Abstract
Introduction
Altered thalamocortical development is hypothesized to be a key substrate underlying neurodevelopmental disabilities in preterm infants. However, the pathogenesis of this abnormality is not well-understood. We combined magnetic resonance spectroscopy of the parietal white matter and morphometric analyses of the thalamus to investigate the association between white matter metabolism and thalamic volume and tested the hypothesis that thalamic volume would be associated with diminished N-acetyl-aspartate (NAA), a measure of neuronal/axonal maturation, independent of white matter injury.
Methods
Data from 106 preterm infants (mean gestational age at birth: 31.0 weeks ± 4.3; range 23–36 weeks) who underwent MR examinations under clinical indications were included in this study.
Results
Linear regression analyses demonstrated a significant association between parietal white matter NAA concentration and thalamic volume. This effect was above and beyond the effect of white matter injury and age at MRI and remained significant even when preterm infants with punctate white matter lesions (pWMLs) were excluded from the analysis. Furthermore, choline, and among the preterm infants without pWMLs, lactate concentrations were also associated with thalamic volume. Of note, the associations between NAA and choline concentration and thalamic volume remained significant even when the sample was restricted to neonates who were term-equivalent age or older.
Conclusion
These observations provide convergent evidence of a neuroimaging phenotype characterized by widespread abnormal thalamocortical development and suggest that the pathogenesis may involve impaired axonal maturation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Worldwide, 10 % of all live births are preterm [1]. Advances in neonatal intensive care during the preceding decades have dramatically increased survival; however, as many as 50 % of survivors continue to display cognitive-behavioral deficits and learning impairments in childhood [2, 3]. The neural substrate underlying these impairments is not well-understood, but the timing of vital developmental processes and the available neuropathological and neuroimaging data all suggest that disruption of the thalamocortical system is a key component [4, 5].
Thalamocortical connections are established during the late second and third trimester of fetal brain development [5, 6]. Quantitative MRI studies have shown that thalamic volume is reduced in preterm infants relative to their term counterparts at term-equivalent age [7–10] and predictive of later neurocognitive functioning [11]. The reduction in thalamic volume is more pronounced in infants with overt signs of white matter injury [7, 8]. Still, even in the absence of severe white matter injury, thalamic volumes remain smaller in preterm infants [8] and associated with altered thalamocortical connectivity [12].
Neuropathological examinations of preterm infants have demonstrated frank neuronal loss in the thalamus of infants with periventricular leukomalacia (PVL) [13, 14]. However, in contrast to the neuroimaging results, the incidence in thalamic pathology is markedly reduced in infants without PVL (19 %; 3/16 cases) compared to infants with PVL (59 %; 13/22 cases) and not associated with decreased neuronal density in the thalamus [13, 14]. This apparent contradiction between the neuroimaging results and the neuropathological data raises the question about the underlying neuroanatomical substrate for the observed alteration in thalamocortical connectivity: that is, if not frank neuronal loss, what accounts for the diminished thalamocortical connectivity in preterm infants without overt white matter injury?
Magnetic resonance spectroscopy (MRS) affords the unique opportunity to investigate cellular-molecular aspects of tissue function in vivo, providing, for example, biomarkers of axonal maturation, membrane synthesis, and energy metabolism. In this study, we focused our investigation first on N-acetyl-aspartate (NAA) in the parietal white matter. NAA is synthesized in the mitochondria of mature neurons and axons [15–19], and prior studies have demonstrated rapid increases in NAA concentration during the perinatal period coincident with neuronal/axonal maturation [20–22]. Other studies have demonstrated decreases in NAA concentration in the setting of preterm white matter injury [23], traumatic brain injury [24], stroke [25], and other hypoxic-ischemic brain injuries [26], signifying diminished neuronal/axonal integrity. Given the neuroimaging and neuropathological evidence cited above, we expected associations among overt white matter injury, white matter NAA, and thalamic volume—i.e., that white matter injury would result in widespread axonopathy and concomitant neuronal loss in the thalamus. Yet, because of the mounting evidence from neuroimaging studies suggesting that thalamocortical development is altered, even in the absence of white matter injury, we also predicted that thalamic volume would be associated with white matter NAA, independent of white matter injury, potentially indicating impaired axonal development or reduced connectivity to and from the thalamus.
