Abstract
Introduction
Patients with tuberous sclerosis complex (TSC) frequently present with neurocognitive deficits which may be related to impaired white matter maturation. The purposes of our study were (a) to evaluate the white matter maturation in children and young adults with TSC by comparing the apparent diffusion coefficient (ADC) values of normal-appearing white matter (NAWM) with age-matched healthy controls and (b) to determine the association of NAWM-ADC values with the severity of neurological symptoms in TSC patients.
Methods
Twenty-three TSC patients who underwent magnetic resonance imaging/diffusion-weighted imaging between January 2000 and January 2009 were studied. ADC values of NAWM were measured in the frontal, parietal, occipital lobes, and in the pons. ADC data were compared with age-matched normative data derived from healthy controls. Patients were neurologically scored by a pediatric neurologist. Two-sample t tests and linear regression were conducted using STATA software.
Results
ADC values of NAWM were higher in TSC patients compared with healthy controls; the increase, however, only reached statistical significance in the frontal white matter and pons in the age group between 96 and 144 months and in the right parietal and occipital white matter in the age group above 144 months. There was no significant change in neurological severity score per unit increase in ADC measurement.
Conclusion
ADC values of NAWM appear increased in TSC patients. The abnormal ADC values suggest that myelination may be delayed/impaired in TSC patients, which could explain global neurocognitive deficits. Larger prospective studies, including diffusion tensor imaging, are necessary to validate our results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tuberous sclerosis is an autosomal dominant disorder and the second most common phakomatosis after neurofibromatosis type 1 [1]. It affects multiple organ systems including the skin, central nervous system, heart, lungs, retina, and kidneys [1, 2]. Due to multiple organ involvement and a genetic heterogeneity, this disorder is called “tuberous sclerosis complex” (TSC) [3]. Traditionally, diagnosis of TSC is based on a clinical triad of facial angiofibromas, seizures, and mental retardation [3]. Nowadays, TSC diagnosis (definite, probably, or possible) is based on “major” and “minor” signs and symptoms according to internationally accepted diagnostic criteria as summarized in the report of the Diagnostic Criteria Committee of the National Tuberous Sclerosis Association [4].
The brain is the most frequently involved organ in TSC [3]. Neurological manifestations are the most common cause of morbidity and mortality in TSC patients [1]. These manifestations include seizures (90%), developmental delay/mental retardation (50%), behavioral problems, and autism (25–50%) [1]. Focal brain lesions are predominantly found in the supratentorial region; a smaller fraction of patients with TSC exhibit lesions in the infratentorial compartment [5–7]. The observed brain lesions and the clinical features of TSC patients are highly variable.
Magnetic resonance imaging (MRI) has become the primary neuroimaging tool in the diagnosis of TSC and in the evaluation of the associated brain lesions [8]. Even though calcification within tubers is still best identified by computer tomography (CT), MRI surpasses CT in the identification of cortical tubers [8], which are the hallmark brain lesions in TSC.
Diffusion-weighted imaging (DWI) is an advanced application of MRI used for the evaluation of the microstructure of tissues [9]. DWI generates image contrast based on the differences of diffusion of water molecules within the brain [9]. DWI sequences allow the calculation of the apparent diffusion coefficient (ADC) which is a measure of the overall magnitude of water diffusion within the brain. With progressive maturation/myelination of the pediatric brain, the overall motion of water molecules within the gray and white matters decreases. Consequently, ADC values decrease with progressive myelination. Moreover, ADC values may be altered by changes in the tissue integrity that occurs as a result of pathologic processes [10]. These pathological changes might not be always detectable on conventional T1- and T2-weighted MRI. Several studies in the past have analyzed the ADC values of the brain lesions in TSC [9, 11–15]. Only a few studies have focused on the normal-appearing white matter (NAWM) beyond the obvious lesions seen on conventional MRI [9, 10, 16, 17]. Garaci et al. [10] and Peng et al. [16] reported that additional abnormalities can be disclosed in patients with TSC by DWI. Makki et al. [17] showed a significant increase in the mean diffusivity of normal-appearing white matter tracts (internal and external capsule and corpus callosum) in six children with TSC (age ranging between 6 and 15 years). They concluded that the noted axonal microstructural changes may be related to changes in myelin packing and myelination defects. They, however, did not study the ADC values of NAWM. In addition, none of the previous studies have analyzed the relationship between the ADC values and the neurological symptoms of children with TSC.
