Abstract
Purpose
Long-term data on aneurysm treatment with flow-diverting stents are still sparse, and follow-up protocols differ widely between institutions. We present long-term results, with a focus on the usefulness of contrast-enhanced MR angiography (ceMRA).
Materials and Methods
Interventions and follow-up imaging of patients with aneurysms treated by flow-diverting stents (“Pipeline,” “Silk” and “FRED” models) without additional coiling were analyzed. All MRI scans included dedicated two-phase ceMRA. Aneurysm occlusion rates, size of the aneurysmal sac and complications were evaluated on MRI and digital subtraction angiography (DSA), where available. The ability of ceMRA to depict aneurysm occlusion and stent patency was graded on a three-point scale.
Results
Twenty-five patients with 102 MRI scans were included. The median duration of follow-up was 830 days. Aneurysm occlusion rates were 52% at 3 months (10 of 19 patients), 72% at 6 months (18/25) and 84% overall (21/25). Shrinkage of the aneurysmal sac was found in 19 patients (76%) and in 12 cases to <50% of the original size (48%). CeMRA assessability of aneurysmal occlusion was graded as good in all cases. When compared to DSA (18 cases), ceMRA had a sensitivity of 100% and specificity of 91% regarding aneurysm remnant detection. Assessability of the stent lumen varied and was limited in most cases.
Conclusions
Flow-diverter treatment achieves high occlusion rates and can cause major aneurysm shrinkage. CeMRA is highly valuable regarding imaging of the aneurysmal sac. There are limitations regarding the assessability of the stent lumen on ceMRA.
Level of Evidence
Level 4, Case Series.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Having been introduced in 2006–2007 [1], flow diverters (FD) are still relatively new devices for the treatment of intracranial aneurysms. Their mode of action differs from the established technique of coiling aneurysmal sacs. FDs are stent-like devices designed to reconstruct the parent artery and change hemodynamics in order to trigger aneurysm thrombosis. This allows therapy of fusiform and wide-neck aneurysms, including cases which would previously have been deemed untreatable [2,3,4,5]. However, long-term data are still sparse [6, 7]. Early studies show that the development of aneurysm thrombosis over time differs widely and cannot be securely predicted [8, 9]. Moreover, delayed complications have been reported, such as in-stent stenosis [10, 11], perianeurysmal edema [12] and delayed hemorrhage [13,14,15,16]. For these reasons, high-quality follow-up imaging is crucial. So far, there are no generally established follow-up intervals and imaging protocols. Digital subtraction angiography is considered the gold standard for follow-up imaging of coiled aneurysms [17] and is the most reliable modality to image the parent vessel and prove aneurysm occlusion. However, DSA is associated with the drawbacks of invasiveness and radiation exposure. Moreover, it is important to depict the brain parenchyma, the surroundings of the aneurysm and a thrombosed aneurysmal sac, particularly in patients treated by flow diversion. For these purposes, cross-sectional imaging is required. At our institution, we have implemented a 3-Tesla MRI follow-up protocol for patients treated with FDs which includes dedicated two-phase contrast-enhanced magnetic resonance angiography (ceMRA). The aim of this study was to evaluate the long-term development of aneurysms after FD therapy and to assess the value of ceMRA as a follow-up imaging tool.
Materials and Methods
We identified all patients treated with FDs for intracranial aneurysms at our institution in the years 2011–2013. Only patients with follow-up imaging including 3T ceMRA after at least 6 months were included. Patients with aneurysms treated with coils additionally to FD placement were excluded in order to create a homogeneous study group. Furthermore, we excluded fusiform vertebrobasilar artery giant aneurysms, as these represent a distinct entity and the data of our patients are already reported elsewhere [18].
The anti-platelet medication regime was identical for all stent types and included dual inhibition with clopidogrel and acetylsalicylic acid (ASA) for 6 months and continued ASA monotherapy lifelong.
At our institution, the standard follow-up protocol after FD treatment includes an MRI scan after 3 months, DSA and MRI after 6 months, MRI after 18 months and further follow-up depending on the findings. However, there were several deviations due to individual patient findings and compliance.
