Abstract
Introduction
The aim of this study was to evaluate whether normal controls and human immunodeficiency virus (HIV) patients with and without planning deficits differ on white matter integrity.
Methods
A total of 34 HIV-positive patients with planning deficits were compared with 13 HIV-positive patients without planning deficits and 19 gender-, age-, and education-matched control subjects. Diffusion tensor imaging (DTI) was performed along 30 noncolinear directions in a 1.5-T scanner. For tract-based spatial statistics analysis, a white matter skeleton was created, and a permutation-based inference with 5000 permutations with a threshold of p < 0.05 was used to identify abnormalities in fractional anisotropy (FA). The median, radial, and axial diffusivities were also projected onto the mean FA skeleton.
Results
Compared with controls, HIV-positive patients with planning deficits had decreased FA in bilateral anterior thalamic radiations, bilateral inferior fronto-occiptal fasciculi, genu and splenium of the corpus callosum, bilateral superior longitudinal fascicule, and bilateral uncinate fasciculi. Compared to HIV-positive patients without planning deficits, patients with planning deficits had decreased FA in bilateral anterior thalamic radiations, bilateral inferior fronto-occiptal fasciculi, genu of the corpus callosum, bilateral superior longitudinal fascicule, and right uncinate fascicule.
Conclusion
DTI can detect extensive white matter abnormalities in the normal-appearing white matter of HIV-positive patients with planning deficits compared with controls and HIV-positive patients without planning deficits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than one third of human immunodeficiency virus (HIV)-positive patients will manifest neurological disorders during the course of the disease. However, conventional magnetic resonance imaging (MRI) sequences show nonspecific changes in late-stage HIV-associated neurocognitive disorders (HAND) [1]. Diffusion tensor imaging (DTI) is an imaging modality that measures, through the amount of anisotropy, the directional nature of water diffusion, which is given by cellular structures, such as myelin and white matter tracts, assessing differences in diffusion of water molecules between normal and pathological tissue [2]. Despite some controversial results [3], most previous studies found a decrease in fractional anisotropy (FA) and an increase in mean diffusivity (MD) and radial diffusivity (RD) values in different white matter regions of HIV-infected patients [4–12].
According to the classification of HAND, the following cognitive domains should be examined, ideally by standardized neuropsychological tests: verbal/language, attention/working memory, abstraction/executive function, memory (learning, recall), speed of information processing, and sensory–perceptual and motor skills [13]. Executive function can be defined as complex cognitive processing, which requires the coordination of several subprocesses to perform complex tasks or goal-directed behaviors. It involves complex cognitive abilities of goal formation, planning, carrying-out goal-directed plans, and effective performance, mainly dependent on the frontal lobes [14, 15]. Planning refers to the ability to identify and organize steps and elements required to achieve a goal, a multifaceted activity that requires complex cognitive demands [15], involving the identification, organization, and completion of sequential behaviors toward the accomplishment of an objective [16].
HIV infection is associated with executive dysfunction [17–19], including planning capacity [16], which is related to impairments in daily functioning, such as inability to plan goals and step-by-step activities. However, no studies have used neuroimaging to correlate planning deficits with the degree of white matter injury in HIV. Understanding the pathophysiology underlying planning deficits in HIV-positive patients will facilitate diagnosis and interventions for this population.
This study used DTI to investigate the integrity of white matter in patients with HIV infection with and without deficits in planning, a subcomponent of executive function. Our hypothesis was that HIV patients with planning deficits would have extensive areas with abnormal FA, predominantly in the frontal lobes, compared with controls and HIV patients without planning deficits.
Materials and methods
Subjects
The Institutional Review Board approved this prospective study, and all participants signed informed consent. Between September 2009 and December 2012, 47 patients with HIV infection, confirmed by enzyme-linked immunosorbant assay and Western blot and lasting at least 5 years, were studied. HIV-positive patients were divided into two groups, based on the presence or absence of planning deficits, assessed using the Wisconsin Card Sorting Test (WCST).
The HIV-positive group with planning deficits included 34 patients (26 men and 8 women), the HIV-positive group without planning deficits included 13 participants (10 men and 3 women) and the control group had 19 healthy volunteers (10 men and 9 women). The three groups were matched for age, sex, years of education, mean Mini-Mental State Examination score, and the HIV-positive patients groups were also matched for years of known infection (Table 1). Years of education were defined as the amount of years that the participant went to school or college, excluding the years of repetition. During the week of the MRI, the HIV-positive with planning deficits group had a mean CD4 count of 693.42 cells/μL; 32 patients had undetectable viral load, one had 717 copies/μL, and one had 580 copies/μL, and all patients were using highly active antiretroviral therapy (HAART); the HIV-positive patients without planning deficits had a mean CD4 count of 606.25 cells/μL, and all had an undetectable viral load in plasma and were using HAART. Based on the Memorial Sloan Kettering (MSK) Ratio Scale [13], 15 HIV-positive patients with planning deficits were rated 0.0, 11 were rated 0.5, and eight were rated 1.0. Eight HIV-positive patients without planning deficits were rated 0.0, three were rated 0.5, and two were rated 1.0.
Exclusion criteria were the following: declared illicit drug use within the past year, neurological disorders, such as current or past CNS infection, MRI contraindications, and abnormal brain MRI findings on conventional fluid attenuation inversion recovery (FLAIR) and sagittal 3D T1-weighted sequences. All MRIs were reviewed by an experienced neuroradiologist (ELG, 15 years of experience).
Neuropsychological assessment
Planning cognitive score was calculated based on two WCST (128 cards) [20, 21] raw scores, which were converted into Z scores (patient score minus normative mean divided by normative standard deviation) [22]. Participants were considered to have planning impairments if they had Z scores ≤1.5 on Learning to Learn score (percent error difference score of consecutive pairs categories) and/or Completed Categories score (number of completed categories trials, maximum of six).
The WCST is one of the most widely used tools to evaluate executive functions. This test requires strategic planning, organization, cognitive flexibility, inhibition, and setting of goal-oriented behavior. Adaptation, validation, and normative studies of WCST for the Brazilian population were published by Cunha et al. [21].
In order to not have our findings biased by other critical cognitive functions involved in planning ability, as suggested by Antinori et al. [13], we assessed and compared our groups on: attention/working memory, memory (learning and recall), speed of information processing, and sensory–perceptual and motor skills domains. Table 2 shows the variables for each cognitive domain and the neuropsychological tests used. Mean Z scores showed no significant differences on performance across the groups for all dimensions, except planning (ANOVA with post hoc Bonferroni), as shown in Table 3. All neuropsychological tests were performed on the same day the MRI was acquired, by one neuropsychologist with specific training on cognitive tests and 8 years of experience.
MRI acquisition
The MRI was acquired on a 1.5-T scanner (Avanto, Siemens, Erlangen, Germany), using an eight-channel phased-array head coil. The conventional MRI protocol included axial FLAIR [repetition time (TR), 9000 ms; echo time (TE), 83 ms; field of view (FOV), 230 mm; matrix, 244 × 256; section thickness, 4.5 mm with a 10 % gap; flip angle, 180°; inversion time, 2500 ms] and sagittal T1 3D magnetization prepared rapid gradient echo (MPRAGE) weighted image (TR, 2730 ms; TE, 3.26 ms; inversion time, 1000 ms; FOV, 256 mm; matrix, 192 × 256; 1.3 mm section thickness, flip angle, 7°; voxel size, 1.3 mm × 1.0 mm × 1.3 mm), and axial diffusion tensor single-shot echo-planar imaging was acquired using bipolar diffusion gradients applied along 30 noncolinear directions (b 0 = 0 and b 1 = 900 s/mm2) (TR, 10,100 ms; TE, 94 ms; FOV, 256 mm; matrix, 122 × 120; 65 slices with 2.1 mm thickness and no gap). Subjects’ heads were stabilized with tape across the forehead and padding around the sides. All MRIs were reviewed by an experienced neuroradiologist (ELG, 15 years of experience) and were of good quality for post-processing.
