Abstract
Inflammatory stenoses of cerebral blood vessels, although rare in general, are an important cause of cerebral ischemia in younger patients. The diagnosis is often difficult. The first step in the diagnostic process is the identification of brain lesions consistent with cerebral vasculitis. Brain lesions are frequently found in this patient group, especially if modern imaging tools such as diffusion and perfusion-weighted imaging are employed. Although no specific pattern for this entity exists, multiple infarcts of various ages in more than one vascular territory should raise this suspicion. The next step in the imaging of patients with suspected vasculitis is the demonstration of the underlying vascular pathology. MR angiography is the mainstay of investigating patients for intracranial vascular stenoses. However, at 1.5 T it is only diagnostic for stenoses of large brain arteries. Hence, conventional angiography is still required to investigate stenoses of medium and small-sized brain arteries. Recent work suggests that MRI can directly demonstrate mural thickening and contrast enhancement in basal brain arteries, rendering biopsy obsolete in this patient group. A classification for cerebral vasculitis is proposed according to the size of the affected brain vessels, analogous to the pertinent nomenclature of primary systemic vasculitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute and chronic inflammatory diseases of the central nervous system (CNS) frequently cause severe neurological deficits or death. The infectious or autoimmunological attack may be directed against various targets such as brain linings, oligodendrocytes, neurons or blood vessels. These changes occur in the course of generalized disorders or are restricted to the CNS. Early diagnosis and differential diagnosis is important as treatment depends on the etiology of the inflammatory disorder and may greatly improve the clinical course. Whereas in cerebral manifestations of systemic disorders the diagnosis usually depends on the identification of the underlying disease by blood tests or body biopsies, the diagnosis of an isolated CNS manifestation is far more demanding [1]. Specific signs are frequently absent in blood samples. The diagnosis often requires invasive tests to gain access to CNS tissue, its linings, vessels or the CSF [2]. Apart from lumbar puncture, these procedures carry a significant risk of complications [3].
Although not regarded as a definitive diagnostic procedure to prove a particular type of inflammatory CNS disorder, MRI has become the most sensitive noninvasive test for the majority of neuroimmunological and infectious disorders. It is now the backbone of the diagnosis of multiple sclerosis, the most frequent inflammatory CNS disease and imaging signs have been established in numerous reports on thousands of patients.
Less is known about MRI findings in patients with inflammatory disorders of the cerebral blood vessels. MRI of the brain parenchyma has been shown to be useful in these patients [4] but often lacks specificity in distinguishing vascular from parenchymal inflammation. However, recent work seems to suggest that in a group of patients with vasculitis, MRI may directly demonstrate vessel wall inflammation, probably with high diagnostic accuracy.
Hence it may be useful to divide the imaging signs of cerebral vasculitis along new lines:
-
Indirect imaging signs of cerebral vasculitis:
-
Cerebral perfusion deficits
-
Ischemic brain lesions
-
Intracerebral or subarachnoid hemorrhage
-
Vascular stenoses unlikely to be atherosclerotic
-
-
Direct imaging signs of cerebral vasculitis:
-
Vessel wall thickening with contrast enhancement
-
Imaging in patients with suspected vasculitis
Indirect imaging signs of cerebral vasculitis
Although reliable epidemiological data on the incidence of cerebral vasculitis are lacking, brain ischemia appears to be far more frequent than vasculitic intracerebral or subarachnoid [5] hemorrhage. MRI is a very sensitive method for the investigation of cerebral ischemia. In our experience, MRI frequently shows ischemic brain lesions even in patients with reversible symptoms.
Multiple infarcts of different ages are thought to be suggestive of cerebral vasculitis, especially if the lesions are located in various vascular territories and do not have a typical embolic pattern. Obviously, the patient’s age and underlying risk factors do have a significant impact on the interpretation of the images. Although a cerebral vasculitis may occur in all age groups, its percentage among patients with ischemic stroke is much higher in young patients and children than in the elderly.
Routine imaging for cerebral ischemia includes T2- and diffusion-weighted sequences [6] in most institutions (Figs. 1a,b and 2a,b). The sensitivity of cerebral MRI can be further increased, if in young patients with reversible neurological symptoms and without other pathology, such as multiple sclerosis, the brain scan is supplemented by a perfusion study (Fig. 1c). For clinical purposes a dynamic susceptibility weighted technique using a contrast bolus is usually preferred. This study employing a T2*-weighted EPI sequence can be performed on most modern MRI scanners.