MRIs were performed on a 3T scanner (Signa HDx, GE Healthcare, Milwaukee, WI, USA) using an eight-channel head coil. CeMRA is a 3D spoiled gradient echo sequence performed after injection of contrast agent (0.1 mmol gadolinium/kg body weight). CeMRA was first executed as a first-pass arterial angiography using bolus tracking. Subsequently, a second ceMRA sequence with a longer scanning time and higher in-plane resolution was performed. In addition to depicting both arterial and venous vasculature, the purpose of the delayed ceMRA is to better delineate small aneurysms and thrombosed aneurysmal sacs due to its higher resolution. The technical parameters of the ceMRA sequences are shown in Table 1. In addition to ceMRA, the MRI protocol included diffusion-weighted imaging (DWI), axial fluid-attenuated inversion recovery (FLAIR) with 5 mm slice thickness and axial T2 with 2 or 3 mm slice thickness. The overall scanning time was 15 min.
The location and shape (saccular, fusiform or “blister”-type) of the aneurysms were assessed. The FD models applied were recorded. Either the Pipeline embolization device (ev3, Irvine, CA, USA), the Silk stent (Balt, Montmorency, France) or the flow-redirection endoluminal device (FRED; MicroVention, Tustin, California) was used. All pre-treatment and follow-up MRI scans were assessed by two readers separately. The perfused parts of the aneurysms were measured in three orthogonal planes and categorized on follow-up scans according to the Kamran scale [19] as modified by Darsaut et al. [20], 1 = unchanged, 2 = reduced perfusion, over 50% of the original lumen, 3 = reduced perfusion, <50% of the original lumen, 4 = almost complete occlusion (remnant of <2 mm diameter) or 5 = complete occlusion. The overall size of the aneurysmal sac, including both perfused and thrombosed parts, was also measured in three orthogonal planes and categorized as 0 = increase in size, 1 = unchanged, 2 = shrinkage to over 50% of the original size or 3 = shrinkage to <50% of the original size. Additionally, the assessability of the ceMRA sequences regarding aneurysm perfusion and stent patency was graded as 1 = no assessability, 2 = limited assessability or 3 = good assessability. All follow-up DSA examinations were evaluated for aneurysm perfusion by the same two readers, and the same categorization as for MRI scans was applied. DSAs were compared to the MRI which was performed on the date closest to the DSA. All complications, both peri-interventional and on follow-up, were recorded.
The gradings of the two readers were compared, and interreader reliability was calculated using Cohen’s Kappa. The few discrepancies (see “Results” section) were solved in consensus. Diagnostic accuracy values with 95% confidence intervals (CI) were calculated regarding the ability of ceMRA to detect aneurysm remnants (grades 1–3) in cases where DSA was available as a reference standard.
Results
Baseline Data and Interventions
Twenty-five patients with 25 aneurysms were included in the study. Nineteen aneurysms originated from the internal carotid artery (ICA), four were located in the intradural vertebral artery (VA), and two at the vertebrobasilar junction. Fifteen aneurysms were classified as saccular, nine as fusiform, and one as “blister” type. Nineteen of the aneurysms were incidental findings, while five were symptomatic with cranial nerve palsies. The blister-type aneurysm had caused a minor subarachnoid hemorrhage. The maximum diameter of the aneurysms ranged from 2 to 47 mm (Median 12 mm).
The stents applied were the Pipeline embolization device in 17 patients, the Silk stent in four patients, and the FRED stent in four patients. Four patients were treated with two, one patient with three and one patient with four Pipeline devices. Two Silk stents were applied in one patient. Eighteen patients were treated with only one FD stent. Baseline data for each patient are presented in Table 2.
Asymptomatic procedural complications included one incomplete stent deployment with stenosis which required post-dilatation, one stent dislocation into the aneurysmal sac and one stent dislocation with subsequent retrieval of the stent. One patient suffered from peri-interventional clinically apparent ischemia (moderate hemiparesis), which was attributed to another stent dislocation and retrieval maneuver.
Follow-up Results
Overall, 102 MRI scans were evaluated. The minimum number of MRIs in one patient was one, and the maximum number was six. Follow-up covered a time span of over 24 months in 17 patients, 12–24 months in five patients and 6–12 months in three patients. The length of follow-up ranged from 182 to 2038 days (Median 830 days). An early MRI scan 3 months after treatment was available in 19 patients. Twenty-two follow-up DSA examinations were performed in 16 patients.
