Abstract
Background
Current treatments in grade III gliomas include surgery, radiotherapy, and chemotherapy. The value of the entity of surgical resection remains an open question. The aim of this evaluation was to analyze the impact of extent of resection (EOR) and residual tumor volume (RTV) on progression-free survival (PFS) and overall survival (OS) in patients with newly diagnosed grade III gliomas.
Methods
Overall, 136 patients were included in this evaluation. EOR and RTV were defined in all patients on postoperative volumetric magnetic resonance imaging, with EOR being defined as the rate of surgical resection, and RTV as contrast-enhancing RTV (CE-RTV) and fluid-attenuated inversion recovery (FLAIR) RTV. A threshold of EOR and RTV was recorded using increments of 2 % and 1 cm3.
Results
EOR and RTV were the only clinical variables influencing PFS and OS. The EOR cut-off value for conditioning survival was 76 %. For EOR ≥76 % or <76 %, the 5- to 10-year PFS was 57 % and 18 % versus 0 % (p = 0.03), and 5- to 10-year OS was 68 % and 42 % versus 0 % (p = 0.06), respectively. Additionally, the RTV cut-off value was 3 cm3; for RTV <3 cm3 or >3 cm3, 5- to 10-year PFS was 64.3 % and 48.2 % versus 42 % and 0 % (p = 0.02), and 5- to 10-year OS was 66.8 % and 33.4 % versus 56 % and 0 % (p = 0.3), respectively. RTV was a more significant parameter conditioning PFS and OS than EOR (p = 0.04), and the presence of CE-RTV was an unfavorable prognostic factor compared with FLAIR-RTV.
Conclusions
In heterogeneous lesions from a radiological point of view as WHO grade III gliomas if a complete removal is not possible, it would be advisable to maximize the removal of enhancing areas, possibly with an EOR >76 % and an RTV <3 cm3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
According to the World Health Organization (WHO), grade III gliomas include the subtypes anaplastic oligodendroglioma (AO), mixed anaplastic oligoastrocytoma (AOA), and anaplastic astrocytoma (AA), and represent <10 % of primary brain tumors. Treatments include surgery, radiation therapy (RT), and chemotherapy, but a standard of care is still lacking. Molecular markers are relevant in the choice of treatment but their role in clinical trials requires validation. Positive O-6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation is associated with a higher response to chemotherapy, and is not apparently correlated with survival.1 1p/19q co-deletion, detected in up to 70 % of AOs/AOAs, was identified as a predictive factor of response to chemotherapy and as an important prognostic biomarker correlated with longer survival.2–4 Recent data indicate that isocitrate dehydrogenase (IDH) mutation status defines biologically distinct groups of tumors. The presence of IDH1 mutation is a favorable prognostic factor in most WHO grade III gliomas, and coexists with 1p-19q co-deletion.5–8 Surgery is one of the main treatment options; however, the impact of the degree of resection on prognosis is still unclear. Several authors reported an improvement in survival in patients who underwent maximal cytoreduction compared with subtotal resection or biopsy alone9 – 12; however, a precise definition of the correlation between surgical resection and survival would be necessary. To estimate the amount of surgical resection, the extent of resection (EOR) measures the percentage of tumor removal and is quantified by comparing the preoperative tumor mass and the postoperative tumor volume, independent of the amount of tumor left after surgery. Alternatively, the amount of detectable abnormal tissue left after surgery in volumetric postoperative magnetic resonance imaging (MRI) measures the amount of residual tumor volume (RTV), independently from the percentage of tumor removal (EOR).13–15 WHO grade III gliomas may harbor areas of contrast enhancement. In this context, the amount of RTV and the contrast-enhancing RTV (CE-RTV) have been introduced and their influence on survival was analyzed. Chaichana et al. showed that progression-free survival (PFS) and overall survival (OS) were improved when EOR was >70 % and RTV <5 cm3.16 Grabowski et al. found that, in primary glioblastomas, postoperative CE-RTV was a stronger indicator of survival than EOR.17,18 Indeed, glioblastomas are characterized radiologically by contrast-enhanced masses, and the measurement of EOR, RTV, and CE-RTV is clinically feasible. On the contrary, in WHO grade III gliomas the impact of EOR, RTV, and CE-RTV has not been largely investigated to date. This may be related to the heterogeneous radiological appearance of the tumors, and the heterogeneous histological and molecular profile, reflecting their variable biological and clinical behavior and response to treatments.
