Abstract
Introduction
Postictal (“Todd’s”) paralysis, or “epileptic hemiplegia,” is a well-known complication of focal or generalized epileptic seizures. However, it is unclear whether the pathophysiology of Todd’s paralysis is related to alterations in cerebral perfusion. We report CT perfusion findings in a patient presenting with postictal aphasia and right hemiparesis.
Methods
A 62-year-old woman with a history of alcohol abuse, closed head injury and posttraumatic epilepsy, presented with acute onset aphasia and right hemiparesis. A non-contrast head CT scan demonstrated no acute hemorrhage. Left hemispheric ischemia was suspected, and the patient was considered for acute thrombolytic therapy. MRI revealed a subtle increase in signal intensity involving the left medial temporal, hippocampal and parahippocampal regions on both T2-weighted FLAIR and diffusion-weighted sequences. CT angiography and CT perfusion study were performed. The CT perfusion study and CT angiography demonstrated a dramatic reduction in cerebral blood flow and blood volume involving the entire left hemisphere, but with relative symmetry of mean transit time, ruling out a large vessel occlusion.
Results
Clinical resolution of the aphasia and hemiparesis occurred within a few hours, and correlated with normalization of perfusion to the left hemisphere (detected by MR perfusion).
Conclusion
This unique case is the first in which clinical evidence of Todd’s paralysis has been correlated with reversible postictal hemispheric changes on CT and MR perfusion studies. This is important because CT perfusion study is being used more and more in the diagnosis of acute stroke, and one needs to be careful to not misinterpret the data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postictal (“Todd’s”) paralysis, or “epileptic hemiplegia,” is a well-known complication of focal or generalized epileptic seizures. However, its pathophysiology is obscure. We report a patient who presented with postictal aphasia and right hemiparesis accompanied by a perfusion deficit to the entire left hemisphere. Clinical resolution of the aphasia and hemiparesis correlated with normalization of perfusion to the left hemisphere. Thus, our case suggests that Todd’s paralysis may be associated with transient postictal hypoperfusion.
Case report
A 62-year-old woman presented with the acute onset of aphasia and right hemiparesis, and was last seen normal one hour prior to arrival at our medical center. At presentation her vital signs showed tachycardia at 118/min and saturation 76% on room air. She was drowsy, but arousable, awake, responded to voice and opened her eyes, but was unable to follow commands. She was nonverbal except she said the word “what” in response to calling her name. She blinked to threat bilaterally. Cranial nerve functions were intact. She tracked across the midline bilaterally. Pupils reacted from 5 mm to 3 mm bilaterally. To motor examination she was uncooperative, but was clearly resisting gravity with both upper extremities and both lower extremities, and moved both lower extremities. She withdrew to pain, but less on the left upper extremity. Her reflexes were 2+ and symmetric at the biceps, triceps, patella, and brachioradialis and achilles. Her left toe was pointed upwards.
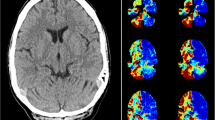
A noncontrast head CT scan (16-slice Lightspeed CT scanner, GE Healthcare) demonstrated bilateral frontal encephalomalacia consistent with prior trauma; no acute hemorrhage was identified (Fig. 1a). The initial diagnostic consideration was left hemispheric ischemia, and the patient was considered for acute thrombolytic therapy (with tissue plasminogen activator, tPA). A CT perfusion (CTP) study and CT angiography (CTA) were performed. The CTP study consisted of a 45-s series with 45 gantry rotations performed in cine mode during intravenous administration of iodinated contrast material. Images were acquired and reconstructed at a temporal sampling rate of one image per second, resulting in a series of 45 images for each assessed slice. Two successive CTP series were performed with an interval of 3–5 min following the noncontrast CT scan and prior to the CTA, affording a total coverage of 40 mm (4 × 10 mm). The two slices of the first CTP series were at the level of the third ventricle and the basal ganglia. The second CTP series was selected at a level 3.5 cm cranial to the first slice of the first series. For each CTP series, a 40-ml bolus of iohexol (Omnipaque, Amersham Health, Princeton, NJ; 300 mg/ml of iodine) was administered into an antecubital vein using a power injector at an injection rate of 5 ml per second. The acquisition parameters were 80 kVp and 100 mAs. CT scanning was initiated 7 s after the start of the contrast bolus injection.
