Abstract
Introduction
Contrast enhancement observable on magnetic resonance (MR) images reflects the destructive features of malignant gliomas. This study aimed to investigate the relationship between radiologic patterns of tumor enhancement, extent of resection, and prognosis in patients with anaplastic gliomas (AGs).
Methods
Clinical data from 268 patients with histologically confirmed AGs were retrospectively analyzed. Contrast enhancement patterns were classified based on preoperative T1-contrast MR images. Univariate and multivariate analyses were performed to evaluate the prognostic value of MR enhancement patterns on progression-free survival (PFS) and overall survival (OS).
Results
The pattern of tumor contrast enhancement was associated with the extent of surgical resection in AGs. A gross total resection was more likely to be achieved for AGs with focal enhancement than those with diffuse (p = 0.001) or ring-like (p = 0.024) enhancement. Additionally, patients with focal-enhanced AGs had a significantly longer PFS and OS than those with diffuse (log-rank, p = 0.025 and p = 0.031, respectively) or ring-like (log-rank, p = 0.008 and p = 0.011, respectively) enhanced AGs. Furthermore, multivariate analysis identified the pattern of tumor enhancement as a significant predictor of PFS (p = 0.016, hazard ratio [HR] = 1.485) and OS (p = 0.030, HR = 1.446).
Conclusion
Our results suggested that the contrast enhancement pattern on preoperative MR images was associated with the extent of resection and predictive of survival outcomes in AG patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaplastic gliomas (AGs) are classified as grade III malignant gliomas by the World Health Organization (WHO). The disease subtypes include anaplastic astrocytoma, anaplastic oligodendroglioma, and anaplastic oligoastrocytoma. The 5-year survival rate of AG patients is less than 40 % despite multimodality treatments such as surgery and adjuvant therapy [1]. The established prognostic factors for AG patients include age at diagnosis, preoperative Karnofsky performance status score (KPS), and the extent of resection [2–4]. Radiological biomarkers have also been utilized for postoperative survival evaluation [5–9]. However, there remains a lack of literature on the role of preoperative magnetic resonance (MR) images in predicting the survival of AG patients.
Contrast enhancement is considered a specific radiologic feature of high-grade gliomas, based on the physiological consequences of the compromised blood-brain barrier. Previous studies have revealed the significance of contrast enhancement on patient survival. Ring-like enhancement observable on computed tomography (CT) is reportedly indicative of poor survival in AG patients [9]. A smaller contrast enhancing area volume has been shown to correlate with longer overall survival (OS) in recurrent AGs [10]. A complete resection of MR contrast-enhanced tissue independently improves the outcomes of patients with oligodendroglioma and anaplastic astrocytoma, irrespective of histological grade [11, 12]. The pattern of contrast enhancement represents the biological characteristic of a tumor and has been observed to vary significantly among individual cases. However, whether the pattern of contrast enhancement on MR images has any impact on the extent of surgical resection and prognosis of AG patients has not been thoroughly investigated. Therefore, the present study aimed to identify a potential association of the tumor contrast enhancement pattern with the extent of resection and survival outcome in patients with AG.
Materials and methods
Patients
The current study spanned the period between May 2007 and August 2010. Clinical and radiologic data of 268 patients with histologically confirmed AG (WHO grade III) were retrospectively collected from our institutional database. Clinical variables included age, sex, preoperative KPS, extent of resection, histopathology, history of seizure, and postoperative adjuvant therapy. The inclusion criteria were as follows: (1) age of ≥18 years, (2) available pre-surgical MR imaging scans (T1-weighted, T2-weighted, and post-contrast T1-weighted), and (3) no previous diagnosis of any brain tumor. Patients were excluded if they had undergone any prior craniotomy or biopsy. Histopathologic diagnoses, including anaplastic astrocytoma, anaplastic oligodendroglioma, and anaplastic oligoastrocytoma, were confirmed by two independent neuropathologists, who were blinded to patients’ clinical information, according to the 2007 WHO classification of brain tumors. The adjuvant therapy was fractionated radiotherapy or chemotherapy using temozolomide. This study was approved by our Institutional Review Board, and written informed consent was obtained from all enrolled patients.