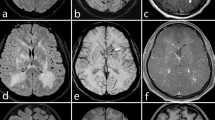
Large vessel vasculitis, presumed PACNS; left hemispheric TIAs with hemiparesis and aphasia. a Normal T2-weighted FLAIR image. b Normal DWI image. c The perfusion-weighted MRI, here the time to peak map, shows hypoperfusion of the left hemisphere in the MCA territory. The basal ganglia are affected. d The TOF-MRA shows an abnormality of the left MCA in its M1 segment, but also some narrowing in the distal ICA and A1 segment around the carotid T. e The unenhanced high-resolution axial T1-weighted image (slice thickness 3 mm, flow compensation) shows wall thickening of the left M1 segment, possibly also some abnormality of the right distal ICA. f The contrast-enhanced T1-weighted image (0.1 mmol/kg Gd-DTPA) with the same sequence parameters shows some contrast enhancement in the wall of the left M1 segment. The vein of Rosenthal (arrow) can clearly be separated from the artery on this high resolution image. g The coronal T1-weighted image through the carotid T several minutes later shows the vessel wall enhancement on the left even more clearly. The changes are also present on the right carotid T and the left distal ICA (arrow). h The carotid angiogram shows tapering of the left ICA and a severe but smooth stenosis of the MCA. The changes of the left A1 segment are better seen on the MRI
Large vessel vasculitis; AIDS and HSV infection; acute left hemispheric stroke with hemiplegia and aphasia; previous right hemispheric stroke. a The T2-weighed image shows a hyperintense lesion in the left MCA territory with minor mass effect. There are signs of previous ischemia in the right hemisphere. b The DWI image shows restricted diffusion in the left MCA territory, indicating cytotoxic edema caused by ischemia. c Even the 5-mm unenhanced T1-weighted image shows abnormal walls of the intracranial vessels bilaterally, although more pronounced on the left. d The contrast-enhanced T1-weighted image shows massive enhancement on the left, indicating acute inflammation with severe stenosis. The vessel wall enhancement is less severe on the right
If an ischemic brain lesion has been detected, the next step is the demonstration of the underlying vascular abnormality. Usually MR angiography (MRA) is added to the brain scan. Today, the routine protocol is mostly based on a large field-of-view contrast-enhanced MRA covering the entire course of the carotid and vertebral vessels as well as the circle of Willis. Whereas this is a good test for the detection of stenoses of the cervical vessels caused by dissection or atherosclerosis and also shows gross pathology of the large intracranial vessels, more subtle stenoses around the circle of Willis may escape detection due to the limited spatial and temporal resolution of this imaging technique. Hence, the addition of an intracranial time-of-flight (TOF) sequence is advisable because it offers high sensitivity for stenoses and a good spatial resolution [7, 8] (Fig. 1d). We prefer to acquire this intracranial TOF angiogram before contrast material is administered. It may be advisable to perform this sequence early during an examination if there is clinical suspicion of vasculitis or if the brain parenchymal lesions are suggestive of vascular inflammation. The increasing availability of 3-T MRI scanners may enhance the role of TOF MRA as it might extend diagnostic vascular imaging beyond the circle of Willis, a region usually not adequately visualized at 1.5 T.
The advances in noninvasive imaging technology have not replaced intraarterial angiography [9] (Fig. 3a). The evaluation of medium sized and small brain vessels still requires this invasive procedure, which not only features unsurpassed spatial but also temporal resolution [10]. This allows the evaluation of cerebral hemodynamics and the assessment of brain perfusion. Angiographic diagnosis of vasculitis is based on the demonstration of one or multiple stenoses of brain vessels. Further signs are abnormal straightening and kinking of arteries caused by vessel wall induration. Unfortunately, the sensitivity and specificity of digital subtraction angiography (DSA) are regarded as fairly low [9].