CTA was performed using the following parameters: 120 kVp, 240 mAs, slice thickness 1.25 mm, slice acquisition interval 1 mm, pitch 1.375:1, intravenous administration of 70 ml iodinated contrast material at a rate of 4–5 ml per second, and an acquisition delay calculated from the CTP study ranging from 14 to 29 s. Data acquisition was performed from the origin of the aortic arch branch vessels to the vertex. The CTP study showed a dramatic decrease in cerebral blood flow (CBF) and blood volume (CBV) involving mainly the cortical gray matter of the left frontal, parietal, temporal, and occipital lobes with relative sparing of the white matter (Fig. 1b,c). Mean transit time (MTT) was relatively symmetric (Fig. 1d). As MTT is a sensitive indicator of acute cerebral ischemia secondary to large vessel occlusion or stenosis, this suggested that the reduction in CBF and CBV was not due to a large vessel occlusion. CTA demonstrated only a mild stenosis of the left M1 segment (Fig. 1e), with normal-appearing internal carotid and left posterior communicating and posterior cerebral arteries. Together, these findings suggested that the large hemispheric perfusion deficit was unlikely to have been secondary to vascular occlusion or embolus.
A 62-year-old woman with acute onset aphasia and right hemiparesis. a Noncontrast head CT scan demonstrates bifrontal encephalomalacia consistent with prior trauma. No signs of acute ischemia or hemorrhage are evident. b–d CTP images demonstrate reduced CBF (b) and CBV (c) in the entire left hemisphere; however, the MTT (d) is only mildly prolonged in the left hemisphere. e Coronal CTA reconstruction demonstrates mild stenosis of the proximal left middle cerebral artery (arrow), but normal cervical and intracranial internal carotid arteries. f–i MR imaging 5 h later, following resolution of aphasia and right hemiparesis. FLAIR image (f) and DW image (g) reveal new left mesial temporal, hippocampal, and parahippocampal hyperintensity associated with low ADC (h) from postictal restricted diffusion. MR perfusion study (i) is normal
Over the next several hours, she improved clinically, showing gradual and complete resolution of her right hemiparesis and aphasia. On further questioning she awoke with an initial phase of confusion and agitation. She stated that she was noncompliant with her phenytoin medication and that she likely had had one of her typical seizures. There was no witness to the ictus; she was found fallen by a bystander who called paramedics and was not available for interview. She was confused for a brief period but had no tongue laceration or incontinence. Further interrogation revealed a history of alcohol abuse, closed head injury and posttraumatic epilepsy. At presentation, her WBC count was 11,400 cells/mm3, serum bicarbonate was 20 meq/l, and a serum prolactin done 3 h after ictus was 17.1 µg/l (normal 1.4–24 µg/l). Her phenytoin level was subtherapeutic (2 mg/l) and she was treated with fosphenytoin. She had a history of generalized tonic-clonic seizures felt to be post-traumatic in origin which was poorly controlled. Her husband indicated that she would have postictal confusion but no aphasia or hemiparesis with her seizures prior to this event.