Finally, in order to determine whether there was convergent evidence of impaired axonal maturation or evidence of other pathogenic mechanisms, we conducted exploratory analyses—comparing putative markers of markers of energy metabolism (creatine, lactate) [27], excitotoxicity (glutamate, glutamine) [23, 28, 29], membrane synthesis (choline) [27, 30, 31], and astrogliosis (myo-inositol) [32] to thalamic volume.
Materials and methods
Case selection
Data from 108 preterm neonates (mean gestational age at birth: 31.0 weeks ± 4.3; range 23–36 weeks; mean postconceptional age at scan: 41.2 weeks ± 6.0; range 25.7–60.7 weeks) were included in this study. Details regarding case selection are available in [23]. Briefly, all preterm infants who underwent clinically indicated MRIs were screened prospectively as part of ongoing longitudinal studies of neurodevelopment in neonates with prematurity. All available cases were included in this study provided: (1) the imaging study had been completed on an infant born before 37 gestational weeks of age; (2) the infant was not older than 60 weeks postconceptional age (PCA; calculated as the interval between the mother’s last menstrual period and birth plus post-natal age) at the time of the MRI; (3) there was no evidence of cerebral abnormality other than punctate white matter lesions (pWMLs) or diffuse excessive high signal intensity (DEHSI) (i.e., large vessel acute or chronic infarction, parenchymal hemorrhage, infection, tumor, or cerebral malformation); and (4) there was no clinical or laboratory evidence of liver failure, hyperbilirubinemia (requiring exchange transfusion), or underlying inborn error of metabolism. We excluded data from two infants for analyses: one for technical reasons and one because it was a statistical outlier (measured thalamic volume was more than four standard deviations larger than the mean); thus, the final sample included only 106 preterm infants (mean gestational age at birth: 31.0 weeks ± 4.3; range 23–36 weeks; mean age at scan: 41.3 weeks ± 6.1; range 25.7–60.7 weeks).
This study was approved by the Children’s Hospital Los Angeles (CHLA) Committee on Clinical Investigations and the University of Pittsburgh Internal Review Board. Written informed consent for use of their child’s clinically acquired MRI data and for participation in additional neurodevelopmental and neuroimaging studies were obtained from parents on behalf of the prospectively recruited patients by a research coordinator. The ethics committee approved this consent process. Additionally, as this study involved a retrospective review of all clinically acquired neonatal data for the period between 2002 and 2008, which included neonates who were not enrolled into prospective studies, approval has also been obtained from the CHLA Committee on Clinical Investigations and the University of Pittsburgh Internal Review Board for the retrospective use of all clinically acquired neonatal MRI data acquired at CHLA between 2002 and 2008. Prior results from this cohort have been published [23, 33].
MR data acquisition
MRI studies were acquired under clinical indications (most often to assess brain injury following preterm birth) on a GE 1.5 T (Signa LX, GE Healthcare, Milwaukee, WI) MR System using a customized neonatal transmit-receive head coil. Some studies were conducted using an MR compatible incubator; however, the majority of the studies were conducted with the neonate wrapped in a blanket and secured in the MR scanner with appropriate physiological monitoring equipment. Per clinical protocol, most infants were sedated with choral hydrate throughout the MR scan. Ear protection was achieved using foam ear plugs in conjunction with MiniMuffs (Natus Medical Inc., San Carlos, CA). Conventional imaging studies were acquired with the MRS studies and included a 3D coronal SPGR sequence (TE = 6 ms, TR = 25 ms, FOV = 18 cm, matrix = 256 × 160, slice thickness 1.5 mm, spacing 0 mm) or axial and sagittal T1-weighted FLAIR sequences (TE = 7.4, TR = 2100, TI = 750, FOV = 20 cm, matrix = 256 × 160), axial T2-weighted FSE sequence (TE = 85 ms, TR = 5000 ms, FOV = 20 cm, matrix = 320 × 160 or 256 × 128) and a diffusion-weighted sequence (TE = 80, TR = 10,000, FOV = 22 cm, matrix = 128 × 128, slice thickness 4.5 mm, spacing 0 mm).
1H spectra were acquired from a single voxel (approximately 3 cm3) placed in the developing parietal white matter dorsolateral to the trigone of the lateral ventricle in the left hemisphere using a point-resolved spectroscopy (PRESS) sequence with a short echo time (TE) of 35 ms, a repetition time (TR) of 1.5 s, 128 signal averages, and a total acquisition time for each spectrum of approximately 5 min, including scanner adjustments. The parietal white matter location was selected because (1) the parietal white matter is known to be a region of vulnerability in preterm infants and (2) numerous developing thalamocortical and corticocortical association pathways traverse that region [34–36].
