Abstract
Introduction
The novel Low-profile Visualized Intraluminal Support (LVIS™, LVIS and LVIS Jr.) device was recently introduced for stent-supported coil embolization of intracranial aneurysms. Periprocedural and midterm follow-up results for its use in stent-supported coil embolization of unruptured aneurysms are presented herein.
Methods
In this prospective multicenter study, clinical and radiologic outcomes were analyzed for 55 patients with saccular aneurysms undergoing LVIS-assisted coil embolization between October 2012 and February 2013. Magnetic resonance angiography or digital subtraction angiography was performed to evaluate midterm follow-up results.
Results
The standard LVIS device, deployed in 27 patients, was more often used in internal carotid artery (ICA) aneurysms (n = 19), whereas the LVIS Jr. (a lower profile stent, n = 28) was generally reserved for anterior communicating artery (n = 14) and middle cerebral artery (n = 8) aneurysms. With LVIS-assisted coil embolization, successful occlusion was achieved in 45 aneurysms (81.8 %). Although no instances of navigation failure or stent malposition occurred, segmentally incomplete stent expansion was seen in five patients where the higher profile LVIS was applied to ICA including carotid siphon. Procedural morbidity was low (2/55, 3.6 %), limited to symptomatic thromboembolism. In the imaging of lesions (54/55, 98.2 %) at 6-month follow-up, only a single instances of major recanalization (1.9 %) occurred. Follow-up angiography of 30 aneurysms (54.5 %) demonstrated in-stent stenosis in 26 (86.7 %), with no instances of stent migration. Only one patient suffered late delayed infarction (modified Rankin Scale 1).
Conclusion
The LVIS device performed acceptably in stent-assisted coil embolization of non-ruptured aneurysms due to easy navigation and precise placement, although segmentally incomplete stent expansion and delayed in-stent stenosis were issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the International Subarachnoid Aneurysm Trial (ISAT) and the International Study of Unruptured Intracranial Aneurysms (ISUIA), endovascular coil embolization has been widely used for treatment of intracranial aneurysms. Device improvements and advanced coiling techniques have also made it possible to treat many cerebral aneurysms with difficult configurations by this method. In particular, the introduction or evolution of various stent systems has greatly broadened the applicability of endovascular therapy in this setting [1–5]. Stent deployment provides mechanical support to prevent coil prolapse, enable dense packing of coil, and potentially divert blood flow around aneurysms, serving as a scaffold for endothelial growth and vessel healing [6–9]. The Low-profile Visualized Intraluminal Support device (LVIS™, Microvention, Tustin, CA, USA) is a novel, self-expandable stent consisting of a single, round-wire nitinol braid with radiopaque proximal/distal markers and helical strands for whole-dimension visualization. There are two variations of the devices (LVIS and LVIS Jr.) with similar structure but different characteristics, which are summarized in Table 1. Presented here is our experience with use of this device for stent-supported coil embolization of wide-neck aneurysms.
Methods and materials
Population
This study is a prospective, nonrandomized, multicenter clinical trial conducted at two institutions (Seoul National University Hospital and Seoul National University Bundang Hospital) in South Korea. Ethical approval was obtained from the relevant ethics committee at each institution, and all patients signed informed consents. The protocol was sanctioned by the Korean Ministry of Food and Drug Safety to seek their approval for marketing this device. The study’s primary endpoint was the success rate of aneurysmal occlusion at 6 months and secondary endpoints were parent artery patency and recurrence rate at 6 months; other outcomes included device performance (stent navigation and positioning), clinical utility, and adverse events (stent migration and in-stent stenosis, etc). At each facility, instructions were to use the LVIS stent within appropriate guidelines. Thus, stent deployment was limited to coil embolization of wide-neck (neck size >4 mm or dome-to-neck ratio <2) unruptured saccular aneurysms arising from parent arteries 2.0–4.5 mm in diameter. Aneurysms of extraordinarily small (<2 mm) or large (>20 mm) size and multiple, fusiform, or ruptured aneurysms were grounds for exclusion. Inclusion and exclusion criteria are summarized in Table 2. A total of 55 patients with 55 aneurysms were registered between October 2012 and February 2013. Clinical and radiologic outcomes of the stipulated treatment were assessed, with periprocedural and midterm follow-up results presented herein.
Endovascular procedure
All procedures were performed under general anesthesia. Aneurysmal configuration and arterial architecture were evaluated via Integris V (Philips Medical Systems, Best, the Netherlands) biplane system, including high-resolution 3D rotational angiography. Patients were given 75 mg of clopidogrel and 100 mg of aspirin for a minimum of 5 days before the procedure or they received loading doses of clopidogrel and aspirin (300 mg each) 1 day prior to the procedure and were supplemented (clopidogrel, 75 mg; aspirin, 100 mg) on the morning of the procedure. A bolus of heparin (3,000 IU) was also infused as the procedure began, with hourly boosting (1,000 IU) sufficient to sustain activated clotting time at 250–300 s. Dual antiplatelet agents were then routinely continued for at least 3 months afterwards, if no major related bleeding ensued. A 0.021-in. microcatheter (with LVIS) or one of 0.0165- to 0.017-in. caliber (with LVIS Jr.) was used for stent delivery.
One experienced neuroradiologist (CHS) independently reviewed angiographic results immediately following coil embolization. Using the thre-point Raymond scale, therapeutic outcomes were classified as follows: complete occlusion (no residual filling of aneurysm by contrast medium), residual neck (limited residual contrast at base of aneurysm), or residual aneurysm (any contrast filling of aneurysmal sac) [10].
The angles of stented parent arteries adjacent to the bifurcation aneurysms were measured on digital subtraction angiography (DSA), before and immediately after stent-assisted procedures. With the aid of 3D reconstruction images, the best available view containing the aneurysmal neck and parent artery was selected for angle measurement. The vascular angles were measured at the intersections of lines parallel to the afferent and efferent arteries [11].
Clinical and radiological follow-up
In all patients, time-of-flight magnetic resonance angiography (TOF-MRA) or conventional angiography was advised at 6 months after coil embolization. Conventional angiography was also recommended when assessing the status of treated aneurysms with MRA was not feasible, or when aneurysmal recanalization was suspected by MRA, in order to decide if further treatment was necessary.
Clinical outcomes were assessed using the modified Rankin Scale (mRS) at the time of 1- and 6-month follow-up visits by independent neurovascular surgeon (JEK). Anatomic follow-up results were also categorized using Raymond scale: complete occlusion, neck remnant, or residual sac. Repeat embolization was recommended for patients showing residual sac, considered as major recanalization.
In-stent stenosis was categorized as follows: mild stenosis (<33 % narrowing relative to non-stented parent artery), moderate stenosis (33–67 %), and severe stenosis (≥67 %) [1].
Results
Study population
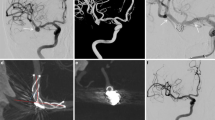
Clinical and demographic patient data are summarized in Table 3. A total of 41 females and 14 males (mean age, 56.7 ± 9.6 years; median age, 55 years) were studied, with maximal mean aneurysmal diameter 5.4 ± 1.9 mm (range, 2.4–13.1 mm; median, 5.1 mm). Three recanalized aneurysms (5.4 %) were included, but all aneurysms were intact and had wide necks (mean diameter at neck, 4.3 ± 1.5 mm (median, 4.1 mm); mean dome-to-neck ratio, 1.3 ± 0.3 (median, 1.2)). Dome-to-neck ratio was <1.5 in 39 aneurysms (71 %). The most common sites involved were internal carotid (ICA; n = 19), anterior communicating (AcomA; n = 15, Fig. 1), and middle cerebral (MCA; n = 8) arteries.