Image acquisition
Pre- and post-surgical MR images were acquired using the standard pulse sequence on a 3.0 T MR scanner (Siemens Trio, Siemens Healthcare, Erlangen, Germany). T2-weighted images were acquired using an echo time (TE) of 140 ms, a repetition time (TR) of 8000 ms, and slice thickness of 5 mm. Post-contrast T1-weighted images were acquired after injection of gadopentetate dimeglumine (Ga-DTPA Injection, Beilu Pharma, Beijing, China) at a dose of 0.1 mmol/kg, using a TE of 15 ms, a TR of 450 ms, and slice thickness of 5 mm. Postoperative MR scans used to determine the extent of resection were acquired 48–72 h after surgery.
Identification of contrast enhancement pattern
Contrast enhancement of the tumor was assessed by two neuroradiologists who were blinded to patients’ clinical data. Non-enhancing tumors were defined as no apparent hyperintensity observed on post-contrast T1-weighted images. Tumor enhancement was defined as an unequivocal increase in signal intensity observed on T1-weighted images following intravenous contrast administration. Patterns of contrast enhancement were categorized into three types according to the morphological properties of the largest enhanced tumor area on contrast-enhanced MR images. The categories were focal enhancement, defined as a largest enhancing focal diameter of ≤1.5 cm with a relatively smooth border; diffuse enhancement, defined as tumor enhancements with maximum diameters of >1.5 cm with rough borders; and ring-like enhancement, defined as cystic necrosis with peripheral enhancement (Fig. 1). Images with classification discrepancy between the two reviewers were re-evaluated by a senior neuroradiologist who decided the pattern category used in the study.
Evaluation of the extent of resection
The extent of resection was assessed by comparing the volumes of pre- and post-surgery T2 hyperintensity and contrast enhancement. Gross total resection (GTR) was defined as no visible contrast-enhancing tumor on postoperative MR images within 72 h after surgery; for tumors with no preoperative contrast enhancement, GTR was defined as removal of all abnormal hyperintense changes on preoperative MR images.
Statistical analysis
The chi-squared test was used to compare the distribution differences of each clinical variable or imaging feature between the contrast enhancement and non-contrast enhancement groups, and among patients with different tumor enhancement patterns. Additionally, log-rank analysis of Kaplan-Meier data was performed for the comparison of progression-free survival (PFS) and OS between patient cohorts. Factors showing significance on univariate analysis (p < 0.05) were further entered into multivariate analysis using the Cox proportional hazards ratio (HR) model. Non-enhancement, focal enhancement, diffuse enhancement, and ring-like enhancement were designated as 0, 1, 2, and 3, respectively on multivariate analysis.
Results
Patient characteristics
A total of 268 AG patients were included in this study, including 73 cases of anaplastic astrocytoma, 47 anaplastic oligodendrogliomas, and 148 anaplastic oligoastrocytomas. Of these, 224 (83.6 %) tumors exhibited post-T1 contrast enhancement. The pattern of tumor contrast enhancement was reviewed for all patients, revealing 40 cases with focal enhancement, 80 with diffuse enhancement, and 104 with ring-like enhancement. The age at diagnosis, preoperative KPS, and extent of resection were significantly different among patients with different tumor contrast enhancement patterns (p < 0.001, chi-squared test; Table 1). Furthermore, 73 patients were diagnosed with anaplastic astrocytoma, 47 with anaplastic oligodendroglioma, and 148 with anaplastic oligoastrocytoma. Of all 268 patients, 212 (79.1 %) received radiotherapy and 165 (61.6 %) received chemotherapy.