Characterization of white matter injury based on conventional MR images
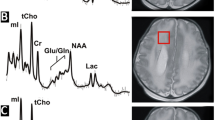
Conventional MRI scans (T1-, T2-, and diffusion-weighted sequences) for all studies were independently reviewed by two investigators (JLW, AP) and scored for the presence of both pWMLs and DEHSI. PWMLs were defined as punctate T1-hyperintense lesions in the developing periventricular white matter and centrum semiovale (Fig. 1) and classified dichotomously (present/absent). DEHSI was classified visually in accordance with a four-point scale modified from [37], with scores reflecting the intensity and extent of signal abnormality within the white matter: no signal abnormality (scored as 0), high signal restricted to the periventricular region only, classified as mild (1), high signal in the periventricular regions extending into the centrum semiovale, classified as moderate (2), and high signal extending from the periventricular white matter into the intragyral white matter (3). (For further details, see [23]).
1H spectra were acquired from a 3 cm3 voxel localized to the left parietal white matter (white square) exemplified here in a case with pWMLs (left image, circled in gray). The acquired 1H-MRS spectra is depicted on the right. The NAA peak is highlighted in black, while other prominent peaks, which were included here in exploratory analyses, are noted in gray. Glu glutamate, gln glutamine, Cho choline, Cr creatine, Lac lactate, mI myoinositol
Measurement of thalamic volume and brain growth
The bilateral regions of the thalamus were manually traced on the 3D coronal SPGR images by a single individual (RCC) under the supervision of a senior pediatric neuroradiologist (AP) using ITK-SNAP as shown in Fig. 2 [38]. The margins of the neonatal thalamus were determined with reference to a standard neuroanatomical atlas [39]. High-resolution axial T2 and coronal 3D SPGR images were co-registered, when available, to help with placement of the contours. Inter- and intra-rater reliabilities were assessed in a subset of cases (n = 5) and determined to be approximately 0.85 and 0.93, respectively.
Metabolites analyzed and data processing
We focused our analyses first on NAA. NAA is synthesized in the mitochondria of mature neurons and axons [15–19], and is used as a marker of axonal maturation. In addition, we included markers of energy metabolism (creatine, lactate), excitotoxicity (glutamate, glutamine), membrane synthesis (choline), and astrogliosis (myo-inositol). Absolute concentration for each metabolite was quantitated from the MRS spectra using LCModel software (Stephen Provencher Inc., Oakville, Ontario, Canada, LCModel Version 6.1-4F). In accordance with prior publications [20, 23, 33, 40], metabolite concentrations were corrected for the varying fractions of cerebrospinal fluid and tissue water content in the parietal white matter region of interest. For absolute quantitation, the signal from unsuppressed water was used as internal concentration reference. MR spectra of low quality were removed by limiting the sample empirically to spectra with a linewidth (measure of field homogeneity) of <5 Hz and signal-to-noise ratio (SNR) ≥ 5. Cramer Rao bounds were typically less than 15 % (as calculated by LCModel).
Statistical analyses
Statistical analyses were carried out in SPSS (V.20/21, IBM Corporation). Standard linear regression models were used to test the associations among various independent variables and thalamic volume (computed separately for right and left thalamus). Analyses were conducted in a sequential fashion. As a first level analysis, gestational age and age at MRI were tested as possible predictors, followed by (second level) MRI indices of white matter abnormalities (pWMLs and DEHSI), then (third level) NAA, and finally (fourth level) other white matter metabolites. To minimize type I error, we included a final model whereby all potential metabolites were tested simultaneously together with age at scan and pWMLs as covariates using stepwise multiple regression with criteria for entry (p < 0.05) and exit (p > 0.10). Effect size was calculated using Cohen’s f2, which can be interpreted as small (f2 = 0.2), medium (f2 = 0.15), or large (f2 = 0.35) [41]. A p value of <0.05 was considered statistically significant.
