Abstract
Introduction
We report here our experience in treating high-flow arteriovenous fistulas (AVFs) of the brain and spine using balloon-assisted glue injection.
Methods
During a 3-year period (2003–2005) five patients with high-flow AVFs were treated at our hospital using transarterial balloon-assisted glue injection. There were two pial AVFs, one dural AVF, one vein of Galen malformation and one perimedullary AVF of the cervical spine. All patients were clinically followed-up for 12–48 months.
Results
Immediate angiographic obliteration was achieved in all patients. The fistulas remained closed in all patients, as ascertained by follow up-angiograms. No new neurological deficits related to the procedure were detected. Clinically, one patient with severe pre-treatment neurological deficit experienced excellent recovery.
Conclusion
Transarterial balloon-assisted glue embolization of high-flow AVFs is a feasible and efficient treatment. This technique affords more control in the glue injection and minimizes the risk of distal embolization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-flow arteriovenous fistulas (AVFs) involving the arteries and veins of the brain and spine are commonly treated using endovascular approach with a variety of embolic materials [1–4]. Although endovascular techniques involving the use of different agents (balloons, coils, glue, among others) have been reported as being successful in treating high-flow fistulas, endovascular treatment is not always successful and safe [5]. For a variety of reasons, it can be technically difficult to deliver the coils, balloons or glue precisely to the site of the fistula.
We report here our experience in treating three high-flow cerebral (two pial and one dural) AVFs, a vein of Galen malformation and a spinal perimedullary AVF of the cervical spine using transarterial balloon-assisted glue embolization.
Methods
Between 2003 and 2005, we treated five patients with high-flow AVFs – two pial, one dural, one vein of Galen and one perimedullary AVF of the cervical spine – endovascularly using the balloon-assisted glue injection technique.
Clinical data on the patients are summarized in Table 1. The patient cohort consists of three males and two females, aged from 7 to 32 years. Their clinical manifestations were headache (n = 2), seizures (n = 2) and tetraparesis (n = 1). Two patients had pial AVF, one patient had a dural AVF, one patient had a vein of Galen malformation and one patient had a perimedullary AVF (Type IVc) of the cervical spine.
The decision to used this technique was based on the angioarchitecture and the hemodynamics of the lesions. All lesions were fistulous-type, single-channel, high-flow lesions, with associated venous varices that could not be treated with glue injection without flow control.
All procedures were performed using the femoral approach, with the patient under general anesthesia. In two patients (patients 1, 5) both femoral arteries were punctured. A 6F (patient 2) or an 8F guiding catheter was positioned in the parent artery (patients 3, 4). In one patient (patient 1) with a cervical spine perimedullary AVF, a 6F guiding catheter was introduced into the right thyrocervical trunk and a 5F angiographic catheter into the left thyrocervical trunk to achieve better angiographic control. In another patient (patient 5) with a vein of Galen malformation a 6F and an 8F guiding catheters were positioned in the left vertebral and left internal carotid artery respectively. Heparin was administered in the flushing solutions (12,500 IU/L). Bolus infusions of heparin were not administered. Activated clotting time measurements were not performed.
A 1.5F microcatheter (Spinnaker Elite; Boston Scientific/Target, Natick, MA) was navigated into the parent artery and the tip placed at the fistula site. A remodeling balloon (Hyperform, 4 × 7, ev3) was placed in the parent artery and inflated in order to provide flow control. In patient 1 (spinal AVF) the balloon was placed into the thyrocervical trunk where the two feeders from both thyrocervical trunks converged; in patient 2 (dural AVF) it was placed into the internal maxillary artery (Fig. 1); in patients 3 and 4 (pial AVFs), it was placed into the inferior trunk of the middle cerebral artery (Fig. 2). In patient 5 (vein of Galen AVF) we positioned two balloons – one into the internal carotid artery and one into the posterior cerebral artery. Advancement of the catheters was supported with a 0.010-inch guidewire (X-pedion; Micro Therapeutics, Irvine, CA).