Post-processing white matter integrity evaluation and statistical analysis
For voxelwise diffusion modeling, diffusion data were analyzed using FMRIB’s Diffusion Toolbox within FSL 5.0 (http://www.fmrib.ox.ac.uk/fsl) [30]. After performing eddy current correction and brain extraction, FA images for all subjects were created by fitting a tensor model onto the raw diffusion data. Voxelwise statistical analysis of the FA data was carried out using Tract-Based Spatial Statistics (TBSS) [31], part of FSL. FA data for all subjects were aligned in a common space using the nonlinear registration tool FNIRT, which uses a b-spline representation of the registration warp field. Next, the mean FA image was created and thinned to create a mean FA skeleton, which represents the centers of all tracts common to the group. Aligned FA data for each subject were then projected onto this skeleton, and the resulting data were fed into voxelwise cross-subject statistics for all voxels with FA ≥ 0.30 to exclude peripheral tracts with significant inter-subject variability and/or partial volume effects with gray matter. By applying the original nonlinear registration of each subject’s FA to standard space, the RD, MD, and axial diffusivity (AD) were also projected onto the mean FA skeleton.
For all diffusion parameters, statistical voxelwise analysis was done using permutation-based inference (5000 permutations), corrected for multiple comparisons (controlling the family wise error) with a threshold-free cluster enhancement (TFCE) and significance level of p < 0.05.
Corrected TFCE p value images were computed to enable identification of differences in FA between pairs of groups: (1) HIV-positive patients with planning deficits versus control subjects, (2) HIV-positive patients with versus without planning deficits, and (3) HIV-positive patients without planning deficits versus control subjects. White matter tracts were then identified using the Johns Hopkins University white matter tractography atlas and the International Consortium for Brain Mapping DTI-81 white matter labels atlas (JHU ICBM DTI-81), available within FSL.
Results
White matter integrity evaluation
HIV-positive patients with planning deficits versus controls
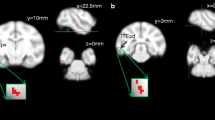
In the voxelwise-based group comparison, based on the JHU ICBM-DTI-81 white matter labels atlas, compared with control subjects, HIV patients with planning deficits had significantly decreased FA (p < 0.05) predominantly in frontal lobes. Such areas of differences in FA were seen in bilateral anterior thalamic radiations, bilateral inferior fronto-occiptal fasciculi (including bilateral anterior corona radiata and external capsule and right retrolenticular portion of the internal capsule and posterior thalamic radiation), genu and splenium of the corpus callosum, bilateral superior longitudinal fascicule, and bilateral uncinate fasciculi (Fig. 1).
Corrected P maps showing the voxels with significantly lower FA values in the brain of HIV-positive patients with planning deficit, compared with control subjects, in red (p < 0.05), in the axial (a), sagittal (b), and coronal (c) planes. It is also shown the lower FA values in the brain of HIV-positive patients with planning deficit, compared with HIV-positive patients without planning deficit subjects, in blue (p < 0.05), in the axial (d), sagittal (e), and coronal (f) planes. Note the predominance of FA value differences in the frontal lobes and genu of the corpus callosum and the similarities between the two comparisons, but with a greater extent in the comparison between HIV positive with planning deficits and control groups. There were no significant differences in mean FA values between HIV-positive patients without planning deficits and controls (not shown)
The mean RD and MD mean values of the HIV-positive group with planning deficit, compared with controls, were increased almost in the same regions where the FA values were reduced (Fig. 2). On the other hand, much less widespread abnormalities were seem on the AD values, being restricted to the left anterior thalamic radiation, left inferior fronto-occiptal, and left superior longitudinal fasciculi, which showed increased AD values in the HIV-positive group with planning deficit.