a Conventional angiography delineating wide-necked anterior communicating artery aneurysm; b after microcatheter (0.017 in.) introduced into ipsilateral A2 branch for stent delivery and separate S-shaped microcatheter (fashioned using steam) advanced into aneurysmal sac for coil delivery, LVIS Jr. (2.5 mm × 16 mm) deployed from ipsilateral A2 to A1 (covering aneurysm neck) for coil insertion under stent protection; c successful occlusion of aneurysm visible by post-procedural angiography; d complete occlusion of the coiled aneurysm and in-stent stenosis visible by 6-month follow-up angiography
Procedural results
Of the 55 patients treated, a standard LVIS stent was deployed in 27 (Fig. 2), using the LVIS Jr. in 28 (Table 4). No navigation failure, stent malposition, or stent migration occurred during the coiling procedures. Immediately following coil embolization, angiographic studies indicated complete occlusion in 8 aneurysms, neck remnants in 37, and residual sacs in 10. All stents were deployed after intra-aneurysmal placement of microcatheters and prior to initial frame coil detachment (i.e., jailed microcatheter technique was first attempted). In three patients, the microcatheters exited during stent deployment but were re-inserted through stent struts. In 14 patients (the aforementioned included), access through stent struts was undertaken because a jailed technique either did not allow compacted filling of aneurysm or multiple microcatheters were required. In this manner, access was successful in eight patients (57 %). Success was highest in aneurysms of basilar tip (3/4, 75 %), MCA bifurcation (2/3, 67 %), and ICA (3/5, 60 %). In two AcomA aneurysms, this approach failed. Among 29 bifurcation aneurysms, mean angle change before and after stent placement was 35.1° ± 19.0° (range, 14–100°) (Fig. 3).
a Conventional angiography illustrating wide-neck aneurysm of basilar tip-note right-sided shallowness of aneurysm, necessitating right posterior cerebral artery stent deployment; b LVIS (3.0 mm × 25 mm) deployed from right P1 to basilar trunk and coil insertion via dual microcatheters under protection of stent; c successful occlusion of aneurysm visible by post-procedural angiography
a Conventional angiography illustrating wide-neck aneurysm of middle cerebral artery bifurcation; b incomplete occlusion of aneurysm visible by post-procedural angiography after coil embolization under protection of LVIS Jr (2.5 mm × 24 mm); c complete occlusion of the coiled aneurysm (by progressive thrombosis) and in-stent stenosis visible by 6-month follow-up angiography. A series of angiography showed that the angle between the stented parent artery and the branch was straightened over time
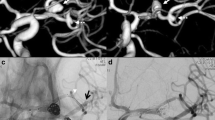
Segmentally incomplete stent expansion was evident in five instances where a standard LVIS was applied to ICA (Fig. 4). In each case, the stent extended from distal ICA to cavernous ICA, through carotid siphon. Proximal segment of stent was involved in four of these and distal segment in one. No apparent complications (e.g., thromboembolism) developed as a consequence, and incomplete expansion was not an issue with the LVIS Jr.
Imaging of incomplete stent expansion. a Post-procedural angiography demonstrating cross of dual radiopaque helical strands on genu portion of cavernous ICA; b, c three-dimensional (b) and illustrating (c) images showing the incomplete apposition of the stent to parent artery; d floatation of the stent visible by source image of TOF MRA; e, f post-procedural angiography illustrating incomplete stent apposition of other patients. Arrows indicate the incomplete apposition of the stents
Procedure-related adverse events included asymptomatic thrombus formation (2/55, 3.6 %) and symptomatic infarction (2/55, 3.6 %). In asymptomatic patients, thrombus resolved with intra-arterial tirofiban infusion, whereas symptomatic patients suffered mild neurologic deficits (mRS 1). No fatalities occurred.
Midterm follow-up results
Follow-up imaging was performed at 6 months in 54 patients (MRA, 24; DSA, 30; follow-up rate, 98.2 %), confirming complete occlusion in 50 aneurysms (92.6 %), neck remnant in 3 (5.5 %), and residual sac in 1 (1.9 %). Among 30 patients (LVIS Jr, 18; LVIS, 12) subjected to DSA, in-stent stenosis was documented in 26 (86.7 %) at mild (18/26, 69.2 %) or moderate (8/26, 30.8 %) levels (see Figs. 1 and 3). No severe in-stent stenosis was observed, and none of the patients affected suffered neurologic symptoms. With the LVIS Jr device, 16 aneurysms (88.9 %) showed in-stent stenosis (mean, 30.7 ± 12.9 %), compared with 10 aneurysms (83.3 %) with the LVIS device (mean, 27.8 ± 13.7 %). The presence and severity of stenosis did not differ statistically by stent type and aneurysm location. There were no instances of stent migration, and only one patient suffered delayed infarction (mRS 1).