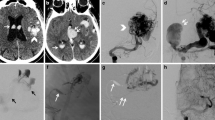
a, b. Right (a) and left (b) external carotid artery (ECA) angiograms, lateral projection, demonstrate a dural arteriovenous fistula (DAVF) supplied from branches of the left external carotid artery (LECA) and from the contralateral middle meningeal artery (MMA). c Superselective injection of the (R) MMA feeder (lateral view) draining directly into an extremely enlarged superficial vein. d The balloon was placed and inflated at the origin of the (right) MMA (arrow) after superselective catheterization of the fistula. e The cast of glue is demonstrated (arrow). The balloon remained inflated to allow polymerization of the glue mixture. f, g Post-embolization right and left ECA angiograms (lateral view), respectively, demonstrate complete occlusion of the fistula. h, i. T2-weighted axial magnetic resonance (MR) images pre- (h) and 6 months post-embolization (i). The enlarged draining vein is not demonstrated in the follow-up MR images
a, b. Towne (a) and lateral (b) view of left internal carotid artery injection demonstrating the left sylvian fissure AVF and associated venous varix. c Superselective injection after superselective catheterization of the fistula and with the balloon inflated in the left middle cerebral artery. d The cast of glue is shown. e, f. Post-embolization Towne (e) and lateral (f) left internal carotid artery injection show complete occlusion of the fistula. g, h Pre- (g) and 6-months post-embolization MR images: coronal FLAIR images. The venous varix is not demonstrated in the post-embolization images
An angiogram through the microcatheter was performed with the balloon inflated to evaluate the flow and to prepare the appropriate mixture of glue (a mixture of 70–100% glue GLUBRAN 2/Lipiodol). The microcatheter withdrawn after the glue had been injected, and the balloon was left inflated for a few seconds to allow adequate polymerization of the glue and to prevent distal embolization.
Results
The results and follow-up findings are shown in Table 1. Complete angiographic obliteration was documented on immediate post-embolization angiograms in four of the five patients. In patient 1, the control angiogram after the first injection showed that the fistula remained patent, although flow was significantly reduced. Catheterization of the anterior spinal artery was then performed and a second injection of glue (without balloon) followed. Complete occlusion of the spinal perimedullary arteriovenous fistula was then achieved.
The results remained stable in all patients, as visualized on follow-up angiograms 6 months after treatment. Follow-up MR imaging, 6 months post-embolization, was also performed in all patients. In one patient (patient 1) a residual thrombosed venous sac was detected on the MR image of the cervical spine.
No procedure-related neurological complications were observed.
One patient (patient 1) showed almost complete resolution of his symptoms within 1 year post-treatment. Two patients (patients 3 and 5) with seizures are now free of symptoms and the antiepileptic drugs have been discontinued. Patient 5 showed significant improvement of the symptoms related to hydrocephalus.
Discussion
Arteriovenous fistulas (cerebral or spinal, pial or dural) are characterized by a direct communication between one or more arterial feeders into a single draining vein without an intervening tangle of vessels (nidus) [5]. This angioarchitecture creates conditions for a rapid high flow. The pathological aspect of AVFs arises principally from their high-flow nature.
These lesions are treated differently than arteriovenous malformations (AVMs). In AVMs, occlusion of just the feeding arteries in not an adequate treatment as it leaves the nidus intact to recruit new arterial feeders. In AVFs, however, disconnection of the AV communication obliterates the fistula and any associated venous varices [5].
Endovascular management of high-flow AVFs is not always successful or safe [5, 6]. Endovascular occlusion of a high flow AVF is not always precise enough to avoid distal embolization into the draining vein. Unintentional distal embolization of embolic material migrating into the venous system with a rapid increase in fistula pressure or pulmonary embolization may have disastrous consequences when the fistula remains patent. In some cases, navigation through the feeding arteries is difficult and if embolization is too proximal, the fistula is only partially obliterated.
In their review of the literature on AVFs published between 1970 and 2000, Hoh [5] et al. found 79 patients, not including nine from their own study, who were treated for pial AVFs (total: 88). Endovascular treatment had been attempted in 50 of these patients (either as a solitary treatment, or before or after surgical treatment); however, in 20 (40%) patients it had failed or the fistulas had to be recanalized, necessitating further treatment. Of the 51 patients who underwent surgery, the attempt failed in only four patients (7.8%). The authors stated that as endovascular technologies advance rapidly, they expect higher success rates with the endovascular approach.
The most common embolic agents employed for the treatment of high-flow AVFs are balloons and coils although liquid embolic material (glue) has also been used occasionally. Other non-adhesive embolic agents (Onyx) are not recommended for fistulous-type lesions [7]. The disadvantage of balloons is that they cannot be guided through tortuous vessels into the position where the fistula is occluded. There are also several reports of the detachable balloon migrating into a varix, into the lung or, most dangerously, into a draining vein [8]. The introduction of the Guglielmi detachable coils (GDCs) has evoked great interest in their use for embolization of high-flow AVFs [2–4, 9]. Detachable coils are useful because they can be repositioned until a stable and safe position is obtained and, therefore, distal embolization can be eliminated or minimized. In addition, they can act as a template for the deposition of other embolic materials, such as glue. However, GDCs are not without disadvantages. They have less space-filling capacity than balloons, which may result in incomplete occlusion, and they are very expensive compared with detachable balloons and glue. Nesbit et al. [3] reported 12 patients with AVFs who were treated with GDCs – six via the transvenous route and six via the transarterial transfistulous route. All patients experienced immediate angiographic obliteration. In 11 of the 12 patients the fistula was shown to have remained closed on the follow-up scan. Repositioning of the coil was required because of migration or proximal herniation of the coil. The authors stated that with the advent of GDCs, the ability of reposition or removal of the coil provides better control and reduces the risks of endovascular treatment. Other descriptions of the use of GDCs have been reported in the treatment of intracranial high-flow pial, dural AVFs and traumatic carotid cavernous fistulas (CCFs). Hoh et al. [5] reported nine patients with pial single channel AVFs who were treated surgically or endovascularly. Treatment was surgical in six patients, endovascular in two and a combined surgical and endovascular in one patient. Embolization of the AVF in the two patients was performed using GDCs. Nevertheless, in the setting of high-flow AVFs, stabilization of the embolic material can be challenging, even when using detachable coils. Weil et al. [10] reported a more sophisticated endovascular approach for the treatment of high-flow AVFs: this group used the Trispan device to control coil deposition and to prevent coil migration during transvenous occlusion of high-flow AVFs.
