half of these cerebral ischemic lesions was 20 µl or smaller and about half were located in the cortex of the cerebral hemispheres. As these lesions were small, they were less likely to lead to new focal neurological deficits [17]. That is probably also part of the explanation for the absence of convincing evidence for a relationship between the size or the number of new ischemic cerebral lesions on post-TAVR DWI and clinical neurocognitive deficits.
The majority of the supratentorial lesions were located in the left cerebral hemisphere. A comparable observation was reported by two other reports [5, 7, 18], with the largest portion of DWI lesions located on the left side of the brain. Two different hypotheses might explain this finding: (1) three major arteries, originating from the aortic arch, are at risk of receiving embolic particles from the calcified aortic valve and ascending aorta: the brachiocephalic, the left common carotid and the left subclavian arteries. The last two major arteries, mainly supplying the left side of the brain, might together receive twice as many emboli as compared to the brachiocephalic artery, supplying the right side of the brain and the right arm; (2) in addition, the left common carotid and the left subclavian arteries also receive emboli dislodged from the aortic arch due to the scratching of the aortic wall by the catheters and the devices. None of these hypotheses are supported by evidence to date and future studies focusing on the embolization process, for instance with the use of TCD during TAVR, are needed. The higher incidence of cerebral DWI lesions at the left side of the brain raises the question of the usefulness of cerebral protection devices, designed specifically for usage during TAVR, that do not protect efficiently the left-sided branch arteries of the aortic arch. For instance, the Embrella Embolic Deflector System (Edwards Lifesciences Ltd., Irvine, CA, USA) and the Claret Medical protection devices lack complete coverage of the left vertebral arterial system, which may reduce the usefulness of these types of cerebral protection devices. Future refinements to cerebral protection devices may offer full coverage of all three branch arteries of the aortic arch and hence better cerebral protection.
With regard to potential risk factors for the development of new cerebral infarcts during TAVR, Fairbairn et al. [7] reported old age and atherosclerotic burden of the aorta as independent predictors of the number of new cerebral ischemic lesions after these procedures. Astarci et al. also found an association between age and the number of post-TAVR cerebral DWI lesions. In line with these previous reports, our study found an independent association between age and the number of post-TAVR cerebral DWI lesions. In addition, our study showed an independent association between hyperlipidaemia at baseline and post-dilatation after prosthesis implantation and the number of new ischemic brain lesions. The association between post-dilatation of the aortic valve prosthesis might be due to an increased interaction between the stent frame of the valve prosthesis and the native aortic valve, which might indeed favor the dislodgment of calcific particles from the native valve. One previous study by Nombela-Franco et al. [19] reported that further stretching of the calcified native valve during balloon post-dilatation is independently associated with a twofold risk of cerebrovascular events immediately or within the first few hours after the procedure. With regard to procedural risk factors, two previous studies [20] showed that high-intensity transient signals (HITS) observed with TCD occurred during all procedural intervals in TAVR, however, the embolic events appeared to peak during prosthesis deployment. Transient expansion and recoil of a metallic stent frame within a partially disrupted native valve (due to pre-dilatation) is probably a particularly efficient way to generate embolic particles, although the prosthesis itself may provide some embolic protection.
In regard to risk factors associated with the total infarct volume after TAVR, only peak transaortic gradient at baseline was found to be independently associated. One previous report by Kahlert et al. studying cerebral embolization during TAVR by use of TCD, identified mean transaortic gradient at baseline as an independent predictor for the number of HITS during TAVR. These findings may be expected as a higher transaortic gradient often reflects a more severe aortic valve stenosis, which is often accompanied by a higher amount of aortic valve calcification. The latter increases the risk of dislodgment of calcific microdebris from the degenerative leaflets. Catheter manipulation of the calcified stenotic valve increases this risk, and it has been shown previously that cerebral embolism as detected by DWI occurs even after valve passage with relatively soft diagnostic catheters [21]. In our study, however, aortic valve calcification was not significantly associated with either the number or volume of the new cerebral ischemic lesions.
Until now, no convincing evidence has related the size or the number of new ischemic lesions on post-TAVR brain imaging to clinical neurocognitive deficits. Although the clinical relevance of these DWI lesions remains uncertain, they may cause decline in cognitive function as suggested by previous reports on silent cerebral lesions involving patients other than the typical TAVR patients [11, 12, 22]. For instance, the population-based Rotterdam Scan Study showed that elderly people with silent brain infarcts have an increased risk of dementia and a steeper decline in cognitive function than those without such lesions [11, 12, 22]. Therefore, based on these previous reports, the high frequency of ischemic lesions on post-TAVR cerebral imaging calls for procedural and technical developments to reduce the risk of peri-procedural embolization. Less traumatic devices, avoidance of extensive manipulation of the calcified aortic valve, omission of post-dilatation after prosthesis implantation, and use of effective cerebral protection devices are currently under consideration [23]. Furthermore, meticulous attention must be paid to valve preparation and thorough sheath and catheter flushing to reduce thrombus formation and gaseous embolization.