Discussion
The development of self-expanding stents dedicated to intracranial use has significantly broadened the applicability of endovascular therapy to include many intracranial aneurysms otherwise unsuitable for this method [12]. After the Neuroform™ stent (Stryker) debuted for treatment of aneurysms, others soon followed, each one dependent on aneurysmal configuration and pattern of parent artery for use. The Neuroform device is a self-expanding open-cell nitinol stent, which has the capacity for segmental expansion and designed to promote stent anchorage and stability. Its competitors, including Enterprise™ (Codman), Leo™ (Balt), and Solitaire™ (eV3), are retrievable stents of closed-cell type, intended to augment navigation, delivery, and stent positioning [1–5, 13, 14]. Because each and all stents differ fundamentally, and none are superior in all functional and physical aspects, the selection of a device is an individualized process based on clinical and technical indications [15]. The LVIS device is a novel, self-expandable braided stent of smaller cell size (~0.9 mm) than any currently available for treatment of intracranial aneurysms (Fig. 5) [16]. It may provide better protection against coil protrusion and yields improved flow diversion (see Fig. 3). Despite the fact that this trial was largely limited to small aneurysms (<10 mm), progressive occlusion was demonstrated during follow-up in a majority of aneurysms that initially showed incomplete occlusion (Fig. 6). However, our success rate in accessing aneurysms through stent struts was fairly low, especially in side-wall aneurysms with tortuous parent arteries (i.e., ICA or AcomA). Its lower porosity may simply equate with a closed-cell stent. Hence, a jailed microcatheter technique should be tried first, given the challenging LVIS construct. The overall risk of procedure-related thromboembolism with use of the LVIS (7.5 %, 4/55) is comparable to rates cited for competitor stents [1–5].
Another advantage of LVIS stents is the capability of delivery through smaller caliber microcatheters. With the standard LVIS stent, a 0.021-in. microcatheter can be used (0.017 in. with LVIS Jr.). This feature facilitates microcatheter navigation (in stent delivery) and accurate stent placement, as shown in our series. The LVIS Jr. is even compatible with the coaxial double lumen balloon catheter system (e.g., Scepter C™, Microvention) [6].
The LVIS stent is well-visualized throughout its course, owing to dual radiopaque helical strands (a triad in LVIS Jr.). Four distal markers (three in LVIS Jr.) are evenly spaced about the device circumference, whereas its four proximal markers are paired. Stability of the stent after deployment is excellent as well. Procedural stent migration never occurred, although incomplete expansion of the stent was evident in five of our patients. In each instance, a higher profile LVIS stent was deployed in ICA, extending through carotid siphon and genu of cavernous ICA. No complications resulted, but the following hazards are possible: (1) delayed thromboembolism due to stent flotation, (2) unprotected coil by floating of stent in aneurysm neck (aneurysmal neck escaped or was overridden by the floating segment in our patients), (3) unfavorable effect in terms of post-procedural endothelization and flow diversion, or (4) access difficulty of re-embolization in instances of major recanalization. Our results suggest that the stent may fold or twist within a tortuous and complex carotid siphon, without full flaring of the braided closed-cell structure. According to Valdivia y Alvarado et al. [17], wire-braided stents have two major mechanical drawbacks: (1) inward crimping of distal and proximal ends of the stents (“fish mouth” or “tulip” phenomenon) during stepwise bending and (2) flattening of the midsection at a curvature, which compromises the stent radius. Although the precise means of incomplete expansion observed in our patients remains elusive, we likewise contend that inherent characteristics of the braided-stent are responsible. It is notable that all such events involved in standard LVIS deployed through long segments of tortuous and large-sized arteries. Thus, caution advised in this context.