Results
Thalamic volume in association with white matter injury and age
Thalamic volume was associated with postconceptional age at MRI (right thalamus: T [104] = 8.622, p < 0.001, effect size = 0.715; left thalamus: T [104] = 8.498, p < 0.001, effect size = 0.695) but not gestational age at birth (p’s > 0.5). Controlling for age at MRI, the presence of pWMLs and DEHSI were tested next as possible predictors of thalamic volume. Reduced thalamic volume was associated with the presence of pWMLs (right thalamus: T [103] = −3.235, p = 0.002, effect size = 0.102; left thalamus: T [103] = −3.577, p = 0.001, effect size = 0.124), but not with DEHSI (right thalamus: T [103] = 0.694, p > 0.25; left thalamus: T [103] = 0.489, p > 0.5).
Thalamic volume in association with NAA, controlling for pWMLs and age
To determine whether thalamic volume was associated with NAA concentration above what could be accounted for by overt white matter injury and age, we used stepwise linear regression to test for the association with NAA, controlling for pWMLs and age at MRI. As hypothesized, NAA concentration was associated with thalamic volume (right thalamus: T [102] = 2.636, p = 0.010, effect size = 0.069; left thalamus: T [102] = 2.631, p = 0.010, effect size = 0.067), that is, the larger the NAA concentration in parietal white matter, the larger the thalamic volume (Fig. 3a).
As an additional test, we excluded the infants with pWMLs from the regression analysis. Again, after controlling for age, NAA concentration was associated with thalamic volume (right thalamus: T [74] = 2.596, p = 0.011, effect size = 0.090; left thalamus: T [74] = 2.503, p = 0.015, effect size = 0.084) (Fig. 3b).
Thalamic volume in association with other white matter metabolites
We also conducted exploratory analyses testing for possible associations among other white matter metabolites and thalamic volume. Controlling for age at MRI, pWMLs, and NAA, only one additional parietal white matter metabolite added significant predictive value to the linear regression on thalamic volume: choline (right thalamus: T [101] = −3.107, p = 0.002, effect size = 0.095; left thalamus: T [101] = −3.237, p = 0.002, effect size = 0.103) (Fig. 4a). No significant associations were found for creatine, lactate, glutamate, glutamine, or myo-inositol (all p’s > 0.2). The final model, which included age at MRI, pWMLs, NAA, and choline, is presented in Table 1.
Residual plots demonstrating the association between choline concentration and the volume of the right thalamus across the full sample of preterm infants, while controlling for PCA, pWMLs, and NAA concentration (a) and in the subsample of preterm infants without pWMLs (n = 75) while controlling for PCA and NAA (b)
As above, we also ran the regression analyses while excluding the preterm infants with pWMLs. In this model, after controlling for age at MRI, choline (right thalamus: T [73] = −2.915, p = 0.005, effect size = 0.116; left thalamus: T [73] = −3.033, p = 0.003; Fig. 4b), and also lactate (right thalamus: T [72] = −2.220, p = 0.030, effect size = 0.069 ; left thalamus: T [72] = −1.991, p = 0.050 (n.s.)], predicted thalamic volume.
Thalamic volume and cerebral metabolism at term-equivalent age
As a final inquiry, we restricted our sample to the cases who were scanned at term-equivalent age or older (postconceptional age ≥ 37.0; n = 87) and repeated the above analyses. In this model, after controlling for age at MRI and pWMLs, NAA (right thalamus: T [83] = 2.440, p = 0.017, effect size = 0.072; left thalamus: T [83] = 2.197, p = 0.031, effect size = 0.058) and choline concentration (right thalamus: T [82] = −1.689; p < 0.1; left thalamus: T [82] = −2.137, p = 0.036, effect size = 0.056) still predicted thalamic volume.
Discussion
The results from this study demonstrated significant associations between NAA and choline concentrations (measured in the parietal white matter) and thalamic volume in preterm infants. These effects were above and beyond the effect of white matter injury (as defined by punctate white matter lesions) and age at MRI and remained evident even when preterm infants with punctate white matter lesions were excluded from the analysis. Recent neuroimaging studies have documented marked differences in thalamocortical connectivity in preterm infants at term-equivalency [12, 42] and demonstrated that diminished thalamic volume in conjunction with white matter abnormalities predict lower neurodevelopmental outcome in early childhood [11]. The association here between white matter NAA concentration and thalamic volume not only provides convergent validity for this neuroimaging phenotype but also, in conjunction with other metabolites, further information regarding pathogenesis.