The association between enhancement pattern and extent of resection
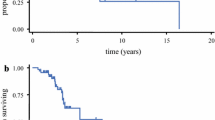
The kappa value for inter-rater agreement on enhancement patterns between the two evaluators was 0.93 (p = 0.016). In general, there were significantly fewer patients with contrast-enhancing AGs who achieved GTR than those with non-enhancing AGs (49.1 % vs. 68.2 % respectively, p = 0.021). Remarkably, the patterns of tumor contrast enhancement were found to be correlated with the extent of resection. A GTR was more likely to be achieved in patients with non-enhanced (68.2 %) or focal (70.0 %) enhanced AGs than those with diffuse (38.8 %) or ring-like (49.0 %) enhanced AGs (p < 0.001; Fig. 2). Of the enhanced AGs, focal-enhanced tumors were more amendable to surgical resection than diffuse (p = 0.001) and ring-like (p = 0.024) enhanced tumors. No significant differences in GTR rate were observed between AGs with focal enhancement and those without enhancement (p = 0.857), or between AGs with diffuse enhancement and those with ring-like enhancement (p = 0.164; Fig. 2).
Gross total resection (GTR) rates for anaplastic gliomas (AGs) with different contrast enhancement patterns. GTR was more likely to be achieved in patients with non-enhanced or focal enhanced AGs than in those with diffuse enhanced or ring-like AGs (p < 0.001). Of the enhanced AGs, focal-enhanced tumors were more amenable to GTR than diffuse enhanced (p = 0.001) and ring-like enhanced (p = 0.024) tumors. No significant differences in GTR rates were observed between non-enhanced and focal enhanced AGs (p = 0.857) or between ring-like and diffuse AGs (p = 0.164)
Association between enhancement pattern, tumor location, necrosis features, and histology
The volumes and ratios of necrosis among patient groups with different enhancement patterns were compared. Volumes and ratios were both found to be significantly different among the three groups (Supplementary Table 1). Employing Fisher’s Least Significant Difference test revealed that tumors with focal enhancement tended to have less volumes of necrosis than those with diffuse and ring-like patterns (Supplementary Table 2). Furthermore, tumors with focal enhancement showed lower ratios of necrosis than tumors with ring-like enhancement (Supplementary Table 3).
According to the histological features of AGs, tumors with and without oligodendroglial components were divided into two subgroups. The difference in proportions of contrast enhancement patterns between the two subgroups was statistically significant (p = 0.042). There was no significant difference in tumor location between AGs with and without an oligodendroglial component (p = 0.389), and the difference in subventricular zone involvement between the two groups was also not statistically significant (p = 0.465) (Supplementary Table 4).
Predictors of survival outcome
During follow-up, 72.8 % of the enrolled patients experienced tumor recurrence, and 82 of 268 (30.6 %) patients with available follow-up data were alive; the median follow-up period was 43 months (range, 25–82 months). On univariate analysis, the age at diagnosis (p < 0.001), preoperative KPS (p = 0.003), tumor enhancement pattern (p = 0.002), necrosis volume (p = 0.023), extent of resection (p = 0.004), and radiotherapy (p = 0.014) were identified as prognostic factors for PFS, these five factors also showed prognostic value for OS (Table 2).
Significant differences in PFS and OS were observed among patients with AGs of different enhancing patterns (focal, diffuse, or ring-like; p = 0.002 for PFS and p = 0.023 for OS, log-rank; Fig. 3). Patients with a focal enhancing tumor had a significantly longer PFS (median, 27 months) than those with a diffuse enhancing tumor (median, 19 months; log-rank, p = 0.025) or a ring-like enhancing tumor (median, 16 months; log-rank, p = 0.008). Similarly, patients with a focal enhancing tumor had a significantly longer OS (median, 30 months) than those with a ring-like enhancing tumor (median, 24 months; log-rank, p = 0.011) or a diffuse enhancing tumor (median, 27 months; log-rank, p = 0.031). There were no significant differences in PFS and OS between patients with a diffuse enhancing tumor and those with a ring-like enhancing tumor (p = 0.159 for PFS and p = 0.278 for OS, log-rank test).