It is well-known that stent placement across bifurcation aneurysms leads to significant biphasic angular remodeling [18]. Progressive stent-induced angular remodeling has the potential to alter peri-aneurysmal hemodynamics, independent of the flow-diverting properties of stent struts, thereby shifting the balance of hemodynamic forces in favor of progressive aneurysmal occlusion [19]. In our series, the angular change in bifurcation aneurysms after LVIS stent deployment was remarkable (mean, 35.1° ± 19.0°), exceeding that of the Enterprise stent at our institution (mean, 27.8° ± 18.5°) [11].
At midterm follow-up (by DSA), there were no instances of LVIS migration, which was notable (4.5 % rate with Enterprise stent) [1], and although the rate of delayed in-stent stenosis was high (86.7 %), instances were mild (18/26, 69.2 %) or moderate (8/26, 30.8 %) only. Previously, we reported a 13.3 % rate of in-stent stenosis with the Enterprise device [1], for which Mocco et al. [20] cited a 3 % rate of significant (>50 %) in-stent stenosis. Fiorella et al. [21] likewise confirmed a 5.8 % rate of delayed in-stent stenosis with Neuroform stents, and Kanaan et al. [22] found that the incidence of stenosis with the Enterprise stent exceeded that of the Neuroform stent. Despite the limited size of our study population, we have shown that the risk of in-stent stenosis is much greater with the LVIS than with the Enterprise stent. In-stent stenosis typically is seen within 3–6 months after stent deployment [23]. Despite its unclear pathophysiology, endothelization and intimal ingrowth are putative factors. Chalouhi et al. [24] reported that younger patients are more likely to develop the stenosis and the neointimal response induced by stent placement is more robust in younger patients. The following influences have also been implicated: (1) background atherosclerosis, (2) endothelial injury after stent deployment, and (3) allergy to metal stent components [1, 20, 25, 26]. The spontaneous resolution of in-stent stenosis was reported by Fiorella et al. and they insisted that the watchful waiting might be effective because some patients experienced partial or complete resolution of the stenosis at follow-up [21]. Recently, Gao et al. [27] reported that Enterprise stent deployment causes a significant dynamic and spontaneously resolvable in-stent stenosis of the parent artery that peaks at 4–6 months and resolves by 12–24 months post-treatment. Similar to the Enterprise device, none of the LVIS-treated patients exhibiting in-stent stenosis suffered ischemic symptoms during follow-up. Long-term DSA monitoring is needed to determine whether or not such stenosis will progress.
Conclusion
The LVIS device performed acceptably in stent-assisted coil embolization of non-ruptured aneurysms, navigating with ease and assuring accurate placement by virtue of lower profile microcatheters. On the other hand, segmentally incomplete expansion of the stent may occur with use of the higher profile LVIS in lesions stemming from tortuous parent vessels, and the high rate of delayed in-stent stenosis warrants follow-up monitoring. Finally, the process of entering an aneurysm with a microcatheter deployed through a stent is a fraught with technical difficulty and may prove challenging.
References
Lee SJ, Cho YD, Kang HS et al (2013) Coil embolization using the self-expandable closed-cell stent for intracranial saccular aneurysm: a single-center experience of 289 consecutive aneurysms. Clin Radiol 68:256–263
Piotin M, Blanc R, Spelle L et al (2010) Stent-assistedcoiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 41:110–115
Gory B, Klisch J, Bonafé A et al (2013) Solitaire AB stent-assisted coiling of wide-necked intracranial aneurysms: short-term results from a prospective, consecutive, European multicentric study. Neuroradiology 55:1373–1378
Kulcsár Z, Göricke SL, Gizewski ER et al (2013) Neuroform stent-assisted treatment of intracranial aneurysms: long-term follow-up study of aneurysm recurrence and in-stent stenosis rates. Neuroradiology 55:459–465
Juszkat R, Nowak S, Smól S et al (2007) Leo stent for endovascular treatment of broad-necked and fusiform intracranial aneurysms. Interv Neuroradiol 13:255–269