Medium vessel vasculitis classified as PACNS; multiple TIAs. a The cerebral angiogram shows the classical findings of cerebral vasculitis with stenoses of multiple medium-sized brain arteries. b The high resolution coronal contrast-enhanced T1-weighted MRI shows massive vessel wall enhancement (arrows) of the pericallosal artery and of arteries in the right sylvian fissure. There is also some minor involvement of the left carotid T. c This slice of the same sequence located more posteriorly shows massive contrast uptake in the enlarged walls of medium-sized brain arteries. There is a transverse cut though an artery in the right sylvian fissure and a longitudinal cut in the left sylvian fissure (arrows)
Inflammatory changes of very small brain arteries which are below the resolution limits of cerebral angiography will escape detection. These patients probably account for only a small proportion of falsely negative angiograms. The main inaccuracy in diagnosing cerebral vasculitis with angiography is caused by the difficulty in identifying vasculitic changes and in differentiating them from other causes of vascular stenoses such as atherosclerosis. Angiographic diagnosis is based on the rarity of intracranial atherosclerotic stenoses especially in young patients. It has been suggested that atherosclerotic stenoses are mainly located at vascular branching points whereas vasculitic lesions do not show this prevalence. However, there remains a considerable degree of uncertainty in many angiographic diagnoses of cerebral vasculitis. It should always be remembered that the presumed gold standard of cerebral angiography is an indirect method, demonstrating the consequences of vessel wall thickening and stiffening. It does not differentiate the underlying cause [9]. Apart from multiple stenoses, there is a further if rather rare angiographic presentation of cerebral vasculitis. Inflammatory changes can present as multiple microaneurysms [11]. This appearance seems to be rather specific for vasculitis.
Although cerebral angiography is still an indispensable tool for the diagnosis of vasculitis, its use has declined with the improvement of MRI and MRA. A significant incidence of ischemic events has been reported in association with angiography in patients with vasculitis [12]. The rate of ischemic stroke was regarded as higher than in angiographic studies for other indications and a premedication with steroids was suggested as a precaution. However, given the relative rarity of these events and the heterogeneity of the patient group regarding age and underlying medical conditions, this is far from certain. In any case, a diagnostic angiogram for suspected vasculitis should be performed by an experienced radiologist and must include high-resolution images of the medium-sized and small brain arteries. As the intravascular contrast agent itself may cause worsening of the vasculitic stenoses, the number of injections should be strictly limited.
Direct imaging signs of cerebral vasculitis
Diagnostic accuracy would be much enhanced if vessel wall inflammation could be imaged directly. In our experience, high-resolution MRI of the skull base can visualize the thickening of arterial walls and demonstrate contrast enhancement in the affected vessels (Figs. 1e–g, 2c,d and 3b,c). Even in small field-of-view thin-section slices (3 mm or less) the walls of intracranial cerebral arteries are invisible or very thin on T1-weighted images. The demonstration of vessel wall thickening is therefore usually abnormal, especially in an area of flow alteration in MRA.
As the volume coverage of high-resolution imaging is usually small in a clinical setting due to scan time limitations and patient movement, high-resolution scans are usually performed in areas of angiographic abnormality. In order to optimize vessel wall visualization, high-resolution imaging should be performed perpendicular to the affected vessel segment. Although it would be helpful to have a longitudinal view of the vessel, the tortuous course of most brain arteries makes it difficult to achieve this. Cerebral vessels with a lumen of more than 2 mm can usually be evaluated in straight segments at 1.5 T. Arteries meeting these criteria are the distal ICAs as well as the M1 and A1 segments. In the posterior fossa, the vertebral arteries and the basilar artery, sometimes even the P1 segment can be adequately imaged. The vessel lumen is indicated by the flow void. A sequence intended to differentiate thickened vessel walls and central flow void has to be free of inflow effects. The application of a saturation pulse or other means of flow compensation may be mandatory.
Contrast enhancement is frequently seen in the wall of acutely inflamed arteries. The degree of enhancement is variable. Occasionally the enhancement is so excessive that it may extend into adjacent leptomeningeal tissue (Figs. 2d and 3b,c). Usually it is restricted to the vessel wall itself. Problems may arise from adjacent veins and flow effects within the arteries mimicking wall thickening. We recommend the application of flow saturation techniques. To avoid confusion with veins, high-resolution imaging (slice thickness 3 mm or less) is necessary. For a reliable diagnosis, vascular thickening should be demonstrated in two planes. This is usually possible if the disease is located around the carotid T. Images before and after administration of contrast material with identical slice positions can be very helpful in identifying the abnormal vessel wall enhancement (Fig. 2c,d). Due to low signal of adjacent soft tissues and long repetition times minimizing inflow effects, fat-suppressed T1-weighted images seem to be particularly sensitive for vessel wall enhancement. The high sensitivity usually offsets the long acquisition time of this sequence.