Complete or nearly complete occlusion of the aneurysm occurred in 21 of the 25 cases (84%). Occlusion rates were 52% after 3 months (10 of 19 aneurysms) and 72% after 6 months (18 of 25 aneurysms). Thus, late occlusion after more than 6 months was found in three patients (12%). In two patients with large aneurysms in whom no occlusion or relevant aneurysm thrombosis had occurred after six and 12 months, respectively, an ICA occlusion was performed for definite aneurysm treatment. In two patients, the aneurysms were only partially occluded at the time of the study. Only two MRI scans were graded discrepantly by the two readers regarding aneurysm occlusion; thus, interreader agreement was very good (Cohen’s Kappa 0.960, 95% confidence interval 0.905–1.000).
Some reduction of the size of the aneurysmal sac was found in 19 aneurysms (76%). Major shrinkage to <50% of the original size occurred in 12 cases (48%). Size reduction was found only in occluded aneurysms. Figure 1 shows imaging examples of different developments of aneurysm size.
A, B CeMRA images of one patient show perfused left ICA aneurysm 3 months after flow diverter (Pipeline) treatment (A) and complete occlusion without shrinkage of the aneurysmal sac at 6 months (B). C, D CeMRA images of one patient show the initial finding of a partially thrombosed left ICA aneurysm (C) and the completely occluded and shrunk aneurysm 6 months after flow diverter (Pipeline) treatment (D). Arrows indicate flow diverter stent; Arrowheads indicate the aneurysmal sac
Regarding delayed complications, we found one small perianeurysmal hemorrhage on follow-up MRI and one in-stent stenosis on MRI and DSA (see Fig. 2). The hemorrhage was detected on a routine MRI scan in the frontobasal parenchyma adjacent to a large ICA aneurysm (Case 7, see Table 2). Its maximum diameter was 8 mm. DSA was performed and demonstrated complete occlusion of the aneurysm. Further MRI follow-up showed no further hemorrhage and resolution of the hematoma. Both hemorrhage and in-stent stenosis were asymptomatic and did not require further treatment.
Three of the five patients with ICA aneurysms and cranial nerve compression syndromes improved clinically: Two had reduced double vision due to oculomotor nerve palsy, and one recovered fully from visual field defects due to optic nerve compression. In all three of them, a major size reduction (>50%) of the aneurysm had occurred. In two patients with ptosis and visual field defects, respectively, no aneurysm occlusion or mass effect resolution was achieved by FD placement and symptoms persisted. These patients were subsequently treated by ICA occlusion. The patient with minor subarachnoid hemorrhage did not suffer from re-bleeding.
Follow-up data of all patients are shown in Table 2.
MRI Assessability
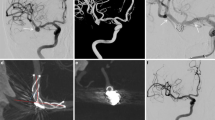
The assessability of aneurysm occlusion on MRI was graded as “good” in all cases by both readers. A direct comparison of a follow-up MRI with DSA was possible in 18 cases. Aneurysm occlusion grades on DSA were “5” in 12 cases and “4,” “3” and “1” in 2 cases each. There were no discrepant findings regarding aneurysm occlusion between DSA and MRI except for one case. In that case, DSA was graded “5” and MRI was graded “3”; thus, MRI was false positive for aneurysm remnant (Fig. 3). Accordingly, sensitivity of ceMRA for the detection of an aneurysm remnant was 100% (CI 59.0–100%), specificity was 90.9% (CI 58.7–99.8%), positive predictive value was 87.5% (CI 47.4–99.7%), and negative predictive value was 100% (CI 69.2–100%). Figure 4 shows concordant findings of DSA and ceMRA.