Spiotta AM, Miranpuri A, Chaudry MI et al (2013) Combined balloon stent technique with the Scepter C balloon and low-profile visualized intraluminal stent for the treatment of intracranial aneurysms. J Neurointerv Surg 5(Suppl 3):iii79–iii82
Wanke I, Forsting M (2008) Stents for intracranial wide-necked aneurysms: more thanmechanical protection. Neuroradiology 50:991–998
Aenis M, Stancampiano AP, Wakhloo AK et al (1997) Modeling of flow in a straight stented and nonstented side wall aneurysm model. J Biomech Eng 119:206–212
Phatouros CC, Sasaki TY, Higashida RT et al (2000) Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery 47:107–113
Roy D, Milot G, Raymond J (2001) Endovascular treatment of unruptured aneurysms. Stroke 32:1998–2004
Cho WS, Kang HS, Kim JE et al (2014) Angle change of the parent arteries after stent-assisted coil embolization of the wide-necked intracranial bifurcation aneurysms. Clin Radiol 69:e63–e70
Mocco J, Snyder KV, Albuquerque FC et al (2009) Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg 110:35–39
Kadkhodayan Y, Rhodes N, Blackburn S et al (2013) Comparison of Enterprise with Neuroform stent-assisted coiling of intracranial aneurysms. AJR Am J Roentgenol 200:872–878
Gentric JC, Biondi A, Piotin M et al (2013) Safety and efficacy of Neuroform for treatment of intracranial aneurysms: a prospective, consecutive, French multicentric study. AJNR Am J Neuroradiol 34:1203–1208
Krischek O, Miloslavski E, Fischer S et al (2011) A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg 54:21–28
Turner RD, Turk A, Chaudry I (2013) Low-profile visible intraluminal support device: immediate outcome of the first three US cases. J Neurointerv Surg 5:157–160
Valdivia y Alvarado M, Ebrahimi N, Benndorf G (2009) Study of conformability of the new leo plus stent to a curved vascular model using flat-panel detector computed tomography (DynaCT). Neurosurgery 64(3 Suppl):130–134
Gao B, Baharoglu MI, Cohen AD et al (2012) Stent-assisted coiling of intracranial bifurcation aneurysms leads to immediate and delayed intracranial vascular angle remodeling. AJNR Am J Neuroradiol 33:649–654
Gao B, Baharoglu MI, Cohen AD et al (2013) Y-Stent coiling of basilar bifurcation aneurysms induces a dynamic angular vascular remodeling with alteration of the apical wall shear stress pattern. Neurosurgery 72:617–629
Mocco J, Fargen KM, Albuquerque FC et al (2011) Delayed thrombosis or stenosis following Enterprise-assisted stent-coiling: is it safe?: midterm results of the Interstate Collaboration of Enterprise Stent Coiling. Neurosurgery 69:908–913
Fiorella D, Albuquerque FC, Woo H et al (2006) Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery 59:34–42
Kanaan H, Jankowitz B, Aleu A et al (2010) In-stent thrombosis and stenosis after neck-remodeling device-assisted coil embolization of intracranial aneurysms. Neurosurgery 67:1523–1532
Yoon KW, Kim YJ (2010) In-stent stenosis of stent assisted endovascular treatment on intracranial complex aneurysms. J Korean Neurosurg Soc 48:485–489
Chalouhi N, Drueding R, Starke RM et al (2013) In-stent stenosis after stent-assisted coiling: incidence, predictors and clinical outcomes of 435 cases. Neurosurgery 72:390–396
Köster R, Vieluf D, Kiehn M et al (2000) Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 356:1895–1897
Saito T, Hokimoto S, Oshima S et al (2009) Metal allergic reaction in chronic refractory in-stent restenosis. Cardiovasc Revasc Med 10:17–22
Gao B, Safain MG, Malek AM (2014) Enterprise stenting for intracranial aneurysm treatment induces dynamic and reversible age-dependent stenosis in cerebral arteries. J Neurointerv Surg. doi:10.1136/neurintsurg-2013-011074
Acknowledgments
This study was supported by Microvention Inc. for clinical trials of LVIS devices.
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Institutional Review Board of Seoul National University Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, Y.D., Sohn, CH., Kang, HS. et al. Coil embolization of intracranial saccular aneurysms using the Low-profile Visualized Intraluminal Support (LVIS™) device. Neuroradiology 56, 543–551 (2014). https://doi.org/10.1007/s00234-014-1363-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1363-x