Human neuropathological studies have identified two predominant abnormalities associated with preterm white matter injury: periventricular leukomalacia (PVL) and diffuse astrogliosis without focal necrosis [14, 43, 44]. Although advances in neonatal intensive care have led to a dramatic reduction in the incidence of large, cystic lesions (i.e., cavitary PVL), non-cystic focal lesions and microcysts (i.e., non-cystic PVL) are still observed in approximately one third of cases at autopsy while the incidence of diffuse white matter gliosis is even higher [14, 44]. Moreover, although it is possible that diffuse astrogliosis and PVL represent a spectrum of preterm white matter injury with increasing severity, it is noteworthy that in neuropathological studies, neuronal loss in gray matter structures, including the thalamus, has been exclusively associated with PVL [13, 14]. Consistent with the neuropathological studies, the results from this study demonstrated a negative association between pWMLs and thalamic volume, that is, the presence of pWMLs predicted a decrease in thalamic volume.
Yet, human neuroimaging studies repeatedly demonstrate that thalamic volume is diminished in preterm infants, even in the absence of focal white matter injury [7, 8, 42]. Moreover, DTI tractography studies have demonstrated diminished connectivity from the thalamus to widespread cortical regions, including frontal cortices, supplementary motor areas, and occipitotemporal gyri [12, 42]. It has been proposed that the decreased thalamic volume, increased thalamic diffusivity, and decreased white matter fractional anisotropy are together compatible with decreased cell numbers in the thalamocortical system [42]. While the decreased NAA in association with diminished thalamic volume would be consistent with this hypothesis, the finding that elevated choline also predicts decreased thalamic volume suggests additional and/or alternative pathogenic mechanisms.
In MRS, the choline signal is generally considered a marker of membranes because it incorporates the precursors or degradation products of membrane phospholipids [31]. Notably, the choline peak, which is comprised largely of indistinguishable signal from two compounds: phosphocholine (PC) and glycerophosphocholine (GPC), reflects only water-soluble choline metabolites. Sphingomyelin and phosphatidylcholine (lecithin), which are large and immobile membrane components, are “MR invisible.” Choline concentration is elevated in fetuses and neonates and declines in infancy, coincident with white matter maturation and myelination [20, 22, 27, 30, 45–47]. Prior research has also demonstrated that choline is elevated in the setting CNS inflammation, with the magnitude of the choline signal related to glial proliferation [48, 49] and demyelination/remyelination [50].
In a prior study, we found no mean difference in choline concentration among preterm infants with and without pWMLs or in relation to increasing DEHSI severity [23]. However, in the present study, after controlling for white matter injury and NAA concentration, we found that elevated choline in the white matter predicted diminished thalamic volume. Moreover, when infants with pWMLs were excluded from the analyses, choline concentration remained a significant predictor of thalamic volume. The fact that pWMLs or DEHSI alone was not associated with a difference in the average choline concentration indicates that these MR biomarkers of white matter injury are not necessarily associated with alterations in the net rate of membrane synthesis or breakdown in the white matter. Nevertheless, the association between choline concentration and thalamic volume observed here does suggest that that the pathogenesis of diminished thalamic volume in preterm neonates is related to a reduction in membrane synthesis, or else, glial proliferation, in the white matter.
Recent studies have demonstrated that injury to and subsequent maturational arrest of the pool of developing oligodendrocytes is the probable pathogenic mechanism underlying cerebral hypomyelination—a hallmark finding in preterm survivors [44, 51–53]. The findings from this study further suggest that the maturational arrest of the oligodendrocytes may not only affect the development of the white matter but also may account for the disrupted thalamocortical development and consequent neurocognitive disabilities. In preparation for myelination, unmyelinated axons rapidly expand in diameter [54, 55] stimulated by signals from mature oligodendroglia [55]. In diffusion tensor imaging, the maturation is represented by increased fractional anisotropy (FA), coincident with the rise in immature oligodendrocytes [56]. In MRS, the maturation is reflected in the increase in NAA concentration [20–22], as well as, as noted above, a decrease in choline. Thus, taken together, the association between thalamic volume and NAA and choline concentrations observed here and the association between decreased thalamic volume and white matter FA observed in previous studies suggests the possibility that the pathogenesis of aberrant thalamocortical development is related to the arrested oligodendrocyte maturation in the white matter.
Additional support for this hypothesis is provided by the observation that in this study, elevated lactate is also associated with diminished thalamic volume when infants with pWMLs are excluded from the analysis. Lactate, a marker of anaerobic metabolism, has been observed in association with active inflammatory lesions in patients with multiple sclerosis, acute HIV infection of the CNS, and correlates with the expression of proinflammatory cytokines in infants with perinatal asphyxia [48, 57, 58]. Moreover, astrogliosis has been shown to potentiate damage to developing oligodendrocytes [59]. Thus, together, the metabolic data provide convergent evidence of an association between impaired corticothalamic development, astrogliosis, and myelination failure.
