Abstract
Introduction
Follow-up of intracranial aneurysms treated by embolisation with detachable coils is mandatory to detect a possible recanalisation. The aim of this study was to compare contrast-enhanced magnetic resonance angiography (CE-MRA) with digital substraction angiography (DSA) used to detect aneurysm recanalisation to determine if DSA is still needed during follow-up.
Materials and methods
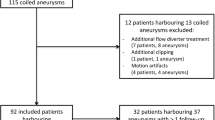
From May 2006 to May 2007, 55 patients with 67 aneurysms were treated by endosaccular coiling with (n = 9) or without (n = 58) an adjunctive stent. Follow-up imaging protocol included MRA at 6 and 12 months and a DSA at 12 months or earlier if a major recanalisation was identified on the 6-month MRA. Two neuroradiologists independently reviewed MRA images (readers 1 and 2) and two other reviewed DSA images.
Results
Follow-up DSA showed stability of the aneurysm occlusion in 52 cases, recanalisation in 14 cases, and further thrombosis in one. On CE-MRA, both readers identified all recanalisations but one (sensitivity of 93%) as they missed a major recanalisation in a 2-mm ruptured aneurysm. There were two false-positive evaluations by reader 1 and three for reader 2. Mean specificity of CE-MRA to detect aneurysm recanalisation was 95.5%.
Conclusion
CE-MRA is accurate to detect aneurysm recanalisation after embolisation with detachable coils. CE-MRA may be proposed as first-intention imaging technique for their follow-up. However, its sensitivity and specificity remain inferior to that of DSA and major recurrences may be missed in very small aneurysms. Therefore, a single DSA remains mandatory during the imaging follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular treatment (EVT) by means of detachable coils is widely used and accepted as an alternative to surgical clipping for patients with unruptured and ruptured intracranial aneurysms [1, 2]. Furthermore, EVT is associated with lower rates of morbidity and mortality in selected cases. However, the outcome of patients treated with detachable coils is poorly documented, and aneurysm recanalisation is not uncommon. These recurrences occur in 8% to 33.6% of patients [3, 4] and may necessitate a re-treatment. In order to evaluate the stability of aneurysm occlusion, patients must therefore be regularly followed-up. Digital substraction angiography (DSA) is currently the standard method for detection and evaluation of intracranial aneurysms [5]. However, this method is invasive, time consuming, relatively expensive and associated with a 0.5% risk of permanent neurological complications [6]. Magnetic resonance angiography (MRA), including time-of-flight (TOF) and contrast-enhanced (CE) techniques, have been suggested as alternatives to DSA [7–24]. These authors have shown that TOF- and CE-MRA are useful for the follow-up of coiled aneurysms [7–24]. In recently published papers [23, 24], it has been shown that both TOF- and CE-MRA have high and comparable sensitivity and specificity to assess immediate aneurysm occlusion after EVT except when the stent-assisted technique is used. In this situation, CE-MRA is superior to TOF-MRA to detect a residual aneurysm and to assess the patency of the parent artery. Therefore, CE-MRA was chosen in the present study as the MR technique for the follow-up of aneurysms treated by embolisation with detachable coils. In order to determine if DSA is still needed for the follow-up of intracranial aneurysms after EVT with detachable coils, the aim of our study was to compare the effectiveness of CE-MRA with that of DSA used to detect aneurysm recanalisation.
Materials and methods
Population
In this retrospective study, patients were included if they harbored one or multiple aneurysms selectively treated with coils and if a follow-up angiography was scheduled according to the usual follow-up protocol. From May 2006 to May 2007, 55 patients (37 females, 18 males; mean age = 46 years, range = 17 to 65 years old) harboring 67 aneurysms treated by endosaccular coiling underwent simultaneous DSA and MRA at follow-up. Twenty-five patients initially presented with SAH and were classified according to the Hunt and Hess scale [25]: 14 patients were graded I or II, two patients were graded III, and nine patients were graded IV or V. In the remaining 30 patients, all aneurysms but one were incidentally diagnosed. One 44-year-old woman presented with a sudden third nerve palsy without SAH on the CT scan.
Aneurysms were located in the anterior circulation in 58 cases [internal carotid artery (n = 25), anterior communicating artery (n = 18), middle cerebral artery (n = 15)] and in the posterior circulation in nine cases [basilar artery (n = 6), posterior inferior cerebellar artery (n = 2), superior cerebellar artery (n = 1)]. Aneurysms size ranged from 2 to 18 mm (mean size = 6.6 mm).