MRI of the brain (1.5 T MR unit, Achieva, Philips Medical Systems) was performed using the following parameters for diffusion (DWI) and perfusion (PWI) sequences. DWI series: echoplanar single-shot spin-echo, TR 3,534 ms, TE 81 ms, b 0 and 1,000, 24 5-mm thick slices with a 0.5-mm gap, matrix size 128 × 128. Average diffusion coefficient (ADC) maps were calculated from the diffusion images. Fluid attenuated inversion recovery (FLAIR) images were subsequently obtained: TR 11,000 ms, TE 140 ms, TI 2,800 ms, 30 5-mm thick slices with a −0.1-mm gap, matrix size 256 × 256. The PWI series was performed immediately afterwards: echoplanar gradient-echo, TR 2,000 ms, TE 50 ms, 12 5-mm thick slices with a 1-mm gap, matrix size 128 × 128, 60 acquisitions obtained every 2 s during intravenous administration of 0.2 mmol/kg Gd-DTPA (Magnevist; Bayer Healthcare), followed by a 15-ml saline flush, at a rate of 4 ml per second into an antecubital vein using a power injector (Spectris MR Injector; Medrad). MRI demonstrated a subtle increase in signal intensity involving the left medial temporal, hippocampal and parahippocampal regions on both T2-weighted FLAIR and diffusion-weighted images (Fig. 1f,g). Apparent diffusion coefficient (ADC) values in the left amygdala, hippocampus and parahippocampal gyrus were low and in the range of 650 μm2/s (Fig. 1h). Five hours after the CT scan, MR PWI demonstrated normalization of CBF in the left hemisphere (Fig. 1i). The patient experienced no further seizures and was discharged home neurologically intact except for a transient postictal memory deficit from which she recovered. Unfortunately, the patient was subsequently lost to follow-up and was unavailable for further investigation. No repeat neuroimaging was performed.
Discussion
Postictal motor deficits were first described by Bravais in 1827 as “l’épilepsie hémiplégique” [1]. In 1849, Todd further described “epileptic hemiplegia,” [2, 3], and it came to bear the eponym “Todd’s paralysis” [4, 5]. Later, Jackson [6] and Gowers [7] acknowledged this clinical entity and ascribed primacy to Todd in describing it.
Despite many theories [4], even today the physiologic explanation for Todd’s paralysis is unknown. Many mechanisms may account for the postictal state, including neurotransmitter depletion, neuronal desensitization, altered local cerebral blood flow [8], and various forms of active inhibition [9]. In 1959, Meyer and Portnoy studied postictal paralysis in nine patients and in monkeys and cats, recording CBF, pH, EEG, and oxygen tension during the postictal period. They found that cortical oxygen tension fell in the affected cortex, and hypothesized that the phenomenon of Todd’s paralysis results from temporary neuronal anoxia [10]. This hypothesis was later questioned by Efron who felt that postictal hemiplegia arose primarily from local electrical inhibition [11].
Even though no EEG was performed at the time of presentation, our patient’s clinical, laboratory, and neuroimaging findings were consistent with the postictal state. Because we documented transient postictal hypoperfusion associated with postictal neurologic deficits, our case offers further insight into the pathophysiology of Todd’s paralysis. Postictal paresis has a strong lateralizing value [12–14]; for example, in a recent study of 29 patients with Todd’s paralysis, 27 of the 29 (93%) had seizure onset contralateral to the hemiparesis [12]. Taking this finding into consideration in our patient, seizure onset was likely to have occurred in the left hemisphere, and postictally this was associated with hypoperfusion to the left hemisphere—manifest as reduced CBF and CBV but with relative preservation of MTT. Following resolution of the aphasia and hemiparesis several hours later, MRI demonstrated left mesial temporal FLAIR hyperintensity and restricted diffusion with low ADC values, indicating vasogenic and cytotoxic edema [15, 16], although perfusion had normalized to the entire left hemisphere. The increased FLAIR signal in the left amygdala and mesial temporal lobe, and the slightly reduced ADC values, are consistent with transient changes classically observed in the immediate postictal period [17, 18]. Such changes can be observed in the absence of a structural lesion such as mesial temporal sclerosis [19]. Ipsilateral cortical hypoperfusion has been previously detected on SPECT studies in patients with postictal hemiplegia [20, 21]. Although a definite mechanism for the reported transient postictal regional hypoperfusion cannot be ascertained in our patient, a possible physiologic explanation is the normal metabolic coupling of perfusion to neuronal activity in a state of postictal exhaustion or inhibition, resulting in a regional reduction in CBF or CBV that recovers with recovery in neural activity. These findings may relate to a “functional deficit zone” and “surround inhibition” described on PET and SPECT studies [22].
Meyer and Portnoy [10] reported a patient with postictal paresis in the setting of prior cerebrovascular disease and noted that the degree of “anoxemia” that the brain suffers in a generalized seizure should be worse distal to a stenosis. It is possible that the mild M1 stenosis in our patient (Fig. 1) contributed to functional cortical ischemia induced by left hemispheric seizure activity. Even a mild stenosis may become clinically significant in the context of increased metabolic demands precipitated by seizure activity.
In our patient a postictal state likely masqueraded as stroke, and several points make clear the value of CT angiography and CT perfusion in such patients particularly those presenting within the 3-h window for tPA therapy. The dramatic reduction in CBF and CBV was seen over the entire left hemisphere (in the left anterior, middle, and posterior cerebral artery distributions); it is difficult to explain a single site of vascular occlusion that would produce this picture especially with a patent left posterior communicating artery and left P1 segment of the posterior cerebral artery. Simultaneous embolism to both of these territories is possible but unlikely, and the patient had no obvious source of embolism. Furthermore, there was a relative preservation of MTT, suggesting that CBF was reduced from a metabolic mechanism rather than restriction of flow. This pattern of findings on CT angiography/CT perfusion is important to recognize, as it might be misinterpreted as cerebral stroke and should dictate caution when considering revascularization therapy acutely.
In summary, our case provides a temporal and spatial correlation of postictal hemispheric cortical hypoperfusion and clinical evidence of Todd’s paralysis. The transient nature of the clinical paralysis correlated with the transient perfusion deficit, suggesting that Todd’s paralysis may be a cerebrovascular phenomenon related to decreased functional cortical substrate demand during postictal neuronal depression. Thus, postictal aphasia may be related to transient hypoperfusion of the language cortex, and other transient postictal syndromes (e.g. postictal apraxia, postictal hemineglect, postictal hemianopsia, ataxic hemiparesis, postictal psychosis) that have been described that may have a similar pathophysiology [23–27]. Indeed, postictal amnesia, which was seen in our patient, has also been well described [28–31]. It will be interesting in future patients with postictal neurologic syndromes to confirm this finding of reversible hypoperfusion and to concurrently localize the affected cortex using modern imaging methods.
References
Bravais LF (1827) Recherches sur les symptômes et le traitement de l’épilepsie hémiplégique. Faculté de Médecine, Paris
Todd RB (1849) On the pathology and treatment of convulsive diseases. London Med Gazette 8:661–671, 724–729, 766–772, 815–822, 837–846
Todd RB (1855) Clinical lectures on paralysis, disease of the brain, and other affections of the nervous system. Lindsay & Blakiston, Philadelphia
Binder DK (2004) A history of Todd and his paralysis. Neurosurgery 54(2):480–486
Reynolds EH (2004) Todd, Faraday, and the electrical basis of epilepsy. Epilepsia 45(8):985–992
Jackson JH (1881) On temporary paralysis after epileptiform and epileptic seizures; a contribution to the study of dissolution of the nervous system. Brain 3:433–451
Gowers WR (1901) Epilepsy and other chronic convulsive diseases: their causes, symptoms, and treatment, 2nd edn. Churchill, London
Weinand ME, Carter LP, El-Saadany WF, Sioutos PJ, Labiner DM, Oommen KJ (1997) Cerebral blood flow and temporal lobe epileptogenicity. J Neurosurg 86:226–232
Fisher RS, Schachter SC (2000) The postictal state: a neglected entity in the management of epilepsy. Epilepsy Behav 1:52–59