Corrected P maps showing the voxels with significantly increased RD values in the brain of HIV positive patients with planning deficit, compared with control subjects in yellow (p < 0.05), in the axial (a), sagittal (b), and coronal (c) planes. It is also shown the increased RD values in the brain of HIV positive patients with planning deficit, compared with HIV-positive patients without planning deficit subjects in brown (p < 0.05), in the axial (d), sagittal (e), and coronal (f) planes. Note the similarity of RD values differences in the two comparisons, including the splenium of the corpus callosum, left uncinate fascicule, and bilateral inferior fronto-occiptal fasciculi
HIV-positive patients with planning deficits versus HIV-positive patients without planning deficits
Similar to the comparison between HIV-positive patients with planning deficits and controls, HIV-positive patients with planning deficits also had significantly decreased FA values (p < 0.05) in relation to those without that deficit in bilateral anterior thalamic radiations, bilateral inferior fronto-occiptal fasciculi (in the anterior corona radiata), genu of the corpus callosum, bilateral superior longitudinal fasciculi, and right uncinate fascicule (Fig. 1), also predominating in frontal lobes. The remaining areas that differed in the previous comparison also had lower FA in HIV-positive patients with planning deficit versus those without this deficit (splenium of the corpus callosum, left uncinate fascicule, remaining of bilateral inferior fronto-occiptal fasciculi, and posterior right thalamic radiation), with a trend of significance (p = 0.07).
The regions with decreased FA values above showed increased RD and MD mean values. In addition, the regions with a trend of significance of the FA analysis showed increased RD and MD mean values (Fig. 2). No significant AD mean values abnormalities were seen between these groups.
HIV-positive patients without planning deficits versus controls
There were no significant differences in mean FA, MD, RD, and AD values of white matter tracts between HIV-positive patients without planning deficits and controls.
Discussion
In this study, we used a voxelwise-based method to assess DTI of white matter tracts in 34 HIV patients with planning deficits, 13 patients without planning deficits, and 19 control subjects. Our results showed no differences in FA, MD, RD, and AD values between HIV-positive patients without planning deficit and the control group. However, we showed significant lower FA and increased MD and RD values in the HIV-positive patients with planning deficit versus HIV-positive patients without this deficit and also versus controls, predominantly in the frontal lobes, and a difference in AD values, only when comparing the HIV-positive group with deficit with the control group.
The differences found among the groups were expected, since the executive functions are mediated primarily by the frontal lobes [15], which develop, select, and execute plans. Planning is a fundamental subcomponent of executive functions that involves a multifaceted process of identification, organization, and integration of the steps required to meet a particular goal [16], but is challenging to operationalize because it requires several component processes like attention, abstract thinking, temporal sequencing, and reasoning [32]. WCST is a standard instrument for executive impairment measures, including planning, although not considered specific for executive and planning dysfunctions, being influenced by other functions such as working memory [33]. We have applied more specific tests to assess other cognitive dysfunctions, in addition to planning, to make the matching of the groups. Therefore, the only significant cognitive difference between the three groups in our study was the planning impairment, detected by the WCST.
Executive dysfunction remains highly prevalent in HIV-positive patients, being detected in approximately 50 % of the patients with neurocognitive impairment. Clinically, less accurate and efficient planners are more likely to be unemployed and to report declines in other instrumental activities of daily living, including medication management [16]. Cattie et al. [16] observed that individuals with HAND show difficulties in various aspects of planning with decreased accuracy and efficiency, slower problem resolution, and less flexibility in problem-solving strategies. Although the frontal lobes have a predominant role in executive function [34], recent studies have implicated a network of brain structures beyond the frontal lobes, and patients without frontal damage often have executive deficits, including planning capacity [35]. Our study found similar results to these neuropsychological studies, since it shows alterations in diffusivity values, predominantly, but not exclusively, in the frontal lobes and the HIV-positive group without planning deficit behaving very similar to the control group.
