Abstract
Introduction
The introduction of the balloon remodeling and stent-assisted technique has revolutionized the approach to coil embolization for wide-neck aneurysms. The purpose of this study was to determine the frequency of thromboembolic events associated with single balloon-assisted, double balloon-assisted, and stent-assisted coil embolization for asymptomatic unruptured aneurysms.
Methods
A retrospective review was undertaken by 119 patients undergoing coiling with an adjunctive technique for unruptured saccular aneurysms (64 single balloon, 12 double balloon, 43 stent assisted). All underwent diffusion-weighted imaging (DWI) within 24 h after the procedure.
Results
DWI showed hyperintense lesions in 48 (40 %) patients, and ten (21 %) of these patients incurred neurological deterioration (permanent, two; transient, eight). Hyperintense lesions were detected significantly more often in procedures with the double balloon-assisted technique (7/12, 58 %) than with the single balloon-assisted technique (16/64, 25 %, p = 0.05). Occurrence of new lesions was significantly higher with the use of stent-assisted technique (25/43, 58 %) than with the single balloon-assisted technique (p = 0.001). Symptomatic ischemic rates were similar between the three groups. The increased number of microcatheters was significantly related to the DWI abnormalities (two microcatheters, 15/63 (23.8 %); three microcatheters, 20/41 (48.8 %) (p = 0.008); four microcatheters, 12/15 (80 %) (p = 0.001)).
Conclusion
Thromboembolic events detected on DWI related to coil embolization for unruptured aneurysms are relatively common, especially in association with the double balloon-assisted and stent-assisted techniques. Furthermore, the number of microcatheters is highly correlated with DWI abnormalities. The high rate of thromboembolic events suggests the need for evaluation of platelet reactivity and the addition or change of antiplatelet agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular coil embolization procedures for the treatment of intracranial aneurysms have been evolving over the past two decades. The indications for treatment of aneurysms have expanded as new techniques and adjunctive devices are used. In 1991, the advent of Guglielmi detachable coils (Target Therapeutics, Boston Scientific, Fremont, CA) significantly improved the endovascular treatment of these lesions by means of a reliable, electrolytically detachable, coiling system [1]. The stent and coil technique was later described by Geremia et al. in 1994 [2]. The use of the balloon remodeling technique was described by Moret et al. in 1997 [3]. These techniques have been proven to be effective, at least in the short term, for aneurysm repair [4–8].
The safety and efficacy of coil insertion procedures have been fairly and rigorously studied, albeit without the benefit of long-term follow-up compared with the “gold standard” of surgical clipping of aneurysms [9–11]. The major complications of endovascular procedures are thromboembolic events [12]. Thromboembolic complications can manifest as the following phenomena: (1) thrombus formation observed during the procedure; (2) clinically recognized ischemia on neurological evaluation, which can be a transient ischemic attack or permanent ischemic infarction; or (3) “silent ischemia,” as represented by diffusion-weighted imaging (DWI) abnormalities on magnetic resonance (MR) imaging.
There is a fairly broad range of the occurrence of silent ischemia reported in the literature. Case series have demonstrated a frequency of silent ischemia as high as 5.5 to 73 % [8, 13–17], while clinical evidence of stroke (transient ischemic attack and ischemic infarcts) can range from 3.8 to 40 % of cases [12, 13, 15, 18]. Although recent studies have found an equal or decrease in the incidence of “clinical” ischemic events compared with conventional coil embolization without adjunctive techniques [4, 14, 19–25], some studies have reported that silent ischemia is detected more frequently in cases with balloon-assisted coil embolization than with conventional coil embolization [15, 17]. However, some studies have reported that there is no significant relationship between adjunctive techniques and silent ischemia [4, 20, 23–25]. However, in these studies, some lacked information on the use of antiplatelet agents, some included mixed ruptured and unruptured cases, and some did not use an antiplatelet agent for the conventional coil treatment group.
The current study was performed to evaluate the frequency of thromboembolic events associated with coil insertion for treatment of unruptured aneurysms in the following situations: single balloon-assisted, double balloon-assisted, and stent-assisted techniques. All procedures were performed using aspirin and/or clopidogrel before treatment. Earnest et al. [26] described in their report that the use of more catheters is significantly associated with neurological complications. Therefore, we hypothesized that the use of adjunctive devices increases the frequency of thromboembolic complications. This study represents a retrospective case series in which we evaluated all saccular unruptured aneurysms that had been treated with endovascular coil embolization using adjunctive techniques at our institution.