The objectives of our study were (a) to study the ADC values of the NAWM of children with TSC in comparison with healthy controls and (b) to determine the association of ADC values of the NAWM with the severity of neurological symptoms.
Materials and methods
Design
Children and young adults under 25 years of age with an established diagnosis of TSC who underwent MRI/DWI at the Johns Hopkins Hospital between January 2000 and January 2009 were retrospectively ascertained using a computer-assisted search of all radiological reports. A search of the computerized hospital discharge records using the ICD-9-CM code for tuberous sclerosis (International Classification of Diseases, Ninth Revision, Clinical Modification, code 759.5) was also performed. Demographic characteristics and information regarding neurological symptoms related to TSC were gathered from electronic patient records. Conventional MR images and calculated ADC maps were available for image and ADC analysis. Patients were excluded from the study if (a) diagnosis of TSC was not confirmed by clinical criteria and/or genetic analysis, (b) MRI or DWI images were incomplete or lacking, (c) images were of poor quality, or (d) if the clinical records were unavailable. Institutional review board approval was obtained for this Health Insurance Portability and Accountability Act compliant study, and a waiver of informed consent was granted.
Imaging and analysis
MRI/DWI studies were reviewed by two experienced pediatric neuroradiologists (TH, AT). The standard departmental protocols were applied in all MRI/DWI studies using 1.5 Tesla MRI units. MRI studies consisted of pre- and postcontrast T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and DWI sequences. All DWI measurements used a balanced diffusion-weighted, single-shot, spin-echo echo-planar sequence sampled along at least three up to maximal 18 different geometric directions. An effective b value of 1,000 mm2/s was used for each of the diffusion encoding directions. An additional measurement without diffusion weighting (b = 0 mm2/s) was performed to allow calculation of the ADC values.
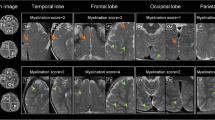
The following anatomical areas were selected for ADC measurements: frontal white matter (FWM), parietal white matter (PWM), occipital white matter (OWM), pons, and cerebellar white matter. All measurements were bilateral. Care was given that all measurements were performed in NAWM excluding pixels affected by TSC lesions. DWI images were used as anatomical reference for the placement of the regions of interest (ROIs). These images were superimposed on the corresponding ADC maps. The ROIs were carefully positioned to exclude pixels affected by focal lesions related to TSC. The ROIs were separated from the focal lesions by at least a 0.5-cm-wide band of normal-appearing white matter. Correlation with FLAIR images was done to ensure that no TSC lesions were included in the ROI. The size of the ROIs varied between 10 and 40 mm2 depending on the patient’s anatomy.
The ADC measurements were compared with 18 age-matched healthy controls who underwent MRI/DWI using the same departmental protocols. The inclusion criteria included normal MRI studies without any significant structural alterations or white matter abnormalities, no neurological deficits, and no developmental delay. Children with organic brain disorders, chronic diseases, or congenital syndromes were excluded from the control group. Follow-up clinical examinations confirmed exclusion of neurological diseases in the control group.
Neurological outcome variables
The neurological outcome variables included presence of seizures, mental retardation, and autism. The clinical neurological scoring system developed for TSC patients by Chou et al. [18] was used in the scoring of neurological severity. This scoring is as follows: one point for seizure, one point for developmental delay and/or mental retardation, and one point for autism. The points were totaled for the neurological severity score. The diagnosis of all neurological outcome variables and neurological scoring was performed by an experienced pediatric neurologist (LJ). The correlation between increased ADC values and the neurological severity score was analyzed.
Statistical analysis
The difference between mean ADC values obtained from the NAWM of TSC patients and the age-matched healthy controls were analyzed using unpaired two-sample t test. The relationship between the ADC values of the NAWM of patients with TSC and the neurological severity score was assessed using linear regression analysis. All reported p values are two-sided; a value of p ≤ 0.05 was considered significant. All statistical computations were performed by a statistician (EK) using the STATA software version 9.0 (STATA Corporation, College Station, TX, USA).
Results
Subjects
A total of 67 children and young adults under 25 years of age were identified with a confirmed or suspected diagnosis of TSC in the surveyed time period. After applying the exclusion criteria, 23 patients (11 males and 12 females) with clinically and/or genetically confirmed diagnosis of TSC who had high-quality MRI/DWI studies were included in the study sample. The age range of these patients at the time of MRI/DWI study was 15 months to 25 years with a mean of 12 years and a SD of 6.8 years.
ADC values compared with age-matched controls
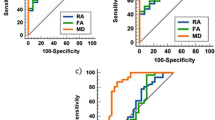
The comparison of measured ADC values in the study sample with the healthy age-matched controls is shown in Table 1. The different age groups considered for the comparison were adapted from a previous study on age correlated normative ADC values and included 12–24, 24-36, 48–96, 96–144, and >144 months [19]. The ROIs considered were the bilateral FWM, PWM, OWM, and pons. Because only one patient was seen in the age group of 12–24 months and one in the age group of 24–36 months, these two patients were excluded because no statistical analysis is possible with a sample size of one patient. Six patients fitted in the 48- to 96-month group, four in the 96- to 144-month group, and 11 in the group of children above 144 months. With the exception of one ROI (left parietal white matter in the child of the 48- to 96-month group), all ADC values were increased comparing patients with TSC and the age-matched controls. Statistically significant increase in ADC values were found in the right and left FWM as well as in the right and left pons in the 96- to 144-month age group. In the >144-month group, a statistically significant ADC increase was found in the right parietal and occipital white matter.
ADC values and neurological outcome variables
The neurological outcome variables were studied in 20 patients (Table 2). One patient was excluded due to poor documentation of the clinical findings. Fourteen (70%) of these patients had seizures, 11 (55%) had developmental delay/mental retardation, and 5 (25%) had autism. The change in neurological severity score per unit increase in ADC measurement is shown in Table 2 for all measured regions. There was no statistical significance in any of the analyzed regions.
Discussion
TSC is a well-described autosomal dominant disorder involving multiple organs including the skin, central nervous system, heart, lungs, retina, and kidneys [1, 2] . Seizures, developmental delay, and behavioral disorders including autism result in significant morbidity [1]. The need for noninvasive biomarkers that may help to predict the future development of children with TSC is obvious in counseling patients and their parents as well in the evaluation of different treatment options.
Few studies have focused on diffusion-weighted imaging which typically gives additional, quantitative information about the microstructural development of the brain, in particular of the white matter myelination. A recent study showed that DWI is able to detect elevated ADC values in normal-appearing white matter of children with neurofibromatosis type 1 [20]. Studies on patients with TSC in the past have also detected altered ADC values in the normal-appearing white matter tracts [10, 16, 17]. These results confirm that DWI/ADC analysis of NAWM can show pathology related to altered/impaired white matter myelination which may go undetected on conventional MR imaging.
Diffuse hypomyelination of the cerebral white matter is thought to occur in patients with TSC [10, 16, 17].The decreased restriction of the diffusion of water molecules within the brain due to hypomyelination is related to an increased ADC. Makki et al. [17] discussed that next to changes in the overall brain water content, disordered myelin sheaths, and astrogliosis, as well as an overall depletion of axons, increased astrocyte cell size and increased number of astrocytes may also explain elevated mean diffusivity in patients with TSC.
Our study extends the previous studies by correlating the measured ADC values with age-matched healthy controls. Our data confirmed that hemispheric NAWM distant to lesions visible on conventional MR imaging in children with confirmed TSC show increased ADC values in nearly all measured anatomical regions. The ADC values of bilateral pons also showed a trend toward elevation compared to the healthy controls. The increase in measured ADC values was, however, only statistically significant in a limited number of anatomical regions. This can at least partly be explained by the relatively small sample size of patients in the different age groups in our study. Larger prospective, longitudinal studies appear manadatory including.diffusion tensor imaging (DTI) data sets with calculation of the degree of anisotropic diffusion.
No statistically significant correlation was found between the measured ADC values and neurological severity scoring. This may have been due to the used neurological scoring which lacks discrimination for subtle delays in development. Due to the retrospective nature of our study, no detailed neurological evaluation was possible. Future prospective studies with longer follow-up periods and highly sensitive neuropsychological evaluations could possibly reveal a correlation between the measured ADC values of NAWM and neurocognitive development.