The aim of this study was to evaluate the impact of EOR, RTV, and CE-RTV on PFS and OS in newly diagnosed WHO grade III gliomas.
Methods
Patients and Procedures
Overall, 136 patients with newly diagnosed WHO grade III gliomas were treated from January 2004 to September 2013, and all patients were treated in accordance with the precepts of the Helsinki Declaration. Inclusion criteria were (i) available clinical and imaging data; (ii) between 18 and 70 years of age; (iii) histological diagnosis of WHO grade III gliomas; (iv) visible and measurable tumor mass in volumetric post-contrast T1 and fluid-attenuated inversion recovery (FLAIR) MR images; (v) available molecular profile; (vi) tumor amenable to surgical resection. Surgery was performed in all patients with the aim of maximally removing the tumor mass according to functional boundaries, with the aid of imaging and brain mapping techniques. Neurophysiological monitoring involved simultaneous acquisition of continuous electroencephalography (EEG), electrocorticography (ECoG), multichannel recording of free-running electromyographic (EMG) activity, and motor-evoked potentials (MEPs) [Comet EEG system, Grass Astro-Med, ISIS-IOM, and Inomed GmbH]. Tumor borders were defined intraoperatively by neuronavigation coupled with ultrasound. Mapping of eloquent language and motor cortex (when needed) was performed to define the cortical safe-entry zone. Subcortical brain mapping (when requested) was then continued, along with tumor resection. Further treatment consisted of adjuvant chemotherapy with temozolomide or concomitant radiotherapy and temozolomide chemotherapy followed by adjuvant temozolomide (Stupp regimen). Generally, for tumors with oligodendroglial features (AO/AOA), 1p/19q co-deletion and IDH1-mutated, the treatment of choice following surgery was adjuvant temozolomide, while in cases of tumors with IDH1 wild-type, and/or any residual tumors after surgery, the treatment choice was RT plus temozolomide. For all astrocytic tumors (AA), treatment following surgery was mostly radiotherapy plus concomitant and adjuvant temozolomide. Chemotherapy consisted of temozolomide administered at 75 mg/mq in the concomitant setting, and at 200–250 mg/mq for 5/28 days in the adjuvant setting. For the RT plan, to precisely delineate the extension of tumor and eventual residual microscopic viable tumor after surgery, both computed tomography (CT) scan and MRI were used and images co-registered. In all patients, the total dose prescribed was 60 Gy.
Residual Tumor Volume and Extent of Resection
The lesion and residual volume was measured onto volumetric MRI acquired through a 3 Tesla scanner (Siemens) 24 and 48 h from surgery, respectively, through a semi-automatic region of interest (ROI) analysis with Iplan Cranial v3.0 software (Brainlab, Feldkirchen, Germany). Three independent physicians with expertise in neuro-oncology performed the measurements. Contrast-enhanced T1 sequences were used for lesions displaying contrast enhancement in the preoperative stage, while FLAIR sequences were used for lesions with no preoperative enhancement. RTV was measured on postoperative MRI performed within 48 h from surgery; postoperative MRI was coregistered with the preoperative dataset. Postoperative diffusion-weighted imaging (DWI) was also coregistered to rule out postoperative ischemic injury. EOR was defined as (preoperative volume − postoperative volume/preoperative volume) × 100 %. According to Sanai and Berger,14 EOR was stratified into four subgroups (i) surgical resection inferior to 70 %; (ii) surgical resection between 71 and 80 %; (iii) surgical resection between 81 and 90 %; and (iv) surgical resection between 91 and 100 %. In addition, we dichotomized EOR and RTV in increments of 1 cm3 and 2 % to evaluate the thresholds for RTV and EOR, respectively.