Log-rank analysis of Kaplan-Meier curves demonstrated the differences in progression-free survival (PFS, p = 0.002) and overall survival (OS, p = 0.023) among patients with different tumor enhancement patterns. Those with focal-enhanced AGs had significantly better PFS and OS than those with diffuse (p = 0.025 and p = 0.031, respectively) or ring-like (p = 0.008 and p = 0.011, respectively) enhanced AGs. No significant differences in PFS (p = 0.159) or OS (p = 0.278) were found between patients with diffuse enhanced tumors and those with ring-like enhanced tumors
Multivariate Cox regression analysis demonstrated that the pattern of tumor enhancement was a significant prognostic factor for PFS and OS in AG patients (p = 0.016 for PFS and p = 0.030 for OS). Other identified adverse prognostic factors for PFS included an age of ≥50 years, preoperative KPS of <80, necrosis volume >10 cm3, and non-GTR. These variables were also independent prognostic factors for OS (Table 3).
Discussion
This study retrospectively reviewed a large cohort of AG patients (n = 268). Specifically, the pattern of tumor enhancement was assessed as a radiological characteristic for all included patients. To our knowledge, the present study is the first to describe the association of tumor enhancement pattern on post-contrast T1 images with the extent of tumor resection and to identify such a pattern as an independent prognostic factor for AGs.
Several studies have revealed the association between aggressive surgical resection and improved survival in AG patients [4, 13–15]. More specifically, a complete resection of enhancing tissue was found to independently improve prognosis in enhancing oligodendrogliomas, irrespective of histological grade or genetic status [12]. For patients with anaplastic astrocytoma, the volume of residual tumor assessed on postoperative contrast-enhanced T1-weighted images was found to be a prognostic factor for PFS and OS [11]. Multivariable analysis in this study also showed that patients undergoing GTR had longer PFS and OS than those with residual tumors. A previous study found that GTR was more likely to be achieved in patients with more favorable tumor characteristics, such as a lack of contrast enhancement [16]. In this study, significant differences in GTR rates were observed between tumors with aggressive enhancement (diffuse and ring-like) and those with no or small (focal) contrast enhancement. Tumors without contrast enhancement or those that display focal enhancing areas disrupt the blood-brain barrier only to a limited degree, and tend to cause less damage to normal brain tissues than tumors exhibiting large areas of diffuse contrast enhancement (e.g., diffuse or ring-like enhancement patterns). Tumors with no diffuse contrast enhancement or those with focal contrast enhancement are more likely to undergo GTR, and thereby indicate a better prognosis.
In addition to clinical characteristics, radiologic features such as contrast enhancing area and relative cerebral blood volume have been suggested to be associated with patient prognosis in high-grade gliomas [5, 10]. A previous study using CT scans demonstrated that ring-like enhancement was an adverse prognostic indicator in AG [9]. Nevertheless, the relationship between tumor enhancement pattern and patient survival outcome has rarely been investigated in AG. The present study addressed this issue by identifying the prognostic value of tumor enhancement patterns in AG patients via both univariate and multivariate analyses. We proposed some possible explanations for these results. First, the appearance of contrast enhancement is based on the blood-brain barrier disruption caused by tumor invasion. Ring-like contrast enhancement pattern indicates a rapid and aggressive proliferation of tumor cells that results in a necrotic core, which is considered to represent extreme malignant behavior and might lead to an unfavorable prognosis. As a common feature of malignant tumors, the extent of necrosis is important for the histological grading of gliomas because of its correlation with poor prognosis [17]. Previous studies showed that necrosis was correlated with the deletion of CDKN2A. Furthermore, anaplastic gliomas showing ring-like enhancements that are indicative of necrosis have been associated with worse survival outcomes [9, 18, 19]. Similarly, tumors with diffuse enhancement, which represents larger areas of damaged brain tissue and immature blood vessels, could possibly have a more invasive behavior than those with focal enhancement. Such differences may contribute to the variances in survival outcome. Second, many previous studies have identified the prognostic role of GTR in patients with high-grade gliomas [4, 13, 15, 20]. We found that GTR was more likely to be achieved in patients with focal tumor enhancement (70.0 %) than in those with ring-like (49.0 %) or diffuse (38.8 %) tumor enhancement (p = 0.005). Therefore, our findings suggested that the role of contrast enhancement pattern in survival prediction might partly be attributed to the prognostic effect by the extent of surgical resection. Finally, the prognostic role of tumor enhancement pattern might be associated with the variations in tumor genetic changes. Recent studies have demonstrated that various molecular subtypes of anaplastic oligodendroglioma, such as the pro-neural, mesenchymal, or neural subtypes, as well as 1p/19q co-deletion status, were associated with different radiological characteristics including tumor location, contrast enhancement, and heterogeneous intratumoral signals [6, 8, 21–23]. Moreover, patients harboring a tumor classified as pro-neural reportedly have a longer survival time compared to those with a mesenchymal subtype tumor [24–26]. Because it reflects the biological features of the tumor, the pattern of tumor enhancement may indicate the genetic specificities that could potentially determine patient survival.