Conclusion
Multiple, clinically silent, small ischemic brain lesions were detected on post-TAVR DWI in 90 % of patients, with half of the lesions being very small (<20 μl) and half located in the cortical regions of the cerebral hemispheres. An independent association was found between age, hyperlipidaemia at baseline and balloon post-dilatation and the number of post-TAVR ischemic brain lesions. Only peak transaortic gradient was independently associated with post-procedural total infarct volume.
Limitations
First, the number of patients included in this study is limited, affecting the sensitivity to find independent predictors of the occurrence of ischemic brain lesions. Second, some (clinical) factors that might be associated with the extent of cerebral ischemic injury during TAVR, such as blood pressure change during the procedure and the presence of aortic atheroma could not be assessed sufficiently due to the retrospective design of this study. In addition, our study lacks data on cognitive performance after TAVR.
References
Haussig S, Schuler G, Linke A (2014) Worldwide TAVI registries: what have we learned? Clin Res Cardiol 103(8):603–612
O’Sullivan CJ, Stortecky S, Buellesfeld L et al (2014) Preinterventional screening of the TAVI patient: how to choose the suitable patient and the best procedure. Clin Res Cardiol 103(4):259–274
Eggebrecht H, Schmermund A, Voigtlander T et al (2012) Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 8(1):129–138
Arnold M, Schulz-Heise S, Achenbach S et al (2010) Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging. JACC Cardiovasc Interv 3(11):1126–1132
Ghanem A, Muller A, Nahle CP et al (2010) Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol 55(14):1427–1432
Rodes-Cabau J, Dumont E, Boone RH et al (2011) Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol 57(1):18–28
Fairbairn TA, Mather AN, Bijsterveld P et al (2012) Diffusion-weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart 98(1):18–23
Kahlert P, Knipp SC, Schlamann M et al (2010) Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 121(7):870–878
Thilo C, Maurer CJ, Berlis A et al (2014) Successful management of cerebral embolism during TAVR. Clin Res Cardiol 103(4):329–331
Reinsfelt B, Westerlind A, Ioanes D et al (2012) Transcranial Doppler microembolic signals and serum marker evidence of brain injury during transcatheter aortic valve implantation. Acta Anaesthesiol Scand 56(2):240–247
Knecht S, Oelschlager C, Duning T et al (2008) Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J 29(17):2125–2132
Vermeer SE, Prins ND, den Heijer T et al (2003) Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348(13):1215–1222
Silent brain infarction in nonrheumatic atrial fibrillation (1996) EAFT Study Group. European Atrial Fibrillation Trial. Neurology 46(1):159–165
Tatu L, Moulin T, Vuillier F et al (2012) Arterial territories of the human brain. Front Neurol Neurosci 30:99–110
Mylonas I, Alam M, Amily N et al (2013) Quantifying coronary artery calcification from a contrast-enhanced cardiac computed tomography angiography study. Eur Heart J Cardiovasc Imaging 15:210–215
Kappetein AP, Head SJ, Genereux P et al (2013) Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 145(1):6–23
Hassell ME, Nijveldt R, Roos YB et al (2013) Silent cerebral infarcts associated with cardiac disease and procedures. Nat Rev Cardiol 10(12):696–706
Astarci P, Glineur D, Kefer J et al (2011) Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans-apical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg 40(2):475–479
Nombela-Franco L, Webb JG, de Jaegere PP et al (2012) Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 126(25):3041–3053
Erdoes G, Basciani R, Huber C et al (2012) Transcranial Doppler-detected cerebral embolic load during transcatheter aortic valve implantation. Eur J Cardiothorac Surg 41(4):778–783 (discussion 783–4)
Omran H, Schmidt H, Hackenbroch M et al (2003) Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: a prospective, randomised study. Lancet 361(9365):1241–1246
Daneault B, Kirtane AJ, Kodali SK et al (2011) Stroke associated with surgical and transcatheter treatment of aortic stenosis: a comprehensive review. J Am Coll Cardiol 58(21):2143–2150
Staubach S, Hein-Rothweiler R, Hochadel M et al (2014) Predictors of minor versus major stroke during carotid artery stenting: results from the carotid artery stenting (CAS) registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Clin Res Cardiol 103(5):345–351
Knipp SC, Kahlert P, Jokisch D et al (2013) Cognitive function after transapical aortic valve implantation: a single-centre study with 3-month follow-up. Interact CardioVasc Thorac Surg 16(2):116–122
Acknowledgments
H.B. van der Worp is supported by a grant from the Dutch Heart Foundation (2010T075).
Conflict of interest
Dr. P.R. Stella is a physician proctor for Edwards Lifesciences. All other co-authors have no relations to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samim, M., Hendrikse, J., van der Worp, H.B. et al. Silent ischemic brain lesions after transcatheter aortic valve replacement: lesion distribution and predictors. Clin Res Cardiol 104, 430–438 (2015). https://doi.org/10.1007/s00392-014-0798-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-014-0798-8