Previous authors had also assessed in vivo white matter integrity in HIV-positive patients. Most of these studies used regions of interest (ROI) manually positioned approaches and showed decreased FA values in the splenium [4, 5] and genu [3, 4] of the corpus callosum, frontal white matter [6], in the FA value of the whole brain [7], or no significant differences [8], and an increased MD value in the genu [3] and splenium [5] of the corpus callosum, in HIV-positive patients, compared to controls. However, ROI-based techniques are susceptible to errors in positioning and do not assess all white matter tracts. More recent studies, which used a voxelwise-based analysis, showed that HIV-positive patients have decreased FA in the body of corpus callosum [9], right posterior limb of the internal capsule, right inferior longitudinal fasciculus, and right optic radiation [10] and in all major white matter regions, mainly distributed in frontal, parietal, and temporal white matter regions [11]. The studies that evaluated the other diffusivity parameters found increased RD and MD values in the body of the corpus callosum, left posterior corona radiata [9], and in all major white matter regions [11], in HIV-positive patients, and increased AD values in the left superior corona radiata [9] and in some white matter regions, but to a lesser extent, in relation to the other diffusivity parameters. These results, similarly to ours, demonstrate a predominance of RD abnormal values over the AD values, on the areas of decreased FA, suggesting that demyelination could play a role on the HIV central nervous system physiopathology [36, 37]. HIV-positive patients with nonspecific macrostructural lesions had substantial FA reduction adjacent to the injured areas, encompassing several white matter tracts, whereas HIV-positive patients without macrostrutural lesions had reduced FA in similar white matter regions, but these effects were not as pronounced [12]. Cognitively impaired HIV-positive patients had significantly decreased FA in the genu and body of the corpus callosum [10], compared with cognitively normal HIV-positive patients. Some authors have found correlations between lower FA values in the whole brain with dementia severity [7], in the splenium of the corpus callosum with dementia severity and motor speed deficits [5], in the basal ganglia with visual and working memory deficits and in centrum semiovale with visuoperceptual deficits [8], and a correlation between an increased MD values in the splenium of the corpus callosum with motor velocity dysfunction [8], in the putamen, with verbal memory deficit, and in the centrum semiovale and visual memory deficit [7]. Similarly, in the present study, HIV-positive patients with cognitive deficits showed widespread white matter abnormalities in FA, RD, and MD values when compared with patients without deficits and controls. These results support the hypothesis that DTI could play a significant role in the evaluation of the normal-appearing white matter of HIV-positive patients and could also be considered as a potential biomarker for cognitive impairment assessment in these patients.
Although our study used a relatively small sample, we assessed a very specific cognitive function, and it was enough to demonstrate abnormalities in the normal-appearing white matter that could indicate an early sign of cognitive impairment. Additionally, all patients were on HAART; thus, we could not compare the differences in DTI of patients treated and untreated. Although, our sample of HIV patients was heterogeneous in terms of viral load, all patients were neurologically asymptomatic, and there were no significant differences in age, sex, education, years of known HIV infection, other cognitive functions, and Mini Mental State Examination; the only difference was the presence or absence of planning deficits. While participants reported that they had not used illicit drugs for at least the previous year, this was not verified by blood tests. Finally, because our study was designed as a cross-sectional examination of DTI in HIV-positive patients, and all patients were on HAART, we could not examine longitudinal effects of treatment, lymphocyte CD4 counts, or viral load.
Conclusion
Using a voxelwise-based technique for analysis of DTI, we found significant abnormalities in the white matter of HIV patients with planning deficits, mainly in the frontal lobes and genu of the corpus callosum. The current study suggests that diffusion tensor MR imaging might play a significant role as a biomarker for the cognitive evaluation of HIV-positive patients associated to specific executive function impairments. These results should be replicated in other studies and extended to other cognitive functions.