Factors that might influence the current results were considered. Different time intervals may be required for the maximum diffusion of contrast agent in tumor tissue. An appropriate scanning time was estimated at around 80 s following contrast agent injection by previous studies [27–29]. To minimize this potential heterogeneity, the time interval between injection of contrast agent and beginning of contrast-enhanced T1-weighted image acquisition was restricted between 75 and 85 s in this study. There are other factors that may influence tumor enhancement on MR images, such as equipment types and contrast agent concentration [30, 31]. In order to achieve a uniform criterion; all images were acquired using a 3.0 T MR scanner (Siemens Trio, Siemens Healthcare, Erlangen, Germany) in this study. Additionally, the contrast agent (Ga-DTPA Injection, Beilu Pharma, Beijing, China) used for all enrolled patients was obtained from the same pharmaceutical company.
Several limitations should be considered in the present study, including its retrospective design and lack of volumetric assessment. Although carefully controlled, a slight discrepancy in the time interval from contrast agent injection to scanning may still exist among individual patients. Such a discrepancy could potentially affect contrast enhancement intensity, although its significance requires further investigation. Future studies are encouraged to investigate the association between contrast enhancement patterns and the genetic characteristics of tumors.
Conclusion
The present study retrospectively reviewed the clinical data of 268 AG patients. In addition to previously reported prognostic factors, this study was the first to demonstrate the association of tumor enhancement patterns with the extent of resection, PFS, and OS in AG patients. Our findings suggest that tumor enhancement patterns could be employed as a non-invasive radiographic marker for the prediction of survival outcomes in patients with AGs.3
References
DeAngelis LM (2009) Anaplastic glioma: how to prognosticate outcome and choose a treatment strategy. J Clin Oncol 27:5861–5862
Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Nuno M, Birch K, Mukherjee D et al (2013) Survival and prognostic factors of anaplastic gliomas. Neurosurgery 73:458–465, quiz 465
Shirai K, Suzuki Y, Okamoto M et al (2010) Influence of histological subtype on survival after combined therapy of surgery and radiation in WHO grade 3 glioma. J Radiat Res 51:589–594
Hu LS, Eschbacher JM, Dueck AC et al (2012) Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol 33:69–76
Jenkinson MD, du Plessis DG, Smith TS et al (2006) Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain 129:1884–1891
Mangla R, Ginat DT, Kamalian S et al (2014) Correlation between progression free survival and dynamic susceptibility contrast MRI perfusion in WHO grade III glioma subtypes. J Neurooncol 116:325–331
Megyesi JF, Kachur E, Lee DH et al (2004) Imaging correlates of molecular signatures in oligodendrogliomas. Clin Cancer Res 10:4303–4306
Tortosa A, Vinolas N, Villa S et al (2003) Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer 97:1063–1071
Chen C, Huang R, MacLean A et al (2013) Recurrent high-grade glioma treated with bevacizumab: prognostic value of MGMT methylation, EGFR status and pretreatment MRI in determining response and survival. J Neurooncol 115:267–276
Keles GE, Chang EF, Lamborn KR et al (2006) Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg 105:34–40
Sankar T, Moore NZ, Johnson J et al (2012) Magnetic resonance imaging volumetric assessment of the extent of contrast enhancement and resection in oligodendroglial tumors. J Neurosurg 116:1172–1181
Nagy M, Schulz-Ertner D, Bischof M et al (2009) Long-term outcome of postoperative irradiation in patients with newly diagnosed WHO grade III anaplastic gliomas. Tumori 95:317–324