It should be noted that a biomarker specific to astrogliosis in preterm neonates remains elusive. In this study as in many previous studies, myo-inositol, an osmolite present in high intracellular concentration in astroglia, was included as a potential surrogate indicator of astrogliosis. However, near term-equivalency, myo-inositol decreases dramatically as a function of age, coincident with the transient proliferation of microglia in the white matter [60]. Thus, myo-inositol is already a challenging marker to use for astrogliosis in a preterm neonate near term-equivalency due to the rapid underlying developmental changes during this period. Furthermore, the role of myo-inositol as an osmolite confounds its potential role as an astroglial marker [33]. More work is needed to develop a more specific marker for astrogliosis in preterm white matter injury.
It should also be noted that prior studies have demonstrated associations between DEHSI and thalamic volume at term-equivalency [7, 8], although such associations were not demonstrated here. Similar inconsistency has been observed in studies attempting to relate DEHSI to long-term neurodevelopmental outcome [61–63]. In this study, DEHSI was classified visually on a qualitative scale. It is possible that a more quantitative measure of white matter signal (such as ADC or T2 relaxation) would relate to thalamic volume.
Also, in this study, a binary classification was used for pWMLs, without quantitating the number of pWMLs or distinguishing isolated pWML lesions from clusters or conglomerates [64]. A remaining question concerns whether these lesions convey a dose-dependent effect on thalamic volume. In this context, it is possible that NAA concentration in infants with pWMLs reflects, in part, a degree of axonopathy that not only predicts thalamic volume but also is linearly related to the extent of overt white matter injury. Further research is needed.
At the same time, recent studies have documented associations between NAA/choline ratios in preterm neonates and later developmental outcome [65–67] (but see also [68]). Although without outcome data, we cannot speculate on the clinical significance of the associations between white matter metabolism and thalamic volume observed in our study, these findings suggest that the observed association between NAA/choline and outcome might be further mediated by changes to the developing corticothalamic system. Moreover, as our parietal white matter voxel included most of the developing white matter from the dorsolateral boundary of the trigone of the lateral ventricle to the intragyral white matter, we also cannot rule out contributions from other developing corticocortical fiber bundles to our findings.
A further limitation of this study is the fact that we did not directly account for brain volume in our analyses of thalamic volume. However, age, which is also strongly associated with growth during this period, was included as covariate.
In summary, we show for the first time that reduced parietal white matter NAA, irrespective of visible white matter injury on conventional MRI, was associated with reduced thalamic volumes in high-risk preterm infants near term-equivalent age. In addition, choline, a marker specific to membrane synthesis, and lactate, measured in the white matter, also predicted thalamic volume. Together, these observations provide convergent evidence of a neuroimaging phenotype characterized by abnormal thalamocortical development and suggest that the pathogenesis may involve impaired axonal maturation in association with arrested oligodendrocyte maturation and myelination failure.
References
Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF (2010) The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88:31–38
[2]. Aarnoudse-Moens CSH, Weisglas-Kuperus N, Goudoever JBv, Oosterlaan J (2009) Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children.