References
Karampekios S, John Hesselink J (2005) Cerebral infections. Eur Radiol 15:485–493
Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R (2004) Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46(5):339–350
Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC (2005) Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. AJNR Am J Neuroradiol 26:2275–2281
Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W (2001) Diffusion tensor imaging of HIV patients and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol 22:277–83
Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB (2006) Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol 27:656–60
Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO (2001) White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res 106(1):15–24
Ragin AB, Storey P, Cohen BA, Epstein LG, Edelman RR (2004) Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR Am J Neuroradiol 25(2):195–200
Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG (2005) Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol 11:292–298
Leite SC, Corrêa DG, Doring TM et al (2013) Diffusion tensor MRI evaluation of the corona radiata, cingulate gyri, and corpus callosum in HIV patients. J Magn Reson Imaging 38(6):1488–1493
Gongvatana A, Schweinsburg BC, Taylor MJ et al (2009) White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 15(2):187–95
Chen Y, An H, Zun H et al (2009) White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. NeuroImage 47:1154–1162
Stubbe-Dräger B, Deppe M, Mohammadi S et al (2012) Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol 12:23
Antinori A, Arendt G, Becker JT et al (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Elliott R (2003) Executive functions and their disorders. Br Med Bull 65:49–59
Jurado MB, Rosselli M (2007) The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 17:213–233
Cattie JE, Doyle K, Weber E et al (2012) Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsychol 34(9):906–918
Reger M, Welsh R, Razani J, Martin DJ, Boone KB (2002) A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 8:410–424
Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S (2008) Variable patterns of neuropsychological performance in HIV-1 infection. J Clin Exp Neuropsychol 30:613–626
Woods SP, Moore DJ, Weber E, Grant I (2009) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19:152–168
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G (1993) Wisconsin card sorting test manual (revised and expanded). Psychological Assessment Resources, Odessa
Cunha JA, Trentini CM, Argimon I, Oliveira MS, Werlang BG, Prieb RGG (2005) Adaptação e padronização brasileira do Manual do Teste Wisconsin de Classificação de Cartas. Casa do Psicólogo
Kavé G, Heled E, Vakil E, Agranov E (2011) Which verbal fluency measure is most useful in demonstrating executive deficits after traumatic brain injury? J Clin Exp Neuropsychol 33(3):358–365
Gauthier L, Dehaut F, Joanette Y (1989) The bells test: a quantitative and qualitative test for visual neglect. J Int Neuropsychol Soc 11:49–54
Fonseca RP, Oliveira C, Gindri G, Zimmermann N, Reppold C (2010) Teste Hayling: um instrumento de avaliação de componentes das funções executivas. In: Hutz C (ed) Avaliação psicológica e neuropsicológica de crianças e adolescente. Casa do Psicólogo, São Paulo, pp 337–364
War Department Adjutant General′s Office (1944) Army individual test battery: manual of directions and scoring. Edited by: Washington DC: War Department Adjutant General′s Office
Wechsler D (1997) Wechsler adult intelligence scale—III. The Psychological Corporation, San Antonio
Salgado JV, Malloy-Diniz LF, Abrantes SSC et al (2011) Applicability of the rey auditory-verbal learning test to an adult sample in Brazil. Rev Bras Psiquiatr 33:234–237
Fonseca RP, Salles JF, Parente MAMP (2009) Instrumento de avaliação neuropsicológica breve NEUPSILIN. Vetor, São Paulo
Fonseca RP, Parente MAMP, Côté H, Ska B, Joanette Y (2008) Bateria montreal de avaliação da comunicação—Bateria MAC. Pró-Fono, São Paulo
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:208–219
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Kramer JH, Mungas D, Possin KL et al (2014) NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc 20:11–19
Gouveia PAR, Brucki SMD, Malheiros SMF, Bueno OFA (2007) Disorders in planning and strategy application in frontal lobe lesion patients. Brain Cogn 63:240–246
Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S (2007) Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68:1515–1523
Grafman J (1995) Similarities and distinctions among current models of prefrontal cortical functions. Ann N Y Acad Sci 769:337–368
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55:302–308
Smith AB, Smirniotopoulos JG, Rushing EJ (2008) From the archives of the AFIP—central nervous system infections associated with human immunodeficiency virus infection: radiologic–pathologic correlation. RadioGraphics 28:2033–2058
Ethical standards and patient consent
We declare that all human and animals studies have been approved by the Ethical Review Board of the Clementino Fraga Filho University Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrêa, D.G., Zimmermann, N., Doring, T.M. et al. Diffusion tensor MR imaging of white matter integrity in HIV-positive patients with planning deficit. Neuroradiology 57, 475–482 (2015). https://doi.org/10.1007/s00234-015-1489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1489-5