Nomiya T, Nemoto K, Kumabe T et al (2007) Prognostic significance of surgery and radiation therapy in cases of anaplastic astrocytoma: retrospective analysis of 170 cases. J Neurosurg 106:575–581
Scoccianti S, Magrini SM, Ricardi U et al (2012) Radiotherapy and temozolomide in anaplastic astrocytoma: a retrospective multicenter study by the Central Nervous System Study Group of AIRO (Italian Association of Radiation Oncology). Neuro Oncol 14:798–807
Schomas DA, Laack NN, Rao RD et al (2009) Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol 11:437–445
Hammoud MA, Sawaya R, Shi W et al (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27:65–73
Sallinen PK, Sallinen SL, Helen PT et al (2000) Grading of diffusely infiltrating astrocytomas by quantitative histopathology, cell proliferation and image cytometric DNA analysis. Comparison of 133 tumours in the context of the WHO 1979 and WHO 1993 grading schemes. Neuropathol Appl Neurobiol 26:319–331
Gutman DA, Cooper LA, Hwang SN et al (2013) MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology 267:560–569
Bradley D, Rees J (2014) Updates in the management of high-grade glioma. J Neurol 261:651–654
Kim JW, Park CK, Park SH et al (2011) Relationship between radiological characteristics and combined 1p and 19q deletion in World Health Organization grade III oligodendroglial tumours. J Neurol Neurosurg Psychiatry 82:224–227
Laigle-Donadey F, Martin-Duverneuil N, Lejeune J et al (2004) Correlations between molecular profile and radiologic pattern in oligodendroglial tumors. Neurology 63:2360–2362
Reyes-Botero G, Dehais C, Idbaih A et al (2014) Contrast enhancement in 1p/19q-codeleted anaplastic oligodendrogliomas is associated with 9p loss, genomic instability, and angiogenic gene expression. Neuro Oncol 16:662–670
Naeini KM, Pope WB, Cloughesy TF et al (2013) Identifying the mesenchymal molecular subtype of glioblastoma using quantitative volumetric analysis of anatomic magnetic resonance images. Neuro Oncol 15:626–634
Phillips HS, Kharbanda S, Chen R et al (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173
Verhaak RG, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Barboriak DP, MacFall JR, Viglianti BL et al (2008) Comparison of three physiologically-based pharmacokinetic models for the prediction of contrast agent distribution measured by dynamic MR imaging. J Magn Reson Imaging 27:1388–1398
Jackson A, Jayson GC, Li KL et al (2003) Reproducibility of quantitative dynamic contrast-enhanced MRI in newly presenting glioma. Br J Radiol 76:153–162
Roberts HC, Roberts TP, Brasch RC et al (2000) Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 21:891–899
Bae KT (2003) Peak contrast enhancement in CT and MR angiography: when does it occur and why? Pharmacokinetic study in a porcine model. Radiology 227:809–816
Kubota T, Yamada K, Kizu O et al (2005) Relationship between contrast enhancement on fluid-attenuated inversion recovery MR sequences and signal intensity on T2-weighted MR images: visual evaluation of brain tumors. J Magn Reson Imaging 21:694–700
Acknowledgments
We would like to thank Dr. Q. Chen and Dr. X. Chen for their efforts in tumor segmentation. We would also acknowledge the financial support from the National High Technology Research and Development Program (2011CB707804 and 2015CB755500) and the National Natural Science Foundation of China (81271541).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human and animal studies have been approved by the Ethics Committee at Beijing Tiantan Hospital, Capital Medical University, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Additional information
YW and KW contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, K., Wang, J. et al. Identifying the association between contrast enhancement pattern, surgical resection, and prognosis in anaplastic glioma patients. Neuroradiology 58, 367–374 (2016). https://doi.org/10.1007/s00234-016-1640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1640-y