Methods
Patient population and characteristics of aneurysms
All patients referred to our institute for endovascular coil embolization of unruptured cerebral aneurysms between January 2009 and April 2012 were retrospectively evaluated. There were 173 aneurysm coil treatment procedures in 160 patients. Patients who did not have a postprocedure MR imaging performed were excluded. If patients had over two aneurysms, these patients were also excluded. Fusiform aneurysms considered as dissecting aneurysms were also excluded. One hundred twenty-one patients with 121 unruptured saccular aneurysms were enrolled. Because most of the procedures were performed using balloon-assisted or stent-assisted techniques to control the coil insertion, to achieve a higher packing ratio and prepare for unexpected bleeding from aneurysms in our institute, only two aneurysms were treated using the conventional technique. Because of the small number of treated aneurysms, these two aneurysms were excluded from this study and 119 patients (30 men, 89 women) with 119 aneurysms were investigated.
Each patient underwent a complete and detailed neurological examination performed by a stroke neurologist before and immediately after embolization. New neurological deficits were noted. Patients were monitored closely in the neuroscience intensive care unit for any clinical change during the first 24–48 h after the procedure was performed.
The aneurysms treated were at the following locations: internal carotid artery (ICA) (n = 66), anterior cerebral artery (ACA) (n = 29), and posterior circulation (n = 24). The size, neck diameter, and dome-to-neck ratio of the aneurysm were measured. Twenty-five aneurysms were small (<10 mm) with a small neck (<4 mm), 70 aneurysms were small with a wide neck (≥4 mm), 21 were large (10–25 mm), and three were giant in size (>25 mm). Characteristics of treated aneurysms are shown in Table 1.
Embolization procedure and clinical and angiographic evaluations
All endovascular treatments were performed by one operator (A.H.). Patients were pretreated with clopidogrel 75 mg/day before single balloon-assisted coil embolization, and with dual antiplatelet agents (clopidogrel 75 mg/day and aspirin 100 mg/day) before double balloon- and stent-assisted coil embolization for a minimum of 4 days. Clopidogrel and/or aspirin were given without any inhibition test made prior to treatment. Interventional procedures were conducted under general anesthesia. Embolization was performed under systemic heparinization to maintain activated clotting time at 2–2.5 times the baseline through the procedure. All flushed saline and contrast medium were heparinized (1,000 IU/100 ml), and a guiding catheter and microcatheter were placed with a continuous heparinized drip.
All patients, except for two patients, had coil embolization performed using a balloon-assisted or stent-assisted technique to control coil insertion, achieve higher packing, and prepare for unexpected bleeding from aneurysms. In cases of wide-neck or large aneurysms, which have two parent arteries to be protected, the double balloon-assisted technique was required. In cases of wide-neck, large, or giant aneurysms, where it was considered that inserted coils were not stabilized, the stent-assisted technique was required.
The procedure was typically performed via a transfemoral route through a 6-F guiding catheter system. When the double balloon-assisted or stent-assisted technique was used, the procedure was performed through either an 8-F guiding catheter system or a 6-F guiding sheath system. Coil embolization of aneurysms was performed with the HyperGlide balloon (ev3 Neurovascular, Irvine, CA), HyperForm balloon (ev3 Neurovascular), or Enterprise stent (Cordis, Miami Lakes, FL). All treated aneurysms were evaluated angiographically during and after embolization by our neurovascular team. The results of the embolization were classified into complete occlusion, residual neck, and residual aneurysms using the Raymond classification scale [27]. Embolized volume was calculated by using the following algebraic equation: embolized volume (%) = (volume of the embolized coil) / (volume of the aneurysm) × 100. The volume of the coil was calculated based on the supposition that the coil is a cylinder. The algebraic equation to calculate the volume of the coil is as follows: volume of coil = π × (diameter of coil / 2)2 × (the length of the coil). The primary diameter of each type of coil is published by each corporation. The volume of the aneurysm was also calculated based on the supposition that the aneurysm is ellipsoid: volume of the aneurysm = 4π/3 × (width / 2)2 × (length / 2)2 × (height / 2)2.
In all cases, an immediate brain computed tomography scan was conducted and the patients were transferred to a neurosurgical intensive care unit. Systemic heparinization was not reversed, and the patients received oral mono or dual antiplatelet agents for 6–12 months postoperatively.
DWI evaluations
MR imaging was scheduled 1 day after coil embolization. The imaging was performed with a 1.5T system (MAGNETOM Avanto; Siemens, Erlangen, Germany) by using a multisection, single-shot, spin-echo echo planar imaging sequence. Diffusion gradients were applied in each of the x, y, and z directions, with 2 b values (0 and 1,000 s/mm2). Imaging parameters included an echo time of 100, field of view of 23 cm, matrix of 96 × 128, section thickness of 4 mm, and intersection gap of 2 mm. Mean apparent diffusion coefficient images were generated online from an MR imaging unit. Conventional spin-echo imaging was also performed at each examination in T1- and T2-weighted conditions and with a fluid-attenuated inversion-recovery sequence.
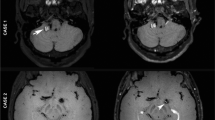
All DWI abnormalities were corrected with the finding of the T2-weighted and fluid-attenuated inversion-recovery images. All MR images were reviewed by independent neurovascular team and divided into two groups: negative or positive. Positive DWI lesions were defined as high-intensity lesions of the treated vascular area. We did not take into consideration the size or number of positive DWI lesions in this study.
Statistical analysis
The association of clinical and angiographic outcomes and new hyperintense lesions in DWI with the use of single balloon-assisted, double balloon-assisted, and stent-assisted techniques were analyzed. Data are shown as mean ± standard deviation (SD). Differences between groups were examined using Fisher’s exact test for categorical variables and the Mann-Whitney or Student’s t test for continuous data, depending on the underlying distribution. Statistical significance was defined as p < 0.05.
Results
Clinical and angiographic outcomes
One hundred nineteen coil embolizations were performed for 119 aneurysms in 119 patients. In this series, 64 aneurysms (54 %) were treated with the single balloon-assisted technique, 12 (10 %) were treated with the double balloon-assisted technique, and 43 (36 %) were treated with the stent-assisted technique. The double balloon-assisted technique was performed significantly more often in men than the other techniques (p = 0.03). ICA unruptured aneurysms treated with the double balloon-assisted technique and ACA unruptured aneurysms treated with the stent-assisted technique were performed significantly less often than ICA and ACA unruptured aneurysms treated with the other techniques, which is considered to be due to anatomical morphology. There were no middle cerebral artery (MCA) aneurysms treated by endovascular therapy because clipping for MCA aneurysm was preferable in our institute. There were no significant differences in aneurysm size and dome-to-neck ratio between the three groups, although small aneurysms with a small neck were treated significantly more frequent by the single balloon-assisted technique than with the other techniques, and there was a tendency for larger and wide-necked aneurysms to be treated with the double balloon and stent-assisted techniques. There were no significant differences in the complete occlusion rate and embolized volume between the groups. Total procedural time from insertion to withdrawal of the guiding catheter was 133.9 ± 59.8 mins in the single balloon-assisted technique, 177.4 ± 53.6 mins in the double balloon-assisted technique, and 154.3 ± 71.2 mins in the stent-assisted technique. Total procedural time in the double balloon-assisted technique was significantly longer than that of single balloon-assisted technique (p = 0.008) (Table 2).
There was one intraoperative aneurysmal rupture during the coil embolization for anterior communicating artery aneurysm using double balloon-assisted technique. After two balloon catheters were placed, microcatheter perforated the aneurysms. Immediately, balloons were inflated, heparin was reversed, and coils were inserted as soon as possible. Fortunately, the postoperative course was uneventful, and the patient was subsequently discharged home, neurologically intact. There were no intraprocedural thrombi. There were ten (8.4 %) symptomatic complications. Eight patients experienced minimum deficits (slight motor weakness in five, dysarthria in three) after the intervention and fully recovered within 12 h. One patient developed slight motor weakness due to pontine infarction after coil embolization with the double balloon-assisted technique for a large basilar apex aneurysm. One patient suffered from slight motor weakness after the procedure for an anterior communicating artery aneurysm and MR imaging demonstrated ACA lesion infarction. One patient died of severe pneumonia after the procedure. Permanent morbidity and mortality rates were 1.7 and 0.8 %, respectively. There were no significant differences between the three groups in permanent and temporary ischemic complications, morbidity rate, and mortality rate (Table 3).
DWI abnormalities
Overall, 40.3 % of the cases (48/119) showed DWI abnormalities within the treated vascular territory. There was a clinical evidence of stroke or transient ischemic attack in 10 (20.8 %) of 48 patients with DWI abnormalities. Hyperintense lesions were observed in 16 (25.0 %) of 64 patients in the single balloon-assisted technique, seven (58.3 %) of 12 in the double balloon-assisted technique, and 25 (58.1 %) of 43 in the stent-assisted technique. The occurrence of new lesions was significantly more frequent with the use of double balloon-assisted technique (p = 0.05) and the stent-assisted technique compared with the single balloon-assisted technique (p = 0.001) (Table 4). Two microcatheters were used in 63 patients at a same time, three were in 41 patients, and four were in 15 patients. Hyperintense lesions were observed in 15 (23.8 %) in two-microcatheter group, 20 (48.8 %) in three-microcatheter group, and 12 (80.0 %) in four-microcatheter group. The occurrence of new lesions was significantly more frequent with use of three microcatheters (p = 0.008) and four microcatheters compared with use of two microcatheters (p = 0.001) (Table 5).
Discussion
We studied ischemic events detected by DWI associated with the use of adjunctive techniques for cerebral unruptured aneurysms. Our results suggest an acceptable rate (1.7 %) of permanent ischemic complications and a high incidence of silent ischemic change (38/119, 31.9 %) after coil embolization. Overall, DWI abnormalities were detected in 40.3 % (48/119) of patients, especially in the use of adjunctive techniques, such as the double balloon-assisted and stent-assisted techniques, which had a higher rate of abnormalities compared with single balloon-assisted coil embolization.
Although recent studies have found an equal or a decrease in the incidence of clinical ischemic events compared with conventional coil embolization without adjunctive techniques [4, 14, 19–25], some studies have reported that silent ischemia is detected more frequently in cases with balloon-assisted coil embolization than with conventional coil embolization [15, 17]. Soeda et al. [15] reported that the overall amount of hyperintense lesions in DWI after coil embolization for asymptomatic aneurysms was 61 % (40/66), and there was a statistically significant association between balloon assistance and development of DWI changes (coil only, 50 %; balloon assistance, 73 %). Cronqvist et al. [17] performed a prospective study in which they looked at the frequency of DWI changes in 40 patients (14 with subarachnoid hemorrhage and 26 with elective procedures). DWI abnormalities were detected in 39.5 % of patients and ischemic lesions occurred more frequently in patients treated with the balloon remodeling technique (6/11, 54.5 %) and patients with ruptured aneurysms. Theoretically, because adjunctive techniques require more catheters, ischemic complications increase in such situations. Our data showed that DWI abnormalities within the treated vascular territory were significantly more frequent when the number of microcatheters increased and supported this theory.
However, some studies have reported that there is no significant relationship between adjunctive techniques and silent ischemia [4, 20, 23–25]. However, in these studies, some lacked detail of the antiplatelet protocol [4, 23, 24], some included mixed ruptured and unruptured cases [4, 20, 24, 25], and some did not use an antiplatelet agent for the conventional coil treatment group [20, 25]. Brooks et al. [20] reported that the presence of DWI abnormalities after coil embolization for ruptured and unruptured aneurysms was 32 % in cases without an adjunctive technique, 18 % in cases with the balloon-assisted technique, and 24 % in cases with the stent-assisted technique. The authors concluded that the use of adjunctive devices in treating aneurysms did not appear to increase the frequency of embolic events. However, in this previous study, dual antiplatelet therapy was used only for stent-assisted coil embolization and their results were probably related to perioperative antiplatelet medical management. Spiotta et al. [23] evaluated 147 coil embolizations (81 balloon, 66 unassisted) for unruptured aneurysms and reported a frequency of 24.7 % for the balloon-assisted technique and a frequency of 19.7 % without an adjunctive technique for DWI changes (there were no significant differences). In their series, perioperative antiplatelet medical management might have affected their results because antiplatelet medications were used in 62 % of balloon-assisted coil embolizations and in 18 % of conventional coil embolizations. In our study, patients were pretreated with clopidogrel 75 mg/day before the single balloon-assisted coil embolization and with dual antiplatelet agents (clopidogrel 75 mg/day and aspirin 100 mg/day) before the double balloon and stent-assisted coil embolization for a minimum of 4 days. If dual antiplatelet agents had been used for single balloon-assisted coil embolization, the differences might have become more significant.
Although our results suggested that coil embolization for unruptured cerebral aneurysms using adjunctive techniques increased the frequency of silent ischemic changes, other reasons should also be considered. One possible reason could be the use of a larger size of guiding catheter when the double balloon or stent-assisted technique was used. Some reports found a lower neurological complication rate when smaller and softer catheters were used [28–33]. In our study, the procedure was performed through a 6-F guiding catheter system for single balloon-assisted coil embolization. When the double balloon-assisted or stent-assisted technique was used, the procedure was performed through either an 8-F guiding catheter system or a 6-F guiding sheath system. Another reason was the total procedural time which was longer in double balloon-assisted technique. It is technically difficult to insert two balloon catheters below the wide-neck aneurysms. Several techniques were used such as catheter exchange technique, sheep technique, and so on. The complicated maneuvers need time and theoretically, thromboembolic complications increase. On the other hand, stent-assisted technique is technically simple and does not take time. However, metal of the stent was always exposed to the blood stream until the neointima covers the stent. The other possible reason for the increased frequency of silent ischemia in our study could be due to of antiplatelet resistance. Several studies have shown considerable interindividual variability in the response to aspirin and clopidogrel, with an inadequate antiplatelet effect in approximately 5 to 71 % of patients for aspirin [34–37] and 5 to 65 % for clopidogrel [38–40]. A few studies have demonstrated that low clopidogrel responders might be associated with thromboembolic events in neurointerventional procedures. Recently, several reports have described about the importance to evaluate aspirin reaction unit for aspirin and P2Y12 reaction units for clopidogrel using VerifyNow (Accumetrics, San Diego, Calf). When the antiplatelet resistance is recognized before endovascular treatment, increase in quantity of the antiplatelet agents or change to another antiplatelet agents (cilostazol, prasugrel, and so on) is an effective method [39, 41, 42]. VerifyNow has not been approved yet in Japan; we could not evaluate the antiplatelet reaction. Although there is no clear evidence or guidelines for perioperative management of antiplatelet agents based on platelet function testing, platelet activity may play an important role in neurointerventional procedures to prevent thromboembolic events.
Recently, many reports have described the efficacy and safety of flow diverter stents for cerebral aneurysms. Unfortunately, flow diverter stents have not been approved yet in Japan, and we have no data of the relationship between flow diverter stents and ischemic complications. A systematic review of pipeline embolization device (ev3 Neurovascular) showed that the symptomatic ischemic complication rate was 3.9 % [43]. Tan et al. [44] reported that presence of DWI abnormalities after pipeline embolization device placement was 50.9 %, and Heller et al. [45] reported 52 %. These reported rates were almost similar to our data treated with stent-assisted coil embolization. To reduce both of the ischemic and hemorrhagic complications, further information is needed including antiplatelet function.
There are several study limitations to this study. First, this study was a nonrandomized, retrospective, single-center trial, and there was a small number of patients. Second, there was a limited number of patients treated with the double balloon-assisted technique. Therefore, more of these cases need to be collected and analyzed. Third, there was no data of antiplatelet reaction in this study, because VerifyNow has not been approved in Japan. Some of the thromboembolic events may be due to suboptimal medical prevention and not merely to the procedure techniques. A large-scale, prospective, multicenter study should be undertaken to verify these preliminary conclusions.
Conclusions
Use of adjunctive devices in treating unruptured saccular cerebral aneurysms does not increase the frequency of symptomatic thromboembolic events, but increases the presence of DWI abnormalities, especially in association with the double balloon-assisted and stent-assisted techniques. Furthermore, the number of microcatheters is very highly correlated with DWI abnormalities. Although permanent deficits are rare, the high rate of thromboembolic events detected by DWI suggests the need for evaluation of platelet reactivity and an addition or change in antiplatelet agents, and more careful in selecting adjunctive technique. The risk and benefit of adjunctive technique must be considered.
References
Guglielmi G, Viñuela F, Sepetka I, Macellari V (1991) Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: electrochemical basis, technique, and experimental results. J Neurosurg 75:1–7
Geremia G, Haklin M, Brennecke L (1994) Embolization of experimentally created aneurysms with intravascular stent devices. AJNR Am J Neuroradiol 15:1223–1231
Moret J, Cognard C, Weill A, Castaings L, Rey A (1997) Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol 24:30–44
Albayram S, Selcuk H, Kara B, Bozdag E, Uzma O, Kocer N, Islak C (2004) Thromboembolic events associated with balloon-assisted coil embolization: evaluation with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 25:1768–1777
Cottier JP, Pasco A, Gallas S, Gabrillargues J, Cognard C, Drouineau J, Brunereau L, Herbreteau D (2001) Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. AJNR Am J Neuroradiol 22:345–351
Lefkowitz MA, Gobin YP, Akiba Y, Duckwiler GR, Murayama Y, Guglielmi G, Martin NA, Viñuela F (1999) Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysma: part II—clinical results. Neurosurgery 45:531–538
Malek AM, Halbach VV, Phatouros CC, Lempert TE, Meyers PM, Dowd CF, Higashida RT (2000) Balloon-assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery 46:1397–1407
Nelson PK, Levy DI (2001) Balloon-assisted coil embolization of wide-necked aneurysms of the internal carotid artery: medium-term angiographic and clinical follow-up in 22 patients. AJNR Am J Neuroradiol 22:19–26
Lanterna LA, Tredici G, Dimitrov BD, Biroli F (2004) Treatment of unruptured cerebral aneurysms by embolization with Guglielmi detachable coils: case-fatality, morbidity, and effectiveness in preventing bleeding—a systematic review of the literature. Neurosurgery 55:767–775
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group (2002) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360:1267–1274
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, International Study of Unruptured Intracranial Aneurysms Investigators (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Pelz DM, Lownie SP, Fox AJ (1998) Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 19:1541–1547
Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN (2000) Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: part II—clinical aspects and recommendations. Neurosurgery 46:1360–1376
Pierot L, Spelle L, Vitry F, ATENA Investigators (2008) Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 39:2497–2504
Soeda A, Sakai N, Sakai H, Iihara K, Yamada N, Imakita S, Nagata I (2003) Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 24:127–132
Rordorf G, Bellon RJ, Budzik RE Jr, Farkas J, Reinking GF, Pergolizzi RS, Ezzeddine M, Norbash AM, Gonzalez RG, Putman CM (2001) Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 22:5–10
Cronqvist M, Wirestam R, Ramgren B, Brandt L, Nilsson O, Säveland H, Holtås S, Larsson EM (2005) Diffusion and perfusion MRI in patients with ruptured and unruptured intracranial aneurysms treated by endovascular coiling: complications, procedural results, MR findings and clinical outcome. Neuroradiology 47:855–873
Derdeyn CP, Cross DT 3rd, Moran CJ, Brown GW, Pilgram TK, Diringer MN, Grubb RL Jr, Rich KM, Chicoine MR, Dacey RG Jr (2002) Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg 96:837–843
Shapiro M, Babb J, Becske T, Nelson PK (2008) Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. AJNR Am J Neuroradiol 29:1777–1781
Brooks NP, Turk AS, Niemann DB, Aagaard-Kienitz B, Pulfer K, Cook T (2008) Frequency of thromboembolic events associated with endovascular aneurysm treatment: retrospective case series. J Neurosurg 108:1095–1100
Ross IB, Dhillon GS (2005) Complications of endovascular treatment of cerebral aneurysms. Surg Neurol 64:12–19
Lubicz B, Lefranc F, Bruneau M, Balériaux D, De Witte O (2008) Balloon-assisted coiling of intracranial aneurysms is not associated with a higher complication rate. Neuroradiology 50:769–776
Spiotta AM, Bhalla T, Hussain MS, Sivapatham T, Batra A, Hui F, Rasmussen PA, Moskowitz SI (2011) An analysis of inflation times during balloon-assisted aneurysm coil embolization and ischemic complications. Stroke 42:1051–1055