RTV was defined as any residual MRI abnormality on postoperative MRI that was evident in the preoperative study. In particular, contrast-enhanced MRI was used to assess enhanced residual masses (CE-RTV), and FLAIR-MRI was used to measure unenhanced residual masses (FLAIR-RTV). The sum of CE-RTV and FLAIR-RTV was recorded for lesions characterized by both tissue components. The presence of aspecific enhancements due to blood deposition, choroid plexus, or vessels were identified and excluded.
Outcome Evaluation
Clinical outcome was evaluated by neurological examination and brain MRI was performed 1 month after RT and then every 3 months. Response was recorded using the Response Assessment in Neuro-Oncology (RANO) criteria.19 Hematologic and non-hematologic toxicities were graded according to the Common Terminology Criteria for Adverse Events version 4.0.
Statistical Analysis
Standard descriptive statistics (mean, standard deviation, and cross-tabulation analysis) were used to describe the general behavior of the data, and survival and recurrence time observations were determined using Kaplan–Meier analysis, starting from the date of surgery. The log-rank test was used for univariate analysis in order to investigate the prognostic role of individual variables. For analysis, the variables analyzed were age, sex, Karnofsky Performance Status (KPS), histology, molecular profile, EOR, and RTV. Groups were defined according to the discrete volume of each variable, and a multivariate Cox model was used for the multivariate tests. Data were split into two groups according to specific thresholds of EOR and RTV. The threshold value was progressively changed and the significant p value (p = 0.05) between groups was evaluated for both OS and PFS. STATA v13.1 statistical software was used to conduct the analyses (StataCorp LP, College Station, TX, USA).
Results
Patients and Treatment
Overall, 136 patients were included in the analysis. Fifty-two (38 %) were female and 84 (62 %) were male, with a median age of 43 years (range 19–78). Forty-six (34 %) patients were diagnosed with AA, 38 (28 %) with AOA and 52 (38 %) with AO. Seventy (51.4 %) patients had a previous history of grade II glioma, who progressed to a grade III glioma at a median time of 4.4 years (range 0.8–26.2). After surgery, 48 (35 %) patients received adjuvant chemotherapy with temozolomide, and 88 (65 %) received RT with concomitant/adjuvant chemotherapy with temozolomide. All patients had a complete molecular profile including IDH1 mutation, 1p19q co-deletion, and MGMT methylation status. IDH1 mutation was observed in 110/136 (81 %) patients—30/46 (65 %) AA patients, 32/38 (84 %) AOA patients, and 48/52 (92 %) AO patients; chromosome 1p/19q co-deletion was found in 66/136 (48.5 %) patients—12/46 (26 %) AA patients, 16/38 (42 %) AOA patients, and 38/52 (73 %) AO patients; and methylated MGMT promoter was detected in 110/136 (81 %) patients—30/46 (65 %) AA patients, 36/38 (95 %) AOA patients, and 44/52 (85 %) AO patients. Patient and tumor characteristics, as well as treatment performed, are summarized in Table 1. The tumor site was frontal in 82 (60 %) patients, parietal in 16 (12 %) patients, and temporal in 38 (28 %) patients. The median preoperative volume was 47.8 cm3 (range 6.9–191.4) and the median RTV was 3.3 cm3 (range 0–67.3). EOR <70 % was obtained in 16 patients (11.8 %), between 71 and 80 % in 10 patients (7.3 %), between 81 and 90 % in 38 patients (27.9 %), and between 90 and 100 % in 72 patients (52.9 %). Among 136 treated patients, 116 (85.2 %) had an RTV; CE-RTV was recorded in 12 patients (8.8 %), and in 22 patients (16.2 %) it was associated with FLAIR abnormality. A pure FLAIR RTV was noted in 82 patients (60.3 %).