Several limitations should be considered. A major limitation was that the lack of correlative pathological–anatomical data did not allow determining the exact etiology of the observed ADC increases in NAWM. We can only speculate that the observed increased ADC values may indicate impaired/delayed myelination/maturation of the hemispheric white matter. However, multiple other microstructural abnormalities and processes have been discussed that could explain increased ADC values like the presence of disordered myelin sheaths, depletion of axons, astrogliosis, increased cell size, increased number of astrocytes, and a pathologic disruption of cell membranes with loss of myelin [17]. An additional major limitation of our study is the relatively small sample size for the different age groups. Future studies should incorporate larger groups of children of any age, including the youngest. It will, however, be difficult to identify TS children at a very young age because frequently, the symptoms become apparent only later during development. Technically, the used ROI analysis is user-dependent. Future studies should use a computational whole brain ADC evaluation which may prevent a user-dependent variance in measurements. Due to the retrospective study design, only DWI data sets with calculated ADC maps were available for data analysis. DTI with calculation of the degree of anisotropic diffusion (e.g., fractional anisotropy) may better help to understand the microstructural pathology present in TSC. Finally, our neurological scoring was limited due to the retrospective study design; prospective studies with standardized highly sensitive neuropsychological evaluations and follow-up examinations are mandatory.
Conclusion
Our study shows that ADC values of hemispheric NAWM are increased in patients with TSC. The obvious findings seen on “conventional” MR imaging most likely reflect only the tip of the iceberg. The abnormal ADC values indicate that the myelination/maturation of the supratentorial brain may be delayed/impaired in TS patients. These findings could explain more global neurocognitive deficits in TS patients. Larger studies, including DTI studies, are necessary to validate our results. Our retrospective study design and the relatively small sample size and limited sensitivity of the applied neurological scoring may have prevented the identification of a correlation between the measured ADC values and neurological scoring.
References
Baskin HJ Jr (2008) The pathogenesis and imaging of the tuberous sclerosis complex. Pediatr Radiol 38:936–952
Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355:1345–1356
Inoue Y, Nemoto Y, Murata R et al (1998) CT and MR imaging of cerebral tuberous sclerosis. Brain Develop 20:209–221
Roach ES, Gomez MR, Northrup H (1998) Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 13:624–628
DiMario FJ Jr (2004) Brain abnormalities in tuberous sclerosis complex. J Child Neurol 19:650–657
Eluvathingal TJ, Behen ME, Chugani HT et al (2006) Cerebellar lesions in tuberous sclerosis complex: neurobehavioral and neuroimaging correlates. J Child Neurol 21:846–851
Barkovich AJ (2000) Pediatric neuroimaging. Lippincott Williams &Wilkins, Philadelphia
Luat AF, Makki M, Chugani HT (2007) Neuroimaging in tuberous sclerosis complex. Curr Opin Neurol 20:142–150
Firat AK, Karakas HM, Erdem G, Yakinci C, Bicak U (2006) Diffusion weighted MR findings of brain involvement in tuberous sclerosis. Diagn Interv Radiol 12:57–60
Garaci FG, Floris R, Bozzao A et al (2004) Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology 232:461–465
Sener RN (2002) Tuberous sclerosis: diffusion MRI findings in the brain. Eur Radiol 12:138–143
Sener RN (2003) Diffusion MR imaging of giant cell tumors in tuberous sclerosis. J Comput Assist Tomogr 27:431–433
Jansen FE, Braun KP, van Nieuwenhuizen O et al (2003) Diffusion-weighted magnetic resonance imaging and identification of the epileptogenic tuber in patients with tuberous sclerosis. Arch Neurol 60:1580–1584
Karadag D, Mentzel HJ, Gullmar D et al (2005) Diffusion tensor imaging in children and adolescents with tuberous sclerosis. Pediatr Radiol 35:980–983
Chandra PS, Salamon N, Huang J et al (2006) FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia 47:1543–1549
Peng SS, Lee WT, Wang YH, Huang KM (2004) Cerebral diffusion tensor images in children with tuberous sclerosis: a preliminary report. Pediatr Radiol 34:387–392
Makki MI, Chugani DC, Janisse J, Chugani HT (2007) Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol 28:1662–1667
Chou IJ, Lin KL, Wong AM et al (2008) Neuroimaging correlation with neurological severity in tuberous sclerosis complex. Eur J Paediatr Neurol 12:108–112
Schneider JF, Il’yasov KA, Hennig J, Martin E (2004) Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology 46:258–266
van Engelen SJ, Krab LC, Moll HA et al (2008) Quantitative differentiation between healthy and disordered brain matter in patients with neurofibromatosis type I using diffusion tensor imaging. AJNR Am J Neuroradiol 29:816–822
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arulrajah, S., Ertan, G., Jordan, L. et al. Magnetic resonance imaging and diffusion-weighted imaging of normal-appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology 51, 781–786 (2009). https://doi.org/10.1007/s00234-009-0563-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0563-2