EVT
EVT was performed under general anaesthesia and systemic heparinisation. The adequacy of systemic anticoagulation was monitored by frequent measurements of the activated clotting time (ACT). A baseline ACT was obtained prior to the bolus infusion of heparin (30 to 50 IU/kg body weight) and hourly thereafter. The bolus infusion of heparin was followed by a continuous drip (1,000 to 1,500 IU/h), with the purpose of doubling the baseline ACT. At the end of the procedure, systemic heparinisation was maintained for 24 h in most patients. All procedures were performed by the same interventional neuroradiologist (BL). All patients were treated by selective embolisation with Microplex coils (MicroVention, Aliso Vieja, CA, USA) or Guglielmi Detachable Coils (Target Therapeutics, Fremont, CA, USA). Technique of endosaccular coiling procedure has already been published in the literature [26–28].
The endovascular procedure consisted of endosaccular coiling in 36 aneurysms, balloon-assisted coiling in 22 aneurysms [29, 30] and stent-assisted coiling in nine aneurysms [31, 32]. At the end of the endovascular procedure, all patients were evaluated by angiography to document aneurysm obliteration. Angiograms included selective injection of internal carotid or vertebral arteries with intracranial views (frontal, sagittal) completed by the working projections used during embolisation. These immediate post-interventional DSA controls served as a reference for imaging follow-up. After endovascular treatment, patients were transferred to the intensive care unit, and fluid balance, neurological status and blood pressure were carefully monitored.
Imaging follow-up
Imaging protocol
A multidisciplinary therapeutic protocol has been established in our institution for the management of patients with ruptured or unruptured intracranial aneurysms. For the follow-up of patients treated by endosaccular coiling, our imaging protocol includes a CE-MRA at 6 and 12 months and a conventional DSA at 12 months. If a recanalisation that is judged to require a re-treatment is observed on the 6-month MRA, a conventional DSA is performed.
DSA
Angiograms included selective injection of internal carotid or vertebral arteries with intracranial views (frontal, sagittal) completed by three-dimensional (3D) rotational angiography and/or additional views when necessary.
CE-MRA
All MR exams were performed on a 1.5-T system (Intera, Philips Medical Systems, Best, the Netherlands) equipped with 100 mT/m/ms slew rate and a maximum gradient amplitude of 21 mT/m. The receiver coil was a synergy head–neck head coil. High-spatial-resolution, intermediate-weighted (proton-density-weighted) images were acquired first with a turbo spin-echo sequence: repetition time millisecond per echo time millisecond of 2,900/14, turbo spin-echo factor of 10, 170 × 260-mm field of view, 354 × 512 acquisition matrix, 36 transverse 2.7-mm-thick sections, no intersection gap, 67.5% half-Fourier acquisition, imaging duration of 3 min 6 s. An MR power injector (Spectris, model SSM 200; MedRad, Pittsburgh, PA, USA) was used for injecting 18 mL of Gadobenate Dimeglumine (Multihance®, Bracco, Milan, Italy) administered via a 20-gauge catheter placed in the left antecubital vein at a rate of 1.8 mL/s and followed immediately with a 20-mL saline solution flush at the same rate. The 3D contrast-enhanced T1-weighted sequence was a 3D spoiled gradient-echo sequence with phase cycling with two successive volumes: The first was oriented in the coronal plane; the second in the transverse plane. The following parameters were used for both stacks: TR/TE 4.2/1.34, 35° flip angle, no flow compensation, 362 Hz per pixel bandwidth, 175 × 350-mm field of view, 212 × 352 acquisition matrix, 85 1.6-mm-thick sections with 0.8-mm overlap and a reconstructed resolution of 0.7 × 0.7 × 0.8 mm. The total imaging duration for the two stacks was 49 s, and a centra k-space filling was used (random sampling of the centre of the k space during the first 4 s followed by a low–high filling of the periphery). The coronal 3D slabs were positioned from transverse intermediate-weighted, turbo spin-echo images and multi-section survey images to include the middle cerebral artery, the circle of Willis, both carotid arteries from the bifurcation and the vertebrobasilar system. The transverse volume encompassed the circle of Willis and the posterior fossa, including the posterior cerebral and superior cerebellar arteries. The acquisition of the 3D contrast-enhanced T1-weighted sequence was interactively started using a single thick-slice real-time tracking sequence (TR/TE/angle 4.5/1.2/40° one image every 0.58 s, quadrature body coil acquisition) when the contrast arrived in the left ventricle.
Image analysis
Image quality of CE-MRA was judged according to the following criteria: image contrast, artefact (coil, motion), vessel overlap. Image contrast was graded as low when the signal intensity in the enhanced arterial lumen was only slightly higher than the signal intensity in the background, as moderate when the signal intensity was clearly higher and as high when the signal intensity was optimal. Artefacts and vessel overlap were judged as minor when they did not prevent the interpretation of images and as major when they degraded the image quality.
According to the Raymond et al. classification [3, 33], both MRA and DSA results were assigned to one of three categories: class 1 = complete obliteration, class 2 = residual neck and class 3 = residual aneurysm. Then, follow-up results were classified as stability (when a similar degree of aneurysm occlusion in multiple projections was found), recanalisation (when an increase of the amount of contrast filling in the aneurysm was observed) and further thrombosis (when the amount of contrast agent filling the aneurysm decreased).
DSA controls served as reference images and were reviewed by two senior neuroradiologists (BL, LC) with a 7- and a 15-year experience in vascular diagnostic neuroradiology. On DSA images, aneurysm recanalisation was judged as minor/major according to the need of a complementary treatment. Indeed, all our patients with an objective recanalisation at DSA are discussed in a multidisciplinary fashion to decide whether or not additional coiling and/or clipping should be performed. Generally, aneurysms with a neck remnant (minor recanalisation) are not re-treated, whereas aneurysms with a residual sac are re-treated (major recanalisation).
MRA images were independently and blindly reviewed by two other senior neuroradiologists (OF, CN) with a 4- and a 9-year experience in vascular diagnostic neuroradiology. Pre-treatment DSA images were shown to MRA readers to orient to the nature of aneurysms.
Results
DSA
Immediate post-treatment results
At the end of EVT, DSA controls were as follows: class 1, 43 aneurysms; class 2, 20 aneurysms; class 3, four aneurysms.
Follow-up results
Follow-up DSA was performed at 6 months for four aneurysms because of a suspected major recanalisation at MRA and at 12 months for the remaining 63 aneurysms. These angiographic controls were as follows: stability, 52 aneurysms; recanalisation, 14 aneurysms; further thrombosis, one aneurysm. Recanalisations occurred in aneurysms located on the ICA (n = 4), the MCA (n = 4), the AcomA (n = 4) and the basilar tip (n = 2). The size of the recanalised aneurysms varied from 4 to 18 mm (mean size = 8.9 mm). These recanalisations were judged as minor in seven cases and major in seven cases. These latter aneurysms were re-treated by endovascular approach without any complication.
CE-MRA
Image quality
The contrast of CE-MRA images was judged high or moderate in all cases by both readers. In all cases, artefacts and/or vessel overlap were judged as minor by both readers because they did not prevent the interpretation of MRA images.
Follow-up results
Diagnostic performances of CE-MRA are detailed in Table 1.
All recanalisations (residual necks or sacs) but one (13/14) were identified by both readers on CE-MRA images. Sensitivity of CE-MRA for the detection of aneurysms recanalisation was thus of 93%. Both readers missed a major recanalisation in a 30-year-old woman with a 2-mm ruptured posterior communicating artery aneurysm that was completely occluded after initial EVT (Fig. 1). Both readers missed this recanalisation on the 6- and 12-month CE-MRA. DSA showed a 50% recanalisation, and the patient was completely re-treated by endovascular approach without any complication. A retrospective review of this case, with the knowledge of DSA results, did not change the interpretation of our two readers. There were two false-positive evaluations by reader 1 and three for reader 2. In these latter cases, both readers identified on CE-MRA a minor recanalisation of an aneurysm that was completely occluded at DSA. Specificity of CE-MRA for the detection of aneurysms recanalisation was thus of 96.4% for reader 1 and of 94.6% for reader 2 (mean specificity of 95.5%).
Subarachnoid haemorrhage in a 30-year-old woman. a Conventional DSA shows a 2-mm aneurysm of the supraclinoidal internal carotid artery with associated significant vasospasm. b After selective endovascular treatment with detachable coils, non-substracted conventional DSA shows a complete aneurysm occlusion. c, d Contrast-enhanced MRA at 6 (c) and 12 months (d) show no obvious recanalisation. e, f However, non-substracted conventional (e) and three-dimensional (f) DSA at 12 months show a significant aneurysm recanalisation that has been re-treated by embolisation
Among 14 recanalisations, one occurred in a patient treated with the stent-assisted technique. This latter patient was treated for an unruptured basilar trunk aneurysm, and both readers identified the recanalisation on CE-MR images.
Discussion
This study shows that CE-MRA may detect with a high sensitivity and specificity a recanalisation in aneurysms treated by embolisation with detachable coils. CE-MRA may thus be proposed as first-intention diagnostic imaging technique for the follow-up of coiled intracranial aneurysms. However, in very small aneurysms, major recurrences requiring re-treatment may be missed. Moreover, CE-MRA sensitivity and specificity still remain inferior to that of DSA. Therefore, our findings suggest that a single DSA remains mandatory during the follow-up of coiled aneurysms.
Aneurysm recanalisation after EVT
Aneurysm recanalisation due to coil compaction occurs in 10% to 40% of embolised intracranial aneurysms [3, 4]. The natural history of these recurrences is still poorly documented, but re-bleeding may occur [1–4, 34]. In a recent series of 466 patients with 501 coiled aneurysms and a mean follow-up period of 31.32 months, three patients (0.8%) re-bled [3]. Therefore, imaging follow-up of coiled aneurysms is mandatory, but the ideal duration of this follow-up is yet unknown. Most authors recommend to follow-up patients for several years as late recanalisation are well described [3, 4, 7–22]. Therefore, non-invasive imaging techniques such as TOF- and CE-MRA have been evaluated and validated to avoid the repeated use of DSA that carries a 0.5% of neurological complication [6].
CE-MRA
It is a current debate in the literature whether or not the use of contrast material is improving the efficacy of MRA.
Most authors have evaluated TOF-MRA for the follow-up of coiled aneurysms [7–14]. They have reported sensitivity for the detection and exclusion of a residual flow within the aneurysm between 71% and 91% and between 89% and 100%, respectively. However, this technique is limited by its sensitivity to both saturation and susceptibility (coil-related) artefacts [7–10, 35]. These latter artefacts are even more significant when coated Nexus coils (Micro Therapeutics, Irvine, CA, USA) are used to occlude the aneurysmal sac [35]. These disadvantages may lead to false positives or false negatives, and it may also explain that some MR exams may not be analysed [7–10, 35]. Finally, TOF sequences require a long acquisition time and may only acquire small acquisition volume. These limitations may be of importance in patients with multiple coiled aneurysms [7–14]. Nevertheless, these authors showed that TOF-MRA is very useful to detect residual neck or aneurysm, and they have suggested to partially replace DSA by MRA for the follow-up of coiled aneurysms.
CE-MRA is a more recent MR technique with several theoretical advantages [15–24]. CE-MRA is less sensitive to flow turbulences and saturation effects than TOF sequences because of the high signal intensity within the arterial lumen caused by the T1-shortening effects. Moreover, contrast enhancement allows the imaging of low-flow signals and that lead to a higher conspicuity of a residual neck or aneurysm. CE-MRA is also less sensitive to coil-related artefacts (susceptibility artefacts) that may decrease image quality and prevent a precise visualisation of a residual neck or aneurysm. The injection of contrast material also allows better visualisation of small arteries because of the appropriate filling of small arteries with gadolinium and because of the short acquisition time of the sequence, which decreases motion artefacts. Finally, this technique allows the assessment of a larger imaging volume than TOF-MRA that is really interesting in patients with multiple coiled aneurysms.
On the other hand, CE-MRA has some disadvantages [15–22]. Venous enhancement occurs at the same time as arterial enhancement because of the short time window between both phases, and this may degrade image quality mostly in case of aneurysms located near the base of the skull or the middle cerebral artery [15, 18, 20]. Another disadvantage is the possibility of a false neck remnant, which may be explained by the peripheral contrast enhancement of the organised thrombus or by the vasa vasorum within the aneurysm wall [16, 17, 21].