Marret S, Marchand-Martin L, Picaud JC, Hascoet JM, Arnaud C, Roze JC, Truffert P, Larroque B, Kaminski M, Ancel PY (2013) Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One 8:e62683
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124
Kostovic I, Judas M (2010) The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr 99:1119–1127
Marin-Padilla M (1970) Prenatal and early postnatal ontogenesis of the human motor cortex: a golgi study. I. The sequential development of the cortical layers. Brain Res 23:167–183
Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, Hajnal J, Allsop JM, Rutherford MA, Edwards AD (2006) Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage 32:70–78
Srinivasan L, Dutta R, Counsell SJ, Allsop JM, Boardman JP, Rutherford MA, Edwards AD (2007) Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 119:759–765
Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294
Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ (1999) Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 46:755–760
Boardman JP, Craven C, Valappil S, Counsell SJ, Dyet LE, Rueckert D, Aljabar P, Rutherford MA, Chew AT, Allsop JM, Cowan F, Edwards AD (2010) A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage 52:409–414
Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, Rueckert D, Edwards AD, Counsell SJ (2013) The influence of preterm birth on the developing thalamocortical connectome. Cortex 49:1711–1721
Ligam P, Haynes RL, Folkerth RD, Liu L, Yang M, Volpe JJ, Kinney HC (2009) Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr Res 65:524–529
Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC (2007) Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol 114:619–631
Clark JB (1998) N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 20:271–276
Patel TB, Clark JB (1979) Synthesis of N-acetyl-l-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J 184:539–546
Patel TB, Clark JB (1980) Lipogenesis in the brain of suckling rats. Studies on the mechansim of mitochondrial-cytosolic carbon transfer. Biochem J 188:163–168
Bhakoo KK, Williams IT, Williams SR, Gadian DG, Noble MD (1996) Proton nuclear magnetic resonance spectroscopy of primary cells derived from nervous tissue. J Neurochem 66:1254–1263
Burri R, Steffen C, Herschkowitz N (1991) N-acetyl-L-aspartate is a major source of acetyl groups for lipid synthesis during rat brain development. Dev Neurosci 13:403–411
Bluml S, Wisnowski JL, Nelson MD Jr, Paquette L, Gilles FH, Kinney HC, Panigrahy A (2013) Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex 23:2944–2955
Xu D, Bonifacio S, Charlton NN, Vaughan CP, Lu Y, Ferriero DM, Vigneron DB, Barkovich AJ (2011) MR spectroscopy of normative premature newborns. J Magn Reson Imaging 33:306–311
Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC, du Plessis AJ (2010) Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121:26–33
Wisnowski JL, Bluml S, Paquette L, Zelinski E, Nelson MD Jr, Painter MJ, Damasio H, Gilles F, Panigrahy A (2013) Altered glutamatergic metabolism associated with punctate white matter lesions in preterm infants. PLoS One 8:e56880
Ross BD, Ernst T, Kreis R, Haseler LJ, Bayer S, Danielsen E, Bluml S, Shonk T, Mandigo JC, Caton W, Clark C, Jensen SW, Lehman NL, Arcinue E, Pudenz R, Shelden CH (1998) 1H MRS in acute traumatic brain injury. J Magn Reson Imaging 8:829–840
Gideon P, Henriksen O, Sperling BK, Christiansen P, Olsen TS, Jorgensen HS, Arlien-Soborg P (1993) Magnetic resonance spectroscopy of acute cerebral infarctions. Ugeskr Laeger 155:3194–3199
Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, Ferriero DM (1999) Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol 20:1399–1405
Cady EB, Penrice J, Amess PN, Lorek A, Wylezinska M, Aldridge RF, Franconi F, Wyatt JS, Reynolds EO (1996) Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med 36:878–886
Johnston MV (2005) Excitotoxicity in perinatal brain injury. Brain Pathol 15:234–240
Vannucci SJ, Hagberg H (2004) Hypoxia-ischemia in the immature brain. J Exp Biol 207:3149–3154
Bluml S, Seymour KJ, Ross BD (1999) Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31)P MRS in in vivo human brain. Magn Reson Med 42:643–654
Miller BL, Chang L, Booth R, Ernst T, Cornford M, Nikas D, McBride D, Jenden DJ (1996) In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 58:1929–1935
Chang L, Munsaka SM, Kraft-Terry S, Ernst T (2013) Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 8:576–593
Wisnowski JL, Schmithorst VJ, Rosser T, Paquette L, Nelson MD, Haynes RL, Painter MJ, Bluml S, Panigrahy A (2014) Magnetic resonance spectroscopy markers of axons and astrogliosis in relation to specific features of white matter injury in preterm infants. Neuroradiology 56:771–779
Kinney HC (2009) The encephalopathy of prematurity: one pediatric neuropathologist's perspective. Semin Pediatr Neurol 16:179–190
Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC (2005) Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol 484:156–167
Volpe JJ (1998) Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol 5:135–151
Maalouf E, Duggan P, Rutherford M, Counsell S, Fletcher A, Battin M, Cowan F, Edwards A (1999) Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr 135:351–357
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128
[39]. Morel A (2013) Stereotactic atlas of the human thalamus and basal ganglia.