A, B Arterial ceMRA (A) and DSA of a patient with right ICA aneurysm before flow diverter treatment. On arterial ceMRA, the aneurysm has the same signal intensity as other depicted arteries. C, D Arterial ceMRA (C) and DSA (D) of the same patient 6 months after flow diverter (Pipeline) treatment. There is hyperintensity of the aneurysmal sac, but the signal intensity is lower compared to other arteries. DSA proves complete aneurysm occlusion. DSA images (B, D) show an untreated right MCA aneurysm as an additional finding. Arrows indicate the aneurysmal sac
The gradings of stent lumen assessability were consistent in the different MRI scans of a single patient in 25 of the 27 cases. Assessability of the parent artery on ceMRA was graded as “not possible” in five cases, limited in 16 patients and good in four cases (Fig. 5). With only three differing gradings, over all MRI scans, interreader agreement regarding assessability of the stent lumen was very good (Cohen’s Kappa 0.933, 95% confidence interval 0.858–1.000).
Discussion
In recent years, a few studies reporting long-term data after FD treatment have been published [21, 22]. These studies focus on complications and angiographic outcome. Follow-up studies which specifically report the data of cross-sectional imaging are even more sparse. With a median follow-up period of 830 days, our study is one of only few detailed reports [8, 23] of long-term data covering more than 2 years after FD therapy. We found that many aneurysms occlude within 3 months (52% in our study) and most within 6 months (72%), but late complete thrombosis can occur in a few cases. There were three cases of aneurysm occlusion between 12 and 24 months in our study. This time course is similar to the one found in the previous long-term studies, who also reported high occlusion rates at 6 months and late thrombosis at up to 29 months in a few cases (1 of 11 and 2 of 35 aneurysms, respectively [23] [8],). These findings indicate that endpoints of six or even 12 months, which have so far been used in most larger studies on FDs, are not sufficient to exactly determine occlusion rates and outcome.
Apart from eliminating the risk of rupture, resolution of mass effect can be another major goal of aneurysm treatment. We specifically included only aneurysms which were treated by flow diversion alone—without additional coiling—in order to be able to determine the development of the size of the aneurysmal sac. Some aneurysm shrinkage was found in 72% of the cases, and major size reduction up to complete resolution of the aneurysm occurred in 48%. Although these rates are not as high as reported by Szikora et al. [24], our findings underline the potential of FDs to decrease the space occupation of aneurysms. When packing an aneurysm with coils, the mass effect can be reduced only to a limited degree. Thus, flow diversion alone should be considered particularly in patients with aneurysms which cause cranial nerve compression syndromes.
Due to the delayed effect of flow diversion, follow-up of patients with aneurysms after FD treatment is even more important than for endovascular coiling or surgical clipping. Many imaging examinations are required to monitor the development of aneurysmal thrombosis and discover potential delayed complications such as in-stent stenosis and perianeurysmal edema. Detecting such complications is important as they might warrant re-interventions or a change in medication regarding anti-platelet drugs or the use of steroids, although there is no reliable data concerning the latter [12]. The use of DSA should be limited because of irradiation exposure and the risk of complications such as cerebral ischemic events and groin hematoma. Although limitations by streak and blooming artefacts caused by stents have been partially overcome by modern dedicated CT angiography [25, 26], CT also carries the disadvantage of ionizing radiation. Moreover, MRI is clearly superior to CT in depicting ischemic, hemorrhagic and inflammatory complications. Thus, MRI should be the modality of choice for frequent follow-up imaging of treated aneurysms. While a good assessability of MR angiography for coiled aneurysms has been shown [27,28,29,30], however, data regarding intracranial stenting and particularly FD stenting are very sparse. Stent braids and radiopaque markers cause susceptibility artefacts and radiofrequency shielding [25], thus potentially limiting assessment of the stent lumen and the adjacent aneurysm. Time-of-flight (TOF) MRA is associated with the additional drawbacks of methemoglobin T1 shine-through effects [27] and signal loss due to spin saturation particularly in large aneurysms with turbulent flow [31]. We therefore prefer ceMRA for imaging of treated intracranial aneurysms which is supported by the findings of Attali et al. [32]., who found ceMRA to be superior to TOF MRA when evaluating aneurysms treated by FDs. Additionally to first-pass ceMRA in arterial phase, our protocol includes a delayed steady-state ceMRA with a longer scanning time. The second ceMRA allows for a higher in-plane resolution and thus further facilitates image interpretation. The combined interpretation of the two ceMRA sequences was found to be very reliable in the assessment of aneurysm occlusion in our study. We did not find stent artefacts to limit evaluation of the adjacent aneurysmal sac, and there was only one discrepant grading between DSA and ceMRA. However, some experience is needed when interpreting the ceMRA sequences. In several cases, delayed ceMRA showed partial enhancement of the aneurysmal sac, while first-pass ceMRA did not reveal inflow. We believe such findings to be caused by thrombus enhancement rather than aneurysm perfusion. Both readers were aware of this phenomenon from clinical routine experience and diagnosed an aneurysm remnant only if inflow was visible on both ceMRA sequences. Hyperintensity of the aneurysmal sac on both sequences led to misinterpretation as aneurysm remnant in one case by both readers. The signal intensity of the aneurysmal sac was lower than that of the parent artery, and DSA did not show any inflow even in venous phase (Fig. 3). Therefore, this finding is retrospectively thought to be due to methemoglobin T1 shine-through, which has not yet been described for ceMRA. Consequently, one should consider including a native T1 sequence when using ceMRA. Moreover, aneurysmal inflow should only be diagnosed if the signal intensity of the aneurysm matches the signal intensity of the parent vessel on arterial ceMRA.
The visibility of the stent lumen varied significantly (Fig. 5). There were cases with complete obscuration of the vessel by stent artefacts and others with very good assessability, even making the correct diagnosis and grading of an in-stent stenosis possible on ceMRA (Fig. 2). In most cases, however, the assessability was limited, with a reduced signal intensity of the stented segment. This variability could be explained by differences regarding the type of flow diverter, the number of flow diverters used and the vessel location and surroundings. Stent visibility on MRI might become a criterion for the selection of the FD model in the future. For now, in most cases DSA will be necessary to reliably depict the stent lumen. Therefore, we believe that at the first control visit (e.g., at 3 or 6 months), both DSA and MRI should be performed, but for further follow-up imaging MRI with ceMRA only will be sufficient in most cases.
Our study has several limitations. Due to the limited number of patients, no definite conclusions can be drawn about the frequency of delayed complications after FD treatment. The study group was inhomogeneous regarding the size and location of the aneurysms. MRI assessability was graded by subjective impressions, and DSA was available as a reference standard in a limited number of cases.
Conclusions
Our study confirms that high occlusion rates as well as resolution of mass effect of intracranial aneurysms can be achieved by flow diversion without additional coiling. At a low percentage, late aneurysm occlusion after more than 6 months occurs. MRI with dedicated ceMRA proved highly valuable in the depiction of aneurysms treated by FDs. The assessability of the stent lumen on ceMRA is limited in most cases.
References
Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke J Cereb Circu. 2007;38(8):2346–52. doi:10.1161/STROKEAHA.106.479576.
Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, Moran CJ, Woo HH, Lopes DK, Berez AL, Cher DJ, Siddiqui AH, Levy EI, Albuquerque FC, Fiorella DJ, Berentei Z, Marosfoi M, Cekirge SH, Nelson PK. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013;267(3):858–68. doi:10.1148/radiol.13120099.
Keskin F, Erdi F, Kaya B, Poyraz N, Keskin S, Kalkan E, Ozbek O, Koc O. Endovascular treatment of complex intracranial aneurysms by pipeline flow-diverter embolization device: a single-center experience. Neurol Res. 2015;37(4):359–65. doi:10.1179/1743132814y.0000000450.
Lubicz B, Van der Elst O, Collignon L, Mine B, Alghamdi F. Silk flow-diverter stent for the treatment of intracranial aneurysms: a series of 58 patients with emphasis on long-term results. AJNR Am J Neuroradiol. 2015;36(3):542–6. doi:10.3174/ajnr.A4143.
Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, Berez AL, Tran Q, Nelson PK, Fiorella D. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632–42. doi:10.1227/01.neu.0000339109.98070.65 (discussion 642–633; quiz N636).
Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013;73(2):193–9. doi:10.1227/01.neu.0000430297.17961.f1 (discussion 199–200).
Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, Maiuri F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J. 2015;28(4):365–75. doi:10.1177/1971400915602803.