Despite many theoretical advantages, only few authors have evaluated CE-MRA for the follow-up of coiled aneurysms [15–22, 24]. Most of them have shown no difference between TOF- and CE-MRA. Indeed, Kwee and Kwee [22] confirmed these findings in a recent meta-analysis showing that there were no statistically significant differences in pooled sensitivity and specificity between TOF-MRA and CE-MRA. Only Leclerc et al. [17] showed a superiority of CE-MRA over TOF-MRA in a series of 20 patients. This difference may be explained by the fact that all patients had aneurysms located on the anterior communicating artery. Indeed, in this particular location, there is no venous opacification that might degrade the image quality. However, we chose to use CE-MRA in our study because it appears superior to TOF-MRA when the stent-assisted technique is used to treat aneurysms [23, 24].
In the present study, sensitivity of CE-MRA to detect aneurysm recanalisation, either minor or major, was of 93% with a mean specificity of 95.5%. Our findings are in agreement with recently published series [16, 17, 20, 21]. These authors showed that sensitivity and specificity of CE-MRA for residual neck and residual aneurysm detection ranged, respectively, from 75% to 100% and from 85.7% to 100%. CE-MRA is thus very useful in the assessment of aneurysmal recanalisation. However, in our series, a major recanalisation was missed in a 2-mm ruptured aneurysm that was completely occluded after EVT (Fig. 1). Despite the fact that the recanalisation was only of 1 mm, it was judged to require re-treatment because it represented 50% of the aneurysmal sac. This limitation of the MR technique has already been described by Pierot et al. [20] who showed that false-negative analysis of MRA correlated with the small size of the residual neck, which was often between 1- and 3-mm wide.
Study limitations
Several shortcomings may be pointed out in the present study. First, the study shares the limitations of all retrospective studies. Second, the CE-MRA did not compare with 3D TOF-MRA used as reference in most of studies. Third, a verification bias is present in four patients who had a control DSA at 6 months because of a significant recanalisation at the MRA. Finally, one may argue that knowledge of the findings of pre-treatment DSA might have influenced the interpretation of the MRA images. We, however, believe that this method of image analysis resembles that in clinical practice and is, therefore, justified.
MR angiography at 3 T
New sequences using higher field strengths (3 T) provide images with higher spatial resolution than that of MRA at 1.5 T, and improvement of sensitivity to detect aneurysm remnants or recurrences is to be expected. Recently, MRA at 3 T have been evaluated in few studies [36–39]. Majoie et al. [36] were the first to report the feasibility and the effectiveness of TOF-MRA at 3 T for the follow-up of coiled aneurysms. Then, Urbach et al. [37] and Buhk et al. [38] showed that TOF-MRA at 3 T might replace DSA as the first-intention diagnostic imaging technique to follow coiled aneurysms. Finally, Anzalone et al. [39] showed that CE-MRA at 3 T is superior to TOF-MRA at 3 T for visualisation of residual patency and is associated with fewer artefacts. Larger numerous studies are thus warranted now to evaluate the place of MRA techniques at 3 T in the imaging protocol of coiled aneurysms.
Follow-up of coiled intracranial aneurysms
Because the sensitivity and the specificity of CE-MRA for the detection of aneurysmal recanalisations are still inferior to DSA, we believe—as most authors do [11–13, 18–20]—that a single DSA is still required during imaging follow-up of coiled aneurysms. Moreover, it is also clearly admitted that DSA complication rate is related to the type of patient who is examined. Indeed, in a meta-analysis by Cloft et al. [40] and in the very large study from Willinsky et al. [41], the DSA neurological complication rate was lower in patients with a SAH, an aneurysm, compared with that in patients with ischaemic stroke because of their younger age and lower cardiovascular risks factors. Therefore, one must consider the risk of DSA very low in patients who will be followed for a coiled aneurysm. Therefore, our previous imaging protocol has not changed: CE-MRA at 6 months and CE-MRA and DSA at 1 year. Six-month MRA is performed to depict a potential aneurysmal recurrence or remnant, for which additional treatment may be necessary. At 1 year, CE-MRA and DSA results are compared, and subsequent follow-up is based on these results.
Conclusion
CE-MRA has high sensitivity and specificity to detect intracranial aneurysm recanalisation after selective EVT with detachable coils. CE-MRA may thus be proposed as first-intention diagnostic imaging technique for the follow-up of coiled aneurysms. However, in very small aneurysms, major recurrences requiring re-treatment may be missed. Moreover, sensitivity and specificity of CE-MRA remain inferior to DSA. Therefore, a single DSA still remains mandatory during the imaging follow-up.
References
International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group (2002) ISAT of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 360:1267–1274 doi:10.1016/S0140-6736(02)11314-6
International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group (2005) ISAT of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366:809–817 doi:10.1016/S0140-6736(05)67214-5
Raymond J, Guilbert F, Weill A et al (2003) Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 34:1398–1403 doi:10.1161/01.STR.0000073841.88563.E9
Cognard C, Weill A, Spelle L et al (1999) Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 212:348–356
Mayberg MR, Batjer HH, Dacey et al (1994) Guidelines for the management of aneurysmal subarachnoid hemorrhage, a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 25:2315–2328
Earnest F, Forbes G, Sandok B et al (1984) Complications of cerebral angiography: prospective assessment of the risk. AJR 142:247–253
Derdeyn CP, Graves VB, Turksi PA, Masaryk AM, Strother CM (1997) MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: preliminary experience. AJNR Am J Neuroradiol 18:279–286
Gonner F, Heid O, Remonda L et al (1998) MR angiography with ultrashort echo time in cerebral aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 19:1324–1328
Kahara VJ, Seppanen SK, Ryymin PS, Mattila P, Kuurne T, Laasonen EM (1999) MR angiography with three-dimensional time-of-flight and targeted maximum-intensity-projection reconstructions in the follow-up of intracranial aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 20:1470–1475
Anzalone N, Righi C, Simionato F et al (2000) Three-dimensional time-of-flight MR angiography in the evaluation of intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 21:746–752
Boulin A, Pierot L (2001) Follow-up of intracranial aneurysms treated with detachable coils: comparison of gadolinium-enhanced 3D time-of-flight MR angiography and digital substraction angiography. Radiology 219:108–113
Weber W, Yousry TA, Felber SR et al (2001) Non-invasive follow-up of GDC-treated saccular aneurysms by MR angiography. Eur Radiol 11:1792–1797 doi:10.1007/s003300000741
Westerlaan HE, Van der Vliet AM, Hew JM et al (2005) Time-of-flight MR angiography in the follow-up of intracranial aneurysms treated with Guglielmi detachable coils. Neuroradiology 47:622–629 doi:10.1007/s00234-005-1395-3
Yamada N, Hayashi K, Murao K, Higashi M, Iihara K (2004) Time-of-flight MR angiography targeted to coiled intracranial aneurysms is more sensitive to residual flow than is digital subtraction angiography. AJNR Am J Neuroradiol 25:1154–1157
Metens T, Rio F, Balériaux D, Roger T, David P, Rodesch G (2000) Intracranial aneurysms: detection with dynamic gadolinium-enhanced three-dimensional MR angiography—initial results. Radiology 216:39–46
Gauvrit JY, Leclerc X, Pernodet M et al (2005) Intracranial aneurysms treated with Guglielmi detachable coils: usefulness of 6-month imaging follow-up with contrast-enhanced MR angiography. AJNR Am J Neuroradiol 26:515–521
Leclerc X, Navez JF, Gauvrit JY, Lejeune JP, Pruvo JP (2002) Aneurysms of the anterior communicating artery treated with Guglielmi detachable coils: follow-up with contrast-enhanced MR angiography. AJNR Am J Neuroradiol 23:1121–1127
Cottier JP, Bleuzen-Couthon A, Gallas S et al (2003) Intracranial aneurysms treated with Guglielmi detachable coils: is contrast material necessary in the follow-up with 3D time-of-flight MR angiography? AJNR Am J Neuroradiol 24:1797–1803
Farb RI, Nag S, Scott JN et al (2005) Surveillance of intracranial aneurysms treated with detachable coils: a comparison of MRA techniques. Neuroradiology 47:507–515 doi:10.1007/s00234-005-1375-7
Pierot L, Delcourt C, Bouquigny F et al (2006) Follow-up of intracranial aneurysms selectively treated with coils: prospective evaluation of contrast-enhanced MR angiography. AJNR Am J Neuroradiol 27:744–749
Gauvrit JY, Leclerc X, Caron S, Taschner C, Lejeune JP, Pruvo JP (2006) Intracranial aneurysms treated with GDC: imaging follow-up with contrast-enhanced MR angiography. Stroke 37:1033–1037 doi:10.1161/01.STR.0000209236.06451.3b
Kwee TC, Kwee RM (2007) MR angiography in the follow-up of intracranial aneurysms treated with Guglielmi Detachable Coils: systematic review and meta-analysis. Neuroradiology 49:703–713 doi:10.1007/s00234-007-0266-5
Lovblad KO, Yilmaz H, Chouiter A et al (2006) Intracranial aneurysm stenting: follow-up with MR angiography. J Magn Reson Imaging 24:418–422 doi:10.1002/jmri.20642
Lubicz B, Levivier M, Sadeghi N, Emonts P, Balériaux D (2007) Immediate intracranial aneurysms occlusion after embolisation with detachable coils: a comparison between MR angiography and intra-arterial digital substraction angiography. J Neuroradiol 34:190–197
Hunt W, Hess R (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 8:14–20
Gulielmi G, Vinuela F, Dion J, Duckwiler G (1991) Electrothrombosis of saccular aneurysms via endovascular approach—part 2: preliminary clinical experience. J Neurosurg 75:8–14
Guglielmi G, Vinuela F, Duckwiler G et al (1992) Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg 77:515–524
Vinuela F, Duckwiler G, Mawad M (1997) Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 86:475–482
Moret J, Cognard C, Weill A, Castaings L, Rey A (1997) The reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results. J Neuroradiol 24:30–44
Lubicz B, Lefranc F, Bruneau M, Balériaux D, De Witte O (2008) Balloon-assisted coiling of intracranial aneurysms is not associated with higher complication rate. Neuroradiology (May):14 (in press)
Alfke K, Straube T, Dörner L, Mehdorn HM, Jansen O (2004) Treatment of intracranial broad-neck aneurysms with a new self-expanding stent and coil embolization. AJNR Am J Neuroradiol 25:584–591
Lubicz B, Leclerc X, Levivier M et al (2006) Retractable self-expandable stent for endovascular treatment of wide-necked intracranial aneurysms: preliminary experience. Neurosurgery 58:451–457
Roy D, Milot G, Raymond J (2001) Endovascular treatment of unruptured aneurysms. Stroke 32:1998–2004 doi:10.1161/hs0901.095600
CARAT Investigators (2006) Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke 37:1437–1442 doi:10.1161/01.STR.0000221331.01830.ce
Kang HS, Moon WJ, Roh HG et al (2007) MR angiographic evaluation is limited in intracranial aneurysms embolized with Nexus coils. Neuroradiology 50:171–178 doi:10.1007/s00234-007-0320-3
Majoie CB, Sprengers ME, van Rooij WJ et al (2005) MR angiography at 3T versus DSA in the follow-up of intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol 26:1349–1356
Urbach H, Dorenbeck U, von Falkenhausen M et al (2008) Three-dimensional Time-Of-Flight MR angiography at 3 T compared to DSA in the follow-up of ruptured and coiled intracranial aneurysms: a prospective study. Neuroradiology 50:383–389 doi:10.1007/s00234-007-0355-5
Buhk JH, Kallenberg K, Mohr A, Dechent P, Knauth M (2008) No advantage of Time-Of-Flight MR angiography at 3 Tesla compared to 1.5 Tesla in the follow-up after endovascular treatment of cerebral aneurysms. Neuroradiology (Jun):4 (in press)
Anzalone N, Scomazzoni F, Cirillo M et al (2008) Follow-up of coiled cerebral aneurysms at 3T: comparison of 3D-Time-Of-Flight MR angiography and contrast-enhanced MR angiography. AJNR Am J Neuroradiol (Jun):12 (in press)
Cloft HJ, Joseph GJ, Dion JE (1999) Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 30:317–320
Willinsky RA, Taylor SM, terBrugge K, Farb RI, Tomlinson G, Montanera W (2003) Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 227:522–528 doi:10.1148/radiol.2272012071
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lubicz, B., Neugroschl, C., Collignon, L. et al. Is digital substraction angiography still needed for the follow-up of intracranial aneurysms treated by embolisation with detachable coils?. Neuroradiology 50, 841–848 (2008). https://doi.org/10.1007/s00234-008-0450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-008-0450-2