Progression-Free Survival and Overall Survival Analysis
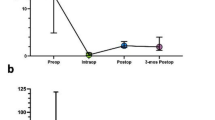
The median follow-up time was 2.6 years (range 0.8–17.6). At the last observation time, 102 patients (75 %) were alive and 34 (25 %) were dead. For the entire cohort, the median 1-, 3-, 5-, and 10-year PFS was 2 years (range 0.8–8.4) [88.1, 51.3, 51.3, and 25.6 %, respectively], and the median 1-, 3-, 5-, and 10-year OS was 2.5 years (range 0.8–17.5) [95.5, 79.9, 62.5, and 39 %, respectively; Fig. 1]. Sex, age, previous history of grade II gliomas, histology, and molecular profile had no statistical relevance. Patients with AO/AOA had better PFS and OS compared with patients with AA; the 5-year PFS was 59.6 % (95 % CI 39.9–74.8) for AO/AOA versus 37.3 % (95 % CI 14.9–60.2) for AA (p = 0.2), while the 5-year OS was 70 % (95 % CI 45.7–85) for AO/AOA versus 49.2 % (95 % CI 20–73.2) for AA (p = 0.07). In relation to the molecular profile, the 5-year PFS was 55 % (95 % CI 36.7–70.5) for IDH1-mutated patients compared with 34.6 % (95 % CI 10–61) for IDH1 wild-type (p = 0.1). The 5-year OS was 68 % (95 % CI 45.7–82.6) for IDH1-mutated versus 42.3 % (95 % CI 12–70) for IDH1 wild-type (p = 0.07). Patients with tumors showing both IDH1 mutation and 1p19q co-deletion had a further improved PFS and OS. The 5-year PFS was 60 % (95 % CI 39–76) in IDH-mutated and co-deleted tumors, and 0 % in non-co-deleted tumors (p = 0.3). The 5-year OS was 74.6 % (95 % CI 48–89) in IDH-mutated and co-deleted tumors, and 0 % in non-co-deleted tumors (p = 0.04).
EOR and RTV were the only clinical variables that influenced PFS and OS. PFS and OS at 1, 3, 5, and 10 years, according to the four EOR subgroups, are summarized in Table 2. In univariate analyses, the cut-off value of EOR conditioning survival was 76 %. Patients with EOR ≥76 % had better PFS and OS compared with patients with lower EOR: the 3-, 5-, and 10-year PFS was 57, 57, and 18 % versus 22.5, 0, and 0 % (p = 0.03), respectively, while the 3-, 5-, and 10-year OS was 81, 68, and 42 % versus 75, 0, and 0 %, respectively (p = 0.06), as shown in Fig. 2. The cut-off value of RTV conditioning survival was 3 cm3. For patients with RTV lower than 3 cm3, the 3-, 5-, and 10-year PFS was 64.3, 64.3, and 48.2 % versus 42, 42, and 0 % for patients with RTV ≥ 3 cm3 (p = 0.02), respectively, and the 3-, 5-, and 10-year OS was 77.6, 66.8, and 33.4 % versus 84, 56, and 0 % (p = 0.3), respectively, as shown in Fig. 3. In multivariate analyses, comparing the value of EOR and RTV, in our series RTV was observed as a more significant parameter conditioning PFS than EOR (p = 0.04). When analyzing RTV, patients with CE-RTV had worse PFS and OS compared with those with FLAIR-RTV only; the 3- and 5-year PFS was 49 % and 49 % versus 66.7 % and 66.7 %, respectively, and the 3- and 5-year OS was 77.6 % and 66.8 % versus 80 % and 80 %, respectively.