Glue has the advantage of being relatively easy to deliver, having good penetration and producing immediate thrombosis and permanent occlusion of the fistula. Glue is also less expensive than coils. There are two major disadvantages of glue when used as an embolic agent for the treatment of high-flow AVFs. First, highly concentrated glue polymerizes rapidly and tends to stick to the microcatheter. The second drawback is the difficulty in controlling the flow and polymerization of glue and its cast formation.
There are reports of the successful embolization of a high-flow arteriovenous fistula with glue by using arterial hypotension in order to avoid distal embolization [11]. In 1979, Kerber et al. [12] reported their technique of using a calibrated-balloon microcatheter and the clinical results of using this approach in 25 patients with arteriovenous malformation. Inflation of the balloon allowed navigation of the catheter and flow control during deposition of synthetic glue. Jayaraman et al. [13] reported one case of traumatic cervical arteriovenous fistula, successfully obliterated with glue injection after flow arrest which was achieved with a balloon occlusion catheter.
In our series of patients, the flow control which was achieved with the inflation of a balloon in the main feeder enabled us to control the injection and thereby avoid distal embolization of the embolic material.
References
Duckwiler G (1992) Dural arteriovenous fistula. Neuroimaging Clin North Am 2:291–307
Guiglielmi G, Vinuela F, Duckwiler G, Dion J, Stocker A (1995) High-flow, small-hole arteriovenous fistulas: treatment with electrodetachable coils. Am J Neuroradiol 16:325–328
Nesbit GM, Barnwell SL (1998) The use of electrolytically detachable coils in treating high-flow arteriovenous fistulas. Am J Neuroradiol 19:1565–1569
Luo C-B, Teng MMH, Chang F-C, Chang C-Y (2006) Endovascular treatment of intracranial high-flow arteriovenous fistulas by Guglielmi detachable coils. J Chin Med Assoc 69:80–85
Hoh BL, Putman CM, Budzik RF, Ogilvy CS (2001) Surgical and endovascular disconnection of intracranial arteriovenous fistulae. Neurosurgery 49:1351–1364
Meyer FB, Grady RE, Abel MD, Nichols DA, Caminha SS, RobbB RA, Bates LM (1997) Resection of a large temporooccipital parenchymal arteriovenous fistula by using deep hypothermic circulatory bypass. J Neurosurg 87:934–939
Mounayer C, Hammami N, Piotin M, Spelle L, Benndorf G, Kessler I, Moret J (2007) Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. Am J Neuroradiol 28:518–523
Vinuela F, Drake CG, Fox AJ, Pelz DM (1987) Giant intracranial varices secondary to high-flow arteriovenous fistulae. J Neurosurg 66:198–203
Yoshimura S, Hashimoto N, Kazekawa K, Nichi S, Sampei K (1995) Embolization of dural arteriovenous fistulas with interlocking detachable coils. Am J Neuroradiol 16:322–324
Weil A, Roy D, Georganos SA, Guibert F, Raymond J (2002) Use of Trispan device to assist coil embolization of high-flow arteriovenous fistulas. Am J Neuroradiol 23:1149–1152
Hermier M, Turjman F, Bozio A, Duquesnel J, Lapras C (1995) Endovascular treatment of an infantile nongalenic cerebral arteriovenous fistula with cyanoacrylate. Child’s Nerv Syst 11:494–498
Kerber CW, Bank WO, Cromwell LD (1979) Calibrated leak balloon microcatheter: a device for arterial exploration and occlusive therapy. AJR Am J Roentgenol 132:207–212
Jayaraman MV, Do HM, Marks MP (2007) Treatment of Traumatic Cervical Arteriovenous Fistulas with N-Butyl-2-Cyanoacrylate. Am J Neuroradiol 28:352–354
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreou, A., Ioannidis, I. & Nasis, N. Transarterial balloon-assisted glue embolization of high-flow arteriovenous fistulas. Neuroradiology 50, 267–272 (2008). https://doi.org/10.1007/s00234-007-0322-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-007-0322-1