Briganti F, Napoli M, Leone G, Marseglia M, Mariniello G, Caranci F, Tortora F, Maiuri F. Treatment of intracranial aneurysms by flow diverter devices: long-term results from a single center. Eur J Radiol. 2014;83(9):1683–90. doi:10.1016/j.ejrad.2014.05.029.
Piano M, Valvassori L, Quilici L, Pero G, Boccardi E. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: a single-center experience. J Neurosurg. 2013;118(2):408–16. doi:10.3171/2012.10.jns112222.
Cohen JE, Gomori JM, Moscovici S, Leker RR, Itshayek E. Delayed complications after flow-diverter stenting: reactive in-stent stenosis and creeping stents. J Clin Neurosci. 2014;21(7):1116–22. doi:10.1016/j.jocn.2013.11.010.
John S, Bain M, Hui F, Hussain MS, Masaryk T, Rasmussen P, Toth G. Long-term follow-up of in-stent stenosis after pipeline flow diversion treatment of intracranial aneurysms. Neurosurgery. 2015;. doi:10.1227/neu.0000000000001146.
Berge J, Tourdias T, Moreau JF, Barreau X, Dousset V. Perianeurysmal brain inflammation after flow-diversion treatment. AJNR Am J Neuroradiol. 2011;32(10):1930–4. doi:10.3174/ajnr.A2710.
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke J Cereb Circ. 2013;44(2):442–7. doi:10.1161/strokeaha.112.678151.
Colby GP, Lin LM, Coon AL. Revisiting the risk of intraparenchymal hemorrhage following aneurysm treatment by flow diversion. AJNR Am J Neuroradiol. 2012;33(7):E107. doi:10.3174/ajnr.A3201 (author reply E108).
Cruz JP, Chow M, O’Kelly C, Marotta B, Spears J, Montanera W, Fiorella D, Marotta T. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol. 2012;33(4):603–8. doi:10.3174/ajnr.A3065.
Kulcsar Z, Houdart E, Bonafe A, Parker G, Millar J, Goddard AJ, Renowden S, Gal G, Turowski B, Mitchell K, Gray F, Rodriguez M, van den Berg R, Gruber A, Desal H, Wanke I, Rufenacht DA. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol. 2011;32(1):20–5. doi:10.3174/ajnr.A2370.
Johnston SC, Higashida RT, Barrow DL, Caplan LR, Dion JE, Hademenos G, Hopkins LN, Molyneux A, Rosenwasser RH, Vinuela F, Wilson CB. Recommendations for the endovascular treatment of intracranial aneurysms: a statement for healthcare professionals from the Committee on Cerebrovascular Imaging of the American Heart Association Council on Cardiovascular Radiology. Stroke J Cereb Circ. 2002;33(10):2536–44.
Ertl L, Holtmannspotter M, Patzig M, Bruckmann H, Fesl G. Use of flow-diverting devices in fusiform vertebrobasilar giant aneurysms: a report on periprocedural course and long-term follow-up. AJNR Am J Neuroradiol. 2014;35(7):1346–52. doi:10.3174/ajnr.A3859.
Kamran M, Yarnold J, Grunwald IQ, Byrne JV. Assessment of angiographic outcomes after flow diversion treatment of intracranial aneurysms: a new grading schema. Neuroradiology. 2011;53(7):501–8. doi:10.1007/s00234-010-0767-5.
Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J. Flow diverters can occlude aneurysms and preserve arterial branches: a new experimental model. AJNR Am J Neuroradiol. 2012;33(10):2004–9. doi:10.3174/ajnr.A3075.
Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, Fiorella D, Jabbour P, Levy E, McDougall C, Siddiqui A, Szikora I, Woo H, Albuquerque F, Bozorgchami H, Dashti SR, Delgado Almadoz JE, Kelly ME, Turner R 4th, Woodward BK, Brinjikji W, Lanzino G, Lylyk P. International retrospective study of pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015;36(1):108–15. doi:10.3174/ajnr.A4111.
Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I, McDougall CG, Szikora I, Lanzino G, Moran CJ, Woo HH, Lopes DK, Berez AL, Cher DJ, Siddiqui AH, Levy EI, Albuquerque FC, Fiorella DJ, Berentei Z, Marosföi M, Cekirge SH, Nelson PK. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. 2016;14:1–8. doi:10.3171/2015.6.JNS15311.