Ishibashi T, Murayama Y, Saguchi T, Ebara M, Irie K, Takao H, Abe T (2006) Thromboembolic events during endovascular coil embolization of cerebral aneurysms. Interv Neuroradiol 12(Suppl 1):112–116
Altay T, Kang HI, Woo HH, Masaryk TJ, Rasmussen PA, Fiorella DJ, Moskowitz SI (2011) Thromboembolic events associated with endovascular treatment of cerebral aneurysms. J Neurointerv Surg 3:147–150
Earnest F 4th, Forbes G, Sandok BA, Piepgras DG, Faust RJ, Ilstrup DM, Arndt LJ (1984) Complications of cerebral angiography: prospective assessment of risk. AJR Am J Roentgenol 142:247–253
Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D (2003) Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 34:1398–1403
Schneider PA, Silva MB Jr, Bohannon WT, Kasirajan K, Caps MT, Nelken N, Santana D (2005) Safety and efficacy of carotid arteriography in vascular surgery practice. J Vasc Surg 41:238–245
Kerber CW, Cromwell LD, Drayer BP, Bank WO (1978) Cerebral ischemia. I. Current angiographic techniques, complications, and safety. AJR Am J Roentgenol 130:1097–1103
Mani RL, Eisenberg RL, McDonald EJ Jr, Pollock JA, Mani JR (1978) Complications of catheter cerebral arteriography: analysis of 5,000 procedures. I. Criteria and incidence. AJR Am J Roentgenol 131:861–865
Eisenberg RL, Bank WO, Hedgcock MW (1980) Neurologic complications of angiography for cerebrovascular disease. Neurology 30:895–897
Wolfel DA, Lovett BP, Ortenburger AI, Johnson LS, Sommerville DL (1984) Outpatient arteriography: its safety and cost effectiveness. Radiology 153:363–364
Saint-Georges G, Aube M (1985) Safety of outpatient angiography: a prospective study. AJR Am J Roentgenol 144:235–236
Gasparyan AY, Watson T, Lip GY (2008) The role of aspirin in cardiovascular prevention: implications of aspirin resistance. J Am Coll Cardiol 51:1829–1843
Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR (2008) Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ 336:195–198
Patrono C, García Rodríguez LA, Landolfi R, Baigent C (2005) Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 353:2373–2383
Tran HA, Anand SS, Hankey GJ, Eikelboom JW (2007) Aspirin resistance. Thromb Res 120:337–346
Feher G, Feher A, Pusch G, Koltai K, Tibold A, Gasztonyi B, Papp E, Szapary L, Kesmarky G, Toth K (2010) Clinical importance of aspirin and clopidogrel resistance. World J Cardiol 2:171–186
Lee DH, Arat A, Morsi H, Shaltoni H, Harris JR, Mawad ME (2008) Dual antiplatelet therapy monitoring for neurointerventional procedures using a point-of-care platelet function test: a single-center experience. AJNR Am J Neuroradiol 29:1389–1394
Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV (2007) Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J 154:221–231
Delgado Almandoz JE, Crandall BM, Scholz JM, Fease JL, Anderson RE, Kadkhodayan Y, Tubman DE (2013) Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the Pipeline Embolization Device. J Neurointerv Surg 5(Suppl 3):iii3–iii10
Kang HS, Kwon BJ, Kim JE, Han MH (2010) Preinterventional clopidogrel response variability for coil embolization of intracranial aneurysms: clinical implications. AJNR Am J Neuroradiol 31:1206–1210
Murthy SB, Shah S, Venkatasubba Rao CP, Bershad EM, Suarez JI (2014) Treatment of unruptured intracranial aneurysms with the pipeline embolization device. J Clin Neurosci 21:6–11
Tan LA, Keigher KM, Munich SA, Moftakhar R, Lopes DK (2014) Thromboembolic complications with Pipeline Embolization Device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg 19. doi:10.1136/neurintsurg-2014-011111
Heller RS, Dandamudi V, Lanfranchi M, Malek AM (2013) Effect of antiplatelet therapy on thromboembolism after flow diversion with the pipeline embolization device. J Neurosurg 119:1603–1610
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Ethical Review Board at Dokkyo Medical University Koshigaya Hospital, Koshigaya, Japan and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takigawa, T., Suzuki, K., Sugiura, Y. et al. Thromboembolic events associated with single balloon-, double balloon-, and stent-assisted coil embolization of asymptomatic unruptured cerebral aneurysms: evaluation with diffusion-weighted MR imaging. Neuroradiology 56, 1079–1086 (2014). https://doi.org/10.1007/s00234-014-1421-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1421-4