Hence, high-resolution T1-weighted images including a fat-suppressed sequence should be performed in patients with suspected cerebral vasculitis to identify vessel wall contrast enhancement. This seems to be the sole direct imaging sign of vessel wall inflammation.
Classification of cerebral vasculitis
Vasculitis can be classified along different lines. The most relevant classification schemes use the size of the affected arteries and the etiology of the wall inflammation [13] (Fig. 4). Obviously imaging can only be used to locate the stenoses and identify vessel wall inflammation as the underlying cause. It therefore supports a classification by arterial size. The etiology has to be established by other methods, such as laboratory studies or biopsy. However, classification by vessel size is an important step in this process as it significantly narrows the range of possible differential diagnoses [14, 15].
Classification of primary vasculitis (ANCA antineutrophil cytoplasmic antibodies, H-S purpura Henoch-Schönlein purpura, MCLN syndrome mucocutaneous lymph node syndrome,SLE systemic lupus erythematosus). From reference [13]
Classifications according to vessel size as proposed by the Chapel Hill nomenclature [15] and by the American College of Rheumatologists classification [14] were developed for systemic disease. Hence, they need to be modified if applied for intracranial vasculitis. Furthermore, in contrast to systemic arteritis, infectious diseases account for a significant and clinically relevant proportion of intracranial stenoses.
Hence, in the present paper we use a different classification of vasculitis than the classifications mentioned before, which regard all intracranial vessels as medium-sized and small vessels.
Large brain vessel vasculitis
Large cerebral vessel vasculitis is defined as affecting the ICA, the M1 and A1 segments, the intracranial vertebral artery, the basilar artery and the P1 segment. These arteries would be considered medium-sized if the Chapel Hill classification were applied [15].
Brain parenchymal changes are present in most patients with a cerebral vasculitis on MRI, regardless of the type of vessels affected. However, the sensitivity of vascular imaging depends on the size of the affected vessels. Large vessel disease is best demonstrated with MRA and MRI (Fig. 1). Vasculitic changes in these patients are commonly found around the carotid T, uni- or bilaterally. This form of vasculitis is frequently encountered in children and young adults. An association with the varicella-zoster virus has been recognized (varicella angiopathy) [16]. However, in many instances it is difficult to establish the etiology beyond doubt as vascular changes occur some time after the varicella infection and the virus remains dormant in the human body. Hence, patients with vasculitis but without established cause have been classified as primary angiitis of the CNS (PACNS) [17, 18] or childhood primary angiitis of the CNS (cPACNS) [4]. Patients with proven varicella-zoster vasculitis fairly uniformly present with large vessel vasculitis, often mainly affecting the M1 segment with ensuing basal ganglia infarcts. As most patients classified as PACNS or cPACNS have identical imaging findings to those with proven zoster vasculopathy, it may be speculated that a significant percentage of PACNS is caused by an undiagnosed zoster infection. Identification of zoster vasculopathy may have a therapeutic and prognostic implication. However, large vessel vasculitis may also be caused by other DNA viruses from the herpes group, such as herpes simplex type I and cytomegaly. The inflammatory response appears to be particularly strong in patients with AIDS [19] (Fig. 2). Other causes of large vessel vasculitis are sarcoid [20], giant cell arteritis, systemic sclerosis and other rheumatological disorders (own unpublished data). Large brain vessels may also be affected in the course of bacterial meningitis such as pneumoccocal [21] and meningococcal disease, meningeal tuberculosis, and Treponema pallidum [22] and Borrelia burgdorferi [23] infections. Moyamoya syndrome is a disorder of unknown, probably inflammatory etiology, causing slowly progressive stenoses of the carotid T and later the basilar artery. Although possibly inflammatory in origin, no imaging signs of wall inflammation have been reported so far.
As mentioned before, large vessel vasculitis is the imaging domain of MRI. This is particularly relevant as the large basal cerebral vessels cannot be biopsied. Furthermore, given the fