Abstract
Introduction
The purpose of this study was to compare the results of perfusion computed tomography (PCT) with those of 15O2/H2 15O positron emission tomography (PET) in a subset of Carotid Occlusion Surgery Study (COSS) patients.
Materials and methods
Six patients enrolled in the COSS underwent a standard-of-care PCT in addition to the 15O2/H2 15O PET study used for selection for extracranial–intracranial bypass surgery. PCT and PET studies were coregistered and then processed separately by different radiologists. Relative measurement of cerebral blood flow (CBF) and oxygen extraction fraction (OEF) were calculated from PET. PCT datasets were processed using different arterial input functions (AIF). Relative PCT and PET CBF values from matching regions of interest were compared using linear regression model to determine the most appropriate arterial input function for PCT. Also, PCT measurements using the most accurate AIF were evaluated for linear regression with respect to relative PET OEF values.
Results
The most accurate PCT relative CBF maps with respect to the gold standard PET CBF were obtained when CBF values for each arterial territory are calculated using a dedicated AIF for each territory (R 2 = 0.796, p < 0.001). PCT mean transit time (MTT) is the parameter that showed the best correlation with the count-based PET OEF ratios (R 2 = 0.590, p < 0.001).
Conclusion
PCT relative CBF compares favorably to PET relative CBF in patients with chronic carotid occlusion when processed using a dedicated AIF for each territory. The PCT MTT parameter correlated best with PET relative OEF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background and purpose
The Carotid Occlusion Surgery Study (COSS) is an ongoing randomized, single-blinded (outcome assessor), controlled trial testing the hypothesis that extracranial–intracranial (EC/IC) bypass surgery in patients with recently symptomatic carotid artery occlusion will significantly reduce the subsequent occurrence of ipsilateral ischemic stroke. Patients eligible for COSS are selected based on the results of an O15O/H2 15O positron emission tomography (PET) imaging study. The latter must show a count-based ratio of ipsilateral to contralateral oxygen extraction fraction (OEF) ratio greater than 1.13 for the patients to be enrolled (COSS: http://dmchost.public-health.uiowa.edu/coss/).
15O2/H2 15O PET imaging is less widely available than other imaging techniques that are used to assess the brain perfusion, such as perfusion computed tomography (PCT). PCT has been shown to give comparable results to H2 15O PET in terms of quantitative cerebral blood flow (CBF) measurements [1] and is more accessible because it requires only a multidetector CT scanner and a power injector, and can thus be performed in most institutions. Other advantages to using PCT over PET include shorter duration of examination and simpler acquisition [2].
The purpose of our study was to compare the results of PCT with those of 15O2/H2 15O PET in a subset of patients enrolled in the COSS trial to determine how PCT compares to O15O/H2 15O PET in patients with chronic carotid occlusion.
Materials and methods
Study design
Inclusion criteria for COSS include (1) transient ischemic attack or ischemic stroke with mild to moderate permanent ischemic neurological deficit (modified Barthel index ≥ 12/20) in the hemispheric carotid territory appropriate to an occluded carotid artery occurring within 120 days before performance of PET, (2) ipsilateral to contralateral PET O15O/H2 15O OEF ratio greater than 1.13, and (3) intra-arterial contrast arteriography demonstrating both the occlusion of an internal carotid artery, and extracranial and intracranial vessels suitable for EC/IC anastamosis (COSS).
Six patients with cervical internal carotid occlusion who were enrolled in COSS at our institution were retrospectively identified as having undergone PCT studies in the month before the PET study. At our institution, PCT is part of a routine stroke CT survey including the following imaging series: noncontrast CT, PCT at two cross-sectional positions, CTA of the cervical and intracranial vessels, and post-contrast cerebral CT, obtained in this chronological sequence. Under the auspices of our institutional review board and with a waiver of patient consent due to the retrospective nature of the study, these six patients were considered for the present study.
Imaging studies
PET was performed using a nonquantitative, count-based method for determining OEF [3]. First, a PET transmission scan was acquired for approximately 10 min. This was followed by acquisition of two 60 × 2-s emission scans each beginning with the administration of radioactivity, one following brief inhalation of 100 mCi O15O and one after bolus intravenous administration of 75 mCi H2 15O. A delay of 15 min between the two emission scans was used to allow radioactivity to decay.
PCT consisted of a 45-s series with 45 gantry rotations performed in cine mode during intravenous administration of iodinated contrast material. Images were acquired and reconstructed at a temporal sampling rate of one image per second, resulting in a series of 45 images for each assessed slice. Two successive PCT series were performed, separated by a time interval of 3–5 min from each other and afforded a total coverage of 40 mm (4 × 10 mm). The two slices of the first PCT series were at the level of the third ventricle and the basal ganglia. The second PCT series was selected at a level 3.5 cm cranial to the first slice of the first series. For each PCT series, a 40-ml bolus of iohexol (Omnipaque, Amersham Health, Princeton, NJ, USA; 300 mg/ml of iodine) was administered into an antecubital vein using a power injector at an injection rate of 5 ml per second for all patients. The acquisition parameters were 80 kVp and 100 mAs. CT scanning was initiated 7 s after start of the injection of the contrast bolus. The effective radiation dose represented by this PCT protocol is 2 mSv, which is comparable to a noncontrast CT study of the brain (2.5 mSv).
PET post-processing
For each PET emission scan, a composite image was created from all 60 2-s frames. A large spherical region of interest (ROI) encompassing the central part of the brain was created on the composite image. This ROI was then applied individually to each of the 2-s frames to create a time–activity curve for the brain. From this curve, the frame in which radioactivity first arrived in the brain was determined visually. This frame and the subsequent 19 frames were used to create a 40-s composite image. These images were filtered with a 3D Gaussian filter to create a final image resolution of 15.6 mm full-width-half-maximum in all three dimensions and transformed to stereotactic atlas space using a nine-parameter fit with the automated image registration (AIR) algorithm to align then to a standard CBF template [4]. AIR is a freeware code written in C that allows automated registration of 2D and 3D images within and across subjects, and within and across imaging modalities (http://bishopw.loni.ucla.edu/AIR5/index.html). In the 40-s H2 15O image, PET counts are linearly proportional to CBF, so this image gives an accurate measurement of relative CBF [5]. Count-based OEF data was derived from dividing the counts in the O15O image by the counts in the H2 15O. These are at least as accurate as quantitative OEF data for predicting stroke risk in patients with unilateral symptomatic carotid artery occlusion [3].
PCT post-processing
PCT data were analyzed utilizing PCT software developed by Philips Medical Systems (Cleveland, OH, USA). This software relies on the central volume principle, which is the most accurate for low injection rates of iodinated contrast material [6]. After motion correction and noise reduction by an anisotropic, edge-preserving spatial filter, the software applies curve fitting by least mean squares to obtain mathematical descriptions of the time–density curves for each pixel. A closed-form (non-iterative) deconvolution that accounts for delay-related errors is then applied to calculate the mean transit time (MTT) map [7]. The deconvolution operation requires a reference arterial input function (AIF). The cerebral blood volume (CBV) map is calculated from the area under the time–density curves [8]. Cerebral blood flow (CBF) is calculated as the CBV/MTT ratio.
PCT datasets in each patient were processed seven times by a trained research assistant. Seven different AIFs were selected semi-automatically by the PCT software within a region of interest drawn by the research assistant: pericallosal anterior cerebral artery (ACA), M1 segment of right middle cerebral artery (MCA), anterior M2 branch of the right MCA, posterior M2 branch of the right MCA, M1 segment of the left MCA, anterior M2 branch of the left MCA, and posterior M2 branch of the left MCA. AIF software detection is based on the detection of an early rise of attenuation, a large increase in density on the tissue density–time curve compared with the adjacent brain parenchyma and a small half width at half maximum of the peak enhancement. An eighth processing using, for each vascular territory, its own AIF was also performed (combined AIF).
Image analysis
PCT and PET studies were co-registered, and the same set of symmetric regions of interest (ROIs) were drawn on matching slices of both imaging datasets. Selected ROIs included the right and left basal ganglia, the right and left ACA, the anterior and posterior MCA, and the posterior cerebral artery (PCA) territories (22 ROIs per patient; ROI size, 5–50 cm2).
The following measurements were recorded in each ROI for each patient: absolute number of counts for the PET O15O scan and the PET H2 15O scan, CBF, CBV, and MTT values for PCT.
Selection of AIFs and selection of ROIs for recording PCT and PET measurements were completely independent from each other. Selection of the AIFs was performed at the time of the processing of the PCT datasets. Once all PCT datasets were processed and all PCT maps obtained, ROIs matching the vascular territories were drawn, and PCT and PET in these ROIs were recorded.
Once all the measurements were recorded by a first radiologist, a second radiologist blinded to the PCT and PET measurements reviewed the CTA obtained at the same time as PCT. Based on the side of the cervical internal carotid artery occlusion and on the anatomy of the Circle of Willis, “ischemic” AIFs (on the side of an occluded cervical internal carotid artery) were distinguished from “nonischemic” AIFs (on the contralateral side), with “ischemic” ROIs similarly distinguished from “nonischemic” ROIs.
Statistical analysis 1: comparison of PCT-relative CBF and PET-relative CBF
Relative PCT CBF values (“ischemic” side divided by “nonischemic” side) were calculated for each of the eight different AIFs. Relative PET CBF from the H2 15O scans (“ischemic” side divided by “nonischemic” side) were also calculated. The PCT CBF ratios were compared to the PET H2 15O ratios by linear regression.
Statistical analysis 2: comparison of PCT relative values and count-based PET OEF values
The relative OEF in each ROI was calculated by dividing the absolute number of regional counts on the PET O15O scan by the absolute number of counts on the PET H2 15O scan. The relative OEF values of the ischemic ROIs were then divided by the relative OEF values in their symmetric counterpart, resulting in a ratio of ischemic to nonischemic OEF for each ROI. Similarly, relative ischemic/nonischemic ratios of PCT CBF, CBV, and MTT were calculated for each data set using the best AIF approach. The PCT ratios were then compared to the gold standard PET OEF ratios by linear regression.
Results
Patients
Six patients (all male; age range, 42–76) with cervical internal carotid occlusion who were enrolled in COSS at our institution were retrospectively identified as having undergone PCT studies in the month before the PET study (time interval between PCT and PET ranged between 2 and 29 days). Stroke CT survey including PCT and CTA was obtained in these six patients to evaluate the carotid arteries given before history of transient ischemic attacks, but none of the patients was symptomatic at the time of the PCT or the PET studies.
PCT results and AIF selection
PCT CBV results were not influenced by the selected AIF. The mean CBV was 4.0 ± 1.0 ml/100g (range, 1.9–6.1 ml/100 g) for nonischemic territories and 3.6 ± 2.1 ml/100 g (range, 0.6–7.6 ml/100 g) for ischemic territories. Using “ischemic” AIFs, MTT values were shorter, and CBF values were increased compared to those obtained with “nonischemic” AIFs (Tables 1 and 2).
Statistical analysis 1: comparison of PCT relative CBF and PET relative CBF
The PET H2 15O count ratios (“ischemic” side divided by “nonischemic” side) were considered as a marker of relative CBF (PET studies were semi-quantitative, without blood sampling, and, as such, only relative CBF rather than absolute CBF values could be measured; no comparison from patient to patient could be performed) and used as a reference for relative PCT CBF measurements to determine the most appropriate AIF for PCT in patients with chronic cervical carotid artery occlusion (Table 3).
The PCT CBF ratios showing the best correlation with PET H2 15O ratios (slope = 0.842, intercept = 0.220, R 2 = 0.797, p < 0.001) were calculated using a combined AIF, i.e., a dedicated AIF for each territory (ACA AIF for ACA territory, right MCA AIF for right MCA territory, left MCA AIF for left MCA territory; Fig. 1).
Statistical analysis 2: comparison of PCT relative values and count-based PET OEF ratios
PCT MTT calculated with a combined AIF is the parameter that shows the best correlation with the count-based PET OEF ratios (slope = 0.124, intercept = 0.676, R 2 = 0.590, p < 0.001; Fig. 2). Correlation for PCT CBF (R 2 = 0.206, p = 0.050) and CBV (R 2 = 0.014, p = 0.613) was poor.
The correlation between PET and PCT was evaluated separately for the two PCT boluses, using a fixed effect for bolus 1 versus bolus 2 in a mixed model. The p value associated with the bolus number turned out not to be significant. We can thus conclude that the measurements obtained during the first PCT series and the second PCT series can be analyzed together.
Discussion
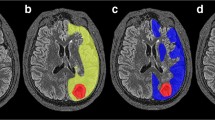
This comparison of PCT and O15O/H2 15O PET in a small retrospective series of six patients with carotid occlusion showed that using a dedicated AIF for each territory provides the best agreement with PET data in patients with carotid occlusion. This is in contrast with PCT datasets obtained in patients with acute stroke, where a single AIF, typically selected in the ACA, is sufficient [9]. In patients with carotid occlusion, selecting such a single AIF leads to overestimated MTT values and underestimated CBF values in the vascular territory dependent on the occluded carotid artery because of the additional delay in arrival time and the dispersion of the contrast agent during its transit to the tissue of interest, reflecting an increase in density, length, and importance of the collateral pathways supplying the brain region at risk [10–12]. A dedicated AIF approach minimizes the delay and dispersion of the contrast bolus between the area of measurement and the tissue of interest, and has the potential advantage of better reflecting the true hemodynamics of the ischemic lesions by taking into account the effects of collateral circulation to the ischemic tissue [12, 13]. The practical, clinical importance of a dedicated AIF selection for the processing of PCT studies obtained in patients with chronic cerebrovascular disease, such as carotid occlusion or EC–IC bypass or a giant carotid aneurysm, is illustrated in Fig. 3.
a Patient with a right carotid artery occlusion (arrows) demonstrated on a volume-rendering image of a CT angiogram viewed from above. b When processed using a single arterial input function (AIF; left column) selected in the anterior cerebral artery (ACA), PCT dataset obtained in the same patient demonstrates significantly asymmetric mean transit time (MTT) and cerebral blood flow (CBF) values, with increased MTT and low CBF on the side of the carotid artery occlusion, suggesting ischemia within the right hemisphere. When processed using each vascular territory’s own AIF (middle column), the same PCT dataset shows almost symmetric MTT and CBF values, which are in agreement with PET H2 15O (third column). Cerebral blood volume (CBV) values are unaffected by the selection of the AIF
The PCT parameter that best matches the PET ischemic-to-nonischemic PET OEF ratios used to select patients with carotid occlusion for bypass therapy is the MTT calculated using a dedicated AIF for each vascular territory. A correlation between 1/MTT and OEF has been previously demonstrated in patients with carotid occlusion as well as in experimental cerebral ischemia [14, 15]. The improved correlation over CBF and CBV alone by using MTT, which combines data from both measurements, reflects the responses to reduced cerebral perfusion pressure of increased CBV and reduced CBF [15, 16]. In head trauma patients, an increase in MTT has also been previously demonstrated to parallel a decrease in intraparenchymal brain tissue oxygen tension as measured using a brain parenchymal oxygen probe [17].
We acknowledge several limitations to our study. First, we evaluated only six patients. Second, several days to weeks elapsed between the PCT and PET studies, and although no clinical event was recorded for any of the patients during this interval, it is possible that changes in brain collaterals could have occurred, making the two studies less comparable. Similarly, end-tidal CO2 was not measured during imaging, and so we cannot rule out the contribution of marked differences in respiratory rate or blood pressure that could confound the comparison of studies done at different times. Finally, the spatial resolution of PCT and PET images are inherently different (7 mm for PCT and 15 mm for PET). Thus, the PET measurements in ROIs were more influenced by the status of the surrounding tissue, an effect that would tend to reduce the magnitude of the correlations. Thus, the correlations that we reported represent a conservative estimate of the actual correlation between the two methods. Furthermore, the effect of the different image resolutions is consistent across different AIF strategies rendering valid our conclusions about the best AIF strategy. Thirdly, we only used side-to-side ratios not quantitative values, so we cannot comment on the correlation of PCT with quantitative CBF or OEF measurements. However, as stated above, PET H2 15O count ratios are linearly proportional to CBF ratios, and OEF count ratios are predictive of subsequent stroke [3, 5].
In conclusion, PCT compares favorably to H2 15O PET in patients with chronic carotid occlusion when processed appropriately. The most accurate PCT CBF maps are obtained with using a dedicated AIF for each vascular territory. PCT MTT is the parameter that shows the best agreement with PET OEF values (R 2 = 0.590). Prospective investigations involving a larger sample size and receiver operating characteristic curve analysis are needed to assess whether PCT can be used as an alternative to PET OEF to identify patients with recently symptomatic carotid artery occlusion who are at high risk for subsequent stroke.
References
Kudo K, Terae S, Katoh C, Oka M, Shiga T, Tamaki N, Miyasaka K (2003) Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H2(15)O positron emission tomography. AJNR Am J Neuroradiol 24:419–426
Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36:e83–e99
Derdeyn CP, Videen TO, Simmons NR, Yundt KD, Fritsch SM, Grubb RL Jr., Powers WJ (1999) Count-based PET method for predicting ischemic stroke in patients with symptomatic carotid arterial occlusion. Radiology 212:499–506
Woods RP, Mazziotta JC, Cherry SR (1993) MRI-PET registration with automated algorithm. J Comput Assist Tomogr 17:536–546
Herscovitch P, Markham J, Raichle ME (1983) Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med 24:782–789
Wintermark M, Maeder P, Thiran JP, Schnyder P, Meuli R (2001) Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol 11:1220–1230
Axel L (1983) Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol 18:94–99
Ladurner G, Zilkha E, Iliff D, du Boulay GH, Marshall J (1976) Measurement of regional cerebral blood volume by computerized axial tomography. J Neurol Neurosurg Psychiatry 39:152–158
Wintermark M, Lau B, Chien J, Arora S. Anterior cerebral artery is an appropriate arterial input function for perfusion-ct processing in acute stroke patients. Neuroradiology (in press)
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Wu O, Ostergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG (2003) Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 50:164–174
Calamante F, Gadian DG, Connelly A (2000) Delay and dispersion effects in dynamic susceptibility contrast MRI: simulations using singular value decomposition. Magn Reson Med 44:466–473
Knutsson L, Larsson EM, Thilmann O, Stahlberg F, Wirestam R (2006) Calculation of cerebral perfusion parameters using regional arterial input functions identified by factor analysis. J Magn Reson Imaging 23:444–453
Gibbs JM, Wise RJ, Leenders KL, Jones T (1984) Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet 1:310–314
Schumann P, Touzani O, Young AR, Morello R, Baron JC, MacKenzie ET (1998) Evaluation of the ratio of cerebral blood flow to cerebral blood volume as an index of local cerebral perfusion pressure. Brain 121(Pt 7):1369–1379
Powers WJ (1991) Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 29:231–240
Hemphill JC III, Smith WS, Sonne DC, Morabito D, Manley GT (2005) Relationship between brain tissue oxygen tension and CT perfusion: feasibility and initial results. AJNR Am J Neuroradiol 26:1095–1100
Acknowledgment
This research was supported by USPHS grant NS42167 (William J. Powers, Principal Investigator).
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamath, A., Smith, W.S., Powers, W.J. et al. Perfusion CT compared to H2 15O/O15O PET in patients with chronic cervical carotid artery occlusion. Neuroradiology 50, 745–751 (2008). https://doi.org/10.1007/s00234-008-0403-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-008-0403-9