Deutschmann HA, Wehrschuetz M, Augustin M, Niederkorn K, Klein GE. Long-term follow-up after treatment of intracranial aneurysms with the Pipeline embolization device: results from a single center. AJNR Am J Neuroradiol. 2012;33(3):481–6. doi:10.3174/ajnr.A2790.
Szikora I, Marosfoi M, Salomvary B, Berentei Z, Gubucz I. Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2013;34(5):935–9. doi:10.3174/ajnr.A3547.
Kovacs A, Mohlenbruch M, Hadizadeh DR, Seifert M, Greschus S, Clusmann H, Willinek WA, Flacke S, Urbach H. Noninvasive imaging after stent-assisted coiling of intracranial aneurysms: comparison of 3-T magnetic resonance imaging and 64-row multidetector computed tomography–a pilot study. J Comput Assist Tomogr. 2011;35(5):573–82. doi:10.1097/RCT.0b013e318224e528.
Schlotzer W, Huber R, Schmitz BL. Stent-assisted intracranial angioplasty: potentials and limitations of pre- and postinterventional CT angiography. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2009;181(2):121–8. doi:10.1055/s-2008-1027889.
Anzalone N, Scomazzoni F, Cirillo M, Righi C, Simionato F, Cadioli M, Iadanza A, Kirchin MA, Scotti G. Follow-up of coiled cerebral aneurysms at 3T: comparison of 3D time-of-flight MR angiography and contrast-enhanced MR angiography. AJNR Am J Neuroradiol. 2008;29(8):1530–6. doi:10.3174/ajnr.A1166.
Deutschmann HA, Augustin M, Simbrunner J, Unger B, Schoellnast H, Fritz GA, Klein GE. Diagnostic accuracy of 3D time-of-flight MR angiography compared with digital subtraction angiography for follow-up of coiled intracranial aneurysms: influence of aneurysm size. AJNR Am J Neuroradiol. 2007;28(4):628–34.
Ferre JC, Carsin-Nicol B, Morandi X, Carsin M, de Kersaint-Gilly A, Gauvrit JY, Desal HA. Time-of-flight MR angiography at 3T versus digital subtraction angiography in the imaging follow-up of 51 intracranial aneurysms treated with coils. Eur J Radiol. 2009;72(3):365–9. doi:10.1016/j.ejrad.2008.08.005.
Gauvrit JY, Leclerc X, Caron S, Taschner CA, Lejeune JP, Pruvo JP. Intracranial aneurysms treated with Guglielmi detachable coils: imaging follow-up with contrast-enhanced MR angiography. Stroke J Cereb Circ. 2006;37(4):1033–7. doi:10.1161/01.STR.0000209236.06451.3b.
Thomas B, Sunaert S, Thamburaj K, Wilms G. Spurious absence of signal on 3D time-of-flight MR angiograms on 1 and 3 tesla magnets in cerebral arteries associated with a giant ophthalmic segment aneurysm the need for alternative techniques. JBR-BTR organe de la Societe royale belge de radiologie (SRBR) = orgaan van de Koninklijke Belgische Vereniging voor Radiologie (KBVR). 2005;88(5):241–4.
Attali J, Benaissa A, Soize S, Kadziolka K, Portefaix C, Pierot L. Follow-up of intracranial aneurysms treated by flow diverter: comparison of three-dimensional time-of-flight MR angiography (3D-TOF-MRA) and contrast-enhanced MR angiography (CE-MRA) sequences with digital subtraction angiography as the gold standard. J Neurointerventional Surg. 2016;8(1):81–6. doi:10.1136/neurintsurg-2014-011449.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest regarding this study.
Ethics and Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This is a retrospective study. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Patzig, M., Forbrig, R., Ertl, L. et al. Intracranial Aneurysms Treated by Flow-Diverting Stents: Long-Term Follow-Up with Contrast-Enhanced Magnetic Resonance Angiography. Cardiovasc Intervent Radiol 40, 1713–1722 (2017). https://doi.org/10.1007/s00270-017-1732-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1732-z