Meyer JS, Portnoy HD (1959) Postepileptic paralysis: a clinical and experimental study. Brain 82:162–185
Efron R (1961) Post-epileptic paralysis: theoretical critique and report of a case. Brain 84:381–394
Kellinghaus C, Kotagal P (2004) Lateralizing value of Todd’s palsy in patients with epilepsy. Neurology 62(2):289–291
Gallmetzer P, Leutmezer F, Serles W, Assem-Hilger E, Spatt J, Baumgartner C (2004) Postictal paresis in focal epilepsies—incidence, duration, and causes: a video-EEG monitoring study. Neurology 62(12):2160–2164
Adam C, Rouleau I, Saint-Hilaire JM (2000) Postictal aphasia and paresis: a clinical and intracerebral EEG study. Can J Neurol Sci 27(1):49–54
Parmar H, Lim SH, Tan NC, Lim CC (2006) Acute symptomatic seizures and hippocampus damage: DWI and MRS findings. Neurology 66(11):1732–1735
Lazeyras F, Blanke O, Zimine I, Delavelle J, Perrig SH, Seeck M (2000) MRI, (1)H-MRS, and functional MRI during and after prolonged nonconvulsive seizure activity. Neurology 55(11):1677–1682
El-Koussy M, Mathis J, Lovblad KO, Stepper F, Kiefer C, Schroth G (2002) Focal status epilepticus: follow-up by perfusion- and diffusion MRI. Eur Radiol 12(3):568–574
Kim JA, Chung JI, Yoon PH, Kim DI, Chung TS, Kim EJ, Jeong EK (2001) Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am J Neuroradiol 22(6):1149–1160
Oh JB, Lee SK, Kim KK, Song IC, Chang KH (2004) Role of immediate postictal diffusion-weighted MRI in localizing epileptogenic foci of mesial temporal lobe epilepsy and non-lesional neocortical epilepsy. Seizure 13(7):509–516
Salih MA, Kabiraj M, Al-Jarallah AS, El Desouki M, Othman S, Palkar VA (1997) Hemiconvulsion-hemiplegia-epilepsy syndrome. A clinical, electroencephalographic and neuroradiological study. Childs Nerv Syst 13(5):257–263
Higuchi T, Inaba Y, Hata Y, Seki C, Ichikawa M (1998) MRI, SPECT and MRS findings in a case of acute hemiplegia syndrome with a marked hemispheric brain edema. No To Hattatsu 30(5):403–409
Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini A, Van Billoen H, Dupont P (2006) Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage 32(2):684–695
Helmchen C, Steinhoff BJ, Dichgans M (1994) Variants of Todd’s paralysis: postictal apraxia and prolonged postictal hemineglect. Nervenarzt 65(10):700–703
Bansal SK, Chopra JS (1991) Reversible postictal ataxic hemiparesis. Ital J Neurol Sci 12(1):75–79
Salmon JH (1968) Transient postictal hemianopsia. Arch Ophthalmol 79(5):523–525
Kosnik E, Paulson GW, Laguna JF (1976) Postictal blindness. Neurology 26(3):248–250
Leutmezer F, Podreka I, Asenbaum S, Pietrzyk U, Lucht H, Back C, et al (2003) Postictal psychosis in temporal lobe epilepsy. Epilepsia 44(4):582–590
Zeman AZ, Boniface SJ, Hodges JR (1998) Transient epileptic amnesia: a description of the clinical and neuropsychological features in 10 cases and a review of the literature. J Neurol Neurosurg Psychiatry 64(4):435–443
Tassinari CA, Ciarmatori C, Alesi C, Cardinaletti L, Salvi F, Rubboli G et al (1991) Transient global amnesia as a postictal state from recurrent partial seizures. Epilepsia 32(6):882–885
Helmstaedter C, Elger CE, Lendt M (1994) Postictal courses of cognitive deficits in focal epilepsies. Epilepsia 35(5):1073–1078
Maheu G, Adam C, Hazemann P, Baulac M, Samson S (2004) A case of postictal transient anterograde and retrograde amnesia. Epilepsia 45(11):1459–1460
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathews, M.S., Smith, W.S., Wintermark, M. et al. Local cortical hypoperfusion imaged with CT perfusion during postictal Todd’s paresis. Neuroradiology 50, 397–401 (2008). https://doi.org/10.1007/s00234-008-0362-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-008-0362-1