Evaluating EOR with regard to molecular profile and different histological subtypes, an EOR ≥76 % and/or an RTV ≤3 cm3 statistically affected PFS and OS in IDH1 wild-type tumors versus IDH1-mutated and co-deleted tumors, and IDH1-mutated and non-co-deleted tumors, as well as in AA patients compared with AO and AOA patients (p < 0.01).
Clinical Outcome and Toxicity
Perioperative complications occurred in 11 (8 %) patients. No perioperative mortality occurred, and all patients completed the scheduled RT planning. No severe hematologic or neurologic toxicity was recorded during treatment, and neurological examination scores remained stable. During chemotherapy, three (2.2 %) patients had grade III thrombocytopenia, seven (5.1 %) patients had grade III neutropenia, and two (1.5 %) patients had grade III anemia. No hematologic grade IV toxicity occurred. Chemotherapy was interrupted in one patient, and was delayed or reduced in 10 (7.4 %) patients.
Treatment at Progression
Overall, 60 (44 %) patients relapsed; 48 had a local progression and 12 had a distant progression, at a median time of 1.8 years (range 0.3–16.4). Salvage treatment was performed in 46 (76.7 %) patients.
Discussion
WHO grade III gliomas are a heterogeneous group of malignant brain tumors with variable biological behavior and propensity to diffuse infiltration of the surrounding brain parenchyma. Recent studies stressed the importance of histology, particularly molecular profiling, in influencing PFS and OS. Entity of surgical resection in patients with newly diagnosed grade III gliomas has been not widely investigated. Numerous retrospective studies suggest that the amount of tumor resection impacts on survival of patients with high- or low-grade gliomas.11, 14,18,20,21 Recent data17,21 showed how the identification of a threshold of EOR and/or RTV could dichotomize patients in a low- and high-risk group with regard to disease progression. In glioblastoma multiforme (GBM) patients, Sanai et al. demonstrated that an EOR threshold >78 % was a factor significantly affecting survival.21 Grabowski et al. evaluated the effect of CE-RTV and T2/FLAIR residual volume (T2/F-RV) on OS in a cohort of patients affected by GBM, and reported a significant benefit in survival with a CE-RTV <2 cm3 or an EOR >98 %.17 To maximize surgical resection while preserving patients’ neurological status, particularly for lesions in eloquent areas, the determination of a critical residual pathological volume could be useful. Although gross total resection significantly influences both PFS and OS, it is not always feasible. The relationship among EOR, RTV, and survival is still unknown. The goal of this study was to evaluate the interaction of EOR and RTV on PFS and OS, and to establish minimum EOR and maximum RTV thresholds influencing both these variables. In our series, patients with newly diagnosed WHO grade III glioma underwent surgical resection with the aim of maximally removing the tumor-preserving functional structures. In this group of patients, the 5- and 10-year PFS was 51.3 and 25.6 %, respectively, and the 5- and 10-year OS was 62.5 and 39 %, respectively. EOR impacted on PFS and OS, with a significant survival advantage seen with an EOR >76 %. In addition, RTV measured on post-contrast and FLAIR MRI appeared as a predictive factor for PFS, independently of age, KPS, histological subtypes, or biomolecular profile, although IDH1 status and co-deletion showed a trend toward an improved PFS and OS. As expected, oligodendroglial tumors had a better outcome compared with astrocytic tumors, with a 5-years OS of 70 % versus 49 %. In our cohort, the predictivity of RTV on PFS was higher than that of EOR (p = 0.04), confirming that RTV has been a stronger predictor of PFS than EOR. Our data indicate that RTV is a stronger predictor of PFS than EOR. Moreover, CE-RTV has proven to be highly predictive of outcome, indicating that, in WHO grade III gliomas, the appearance of areas of enhancing reflects the onset of a more malignant phenotype. This may suggest that when a large resection is not feasible, surgery might be focused on the removal of all enhancing areas. In our series, the critical RTV recorded as influencing survival was 3 cm3, confirming the hypothesis reported several times in the literature that the residual tumor’s response to subsequent radiation and chemotherapy may be non-linear, with smaller, scattered RTVs having a more favorable response.17 This point confirms that RTV has a greater value compared with EOR with regard to the biological activity of WHO grade III gliomas.