Bluml S, Wisnowski JL, Nelson MD Jr, Paquette L, Panigrahy A (2014) Metabolic maturation of white matter is altered in preterm infants. PLoS One 9:e85829
Cohen J (1988) Statistical power analysis for the behavioral sciences. L. Erlbaum Associates, Hillsdale
Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ (2012) The effect of preterm birth on thalamic and cortical development. Cereb Cortex 22:1016–1024
Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA (2011) The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci 29:423–440
Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA (2012) Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 71:93–109
Dezortova M, Hajek M (2008) (1)H MR spectroscopy in pediatrics. Eur J Radiol 67:240–249
Kok RD, van den Berg PP, van den Bergh AJ, Nijland R, Heerschap A (2002) Maturation of the human fetal brain as observed by 1H MR spectroscopy. Magn Reson Med 48:611–616
Girard N, Fogliarini C, Viola A, Confort-Gouny S, Fur YL, Viout P, Chapon F, Levrier O, Cozzone P (2006) MRS of normal and impaired fetal brain development. Eur J Radiol 57:217–225
Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Bruck W (1999) Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol 20:1619–1627
Brenner RE, Munro PM, Williams SC, Bell JD, Barker GJ, Hawkins CP, Landon DN, McDonald WI (1993) The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med 29:737–745
Degaonkar MN, Khubchandhani M, Dhawan JK, Jayasundar R, Jagannathan NR (2002) Sequential proton MRS study of brain metabolite changes monitored during a complete pathological cycle of demyelination and remyelination in a lysophosphatidyl choline (LPC)-induced experimental demyelinating lesion model. NMR Biomed 15:293–300
Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302–1312
Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, Ligon KL, Volpe JJ, Kinney HC (2008) Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol 18:153–163
Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA (2008) Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol 63:520–530
Alix JJ, Zammit C, Riddle A, Meshul CK, Back SA, Valentino M, Fern R (2012) Central axons preparing to myelinate are highly sensitivity to ischemic injury. Ann Neurol 72:936–951
Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA (1996) Oligodendroglia regulate the regional expansion of axon caliber and local. J Neurosci 16:5095–5105
Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S (2005) Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 25:5988–5997
Bartha AI, Foster-Barber A, Miller SP, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM (2004) Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res 56:960–966
Pavlakis SG, Lu D, Frank Y, Wiznia A, Eidelberg D, Barnett T, Hyman RA (1998) Brain lactate and N-acetylaspartate in pediatric AIDS encephalopathy. AJNR Am J Neuroradiol 19:383–385
Kim S, Steelman AJ, Zhang Y, Kinney HC, Li J (2012) Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol 22:41–57
Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, Kinney HC (2006) Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol 497:199–208
Dyet L, Kennea N, Counsell S, Maalouf E, Ajayi-Obe M, Duggan P, Harrison M, Allsop J, Hajnal J, Herlihy A, Edwards B, Laroche S, Cowan F, Rutherford M, Edwards A (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Jeon TY, Kim JH, Yoo SY, Eo H, Kwon JY, Lee J, Lee M, Chang YS, Park WS (2012) Neurodevelopmental outcomes in preterm infants: comparison of infants with and without diffuse excessive high signal intensity on MR images at near-term-equivalent age. Radiology 263:518–526
Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE (2011) High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am J Neuroradiol 32:2005–2010
Raybaud C, Ahmad T, Rastegar N, Shroff M, Al Nassar M (2013) The premature brain: developmental and lesional anatomy. Neuroradiology 55(Suppl 2):23–40
Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP (2013) Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81:2082–2089
Bapat R, Narayana P, Zhou Y, Parikh N (2014) Magnetic resonance spectroscopy at term equivalent age in extremely preterm infants: association with cognitive and language development. Pediatr Neurol 51:53–59
Kendall GS, Melbourne A, Johnson S, Price D, Bainbridge A, Gunny R, Huertas-Ceballos A, Cady EB, Ourselin S, Marlow N, Robertson NJ (2014) White matter NAA/Cho and Cho/Cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology 271:230–238
Augustine EM, Spielman DM, Barnes PD, Sutcliffe TL, Dermon JD, Mirmiran M, Clayton DB, Ariagno RL (2008) Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatol 28:611–618
Acknowledgments
The authors would like to thank Julia Castro for organizing the data, the NICU and Radiology staff at Children’s Hospital Los Angeles, and the families for their participation in our research studies.
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Children’s Hospital Los Angeles Committee on Clinical Investigations and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in the study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wisnowski, J.L., Ceschin, R.C., Choi, S.Y. et al. Reduced thalamic volume in preterm infants is associated with abnormal white matter metabolism independent of injury. Neuroradiology 57, 515–525 (2015). https://doi.org/10.1007/s00234-015-1495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1495-7