Conclusions
RTV has proven to be a reliable indicator of surgical radicality and factors significantly affecting PFS and OS compared with EOR. In particular, CE-RTV demonstrated more predictive factors on PFS and OS with respect to FLAIR-RTV. In heterogeneous lesions from a radiological point of view as WHO grade III gliomas if a complete removal is not possible, it would be advisable to maximize the removal of enhancing areas, possibly with an EOR ≥76 % and an RTV <3 cm3.
References
Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-methylguanine DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin Cancer Res. 2005;11:5167–74.
Bauman G, Ino Y, Ueki K, et al. Allelic loss of chromosome 1p and radiotherapy plus chemotherapy in patients with oligodendrogliomas. Int J Radiat Oncol Biol Phys. 2000;48:825–30.
Chahlavi A, Kanner A, Peereboom D, Staugaitis SM, Elson P, Barnett G. Impact of chromosome 1p status in response of oligodendroglioma to temozolomide: preliminary results. J Neurooncol. 2003;61:267–73.
Thiessen B, Maguire JA, McNeil K, Huntsman D, Martin MA, Horsman D. Loss of heterozygosity for loci on chromosome arms 1p and 10q in oligodendroglial tumors: relationship to outcome and chemosensitivity. J Neurooncol. 2003;64:271–78.
Jiang H, Ren X, Cui X, et al. 1p/19q codeletion and IDH1/2 mutation identified a subtype of anaplastic oligoastrocytomas with prognosis as favorable as anaplastic oligodendrogliomas. Neuro Oncol. 2013;15:775–82.
Frenel J, Leux C, Loussouarn D, et al. Combining two biomarkers, IDH1/2 mutations and 1p/19q codeletion, to stratify anaplastic oligodendroglioma in three groups: a single-center experience. J Neurooncol. 2013;114:85–91.
Olar A, Wani K, Alfaro-Munoz K, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. 2015;129:585–96.
The Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481–98.
Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. J Clin Oncol. 2009;35:5874–80.
Yamaguchi S, Kobayashi H, Terasaka S, et al. The impact of extent of resection and histological subtype on the outcome of adult patients with high-grade gliomas. Jpn J Clin Oncol. 2012;42(4)270–77.
McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–62.
Cohen ZR, Suki D, Shi W, et al. Surgical resection of anaplastic astrocytoma: prognostic factors and outcome. Neuro Oncol. 2002;4:367–72
Castellano A, Bello L, Michelozzi C, et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol. 2012;14:192–202.
Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–64.
Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–45.
Chaichana KL, Jusue-Torres L, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16:113–22.
Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121:1115–23.
Smith JS, Cha S, Mayo MC, et al. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: Distinguishing tumor recurrence from postresection injury. J Neurosurg. 2005;103:428–38.
Wen P, Macdonald D, Reardon D, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72.
Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurg. 2008;62:564–76.
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8
Disclosure
Luca Cozzi acts as a Scientific Advisor to Varian Medical Systems and is Clinical Research Scientist at Humanitas Cancer Center. Federico Pessina, Pierina Navarria, Anna Maria Ascolese, Matteo Simonelli, Armando Santoro, Stefano Tomatis, Marco Riva, Enrica Fava, Marta Scorsetti, and Lorenzo Bello have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pessina, F., Navarria, P., Cozzi, L. et al. Value of Surgical Resection in Patients with Newly Diagnosed Grade III Glioma Treated in a Multimodal Approach: Surgery, Chemotherapy and Radiotherapy. Ann Surg Oncol 23, 3040–3046 (2016). https://doi.org/10.1245/s10434-016-5222-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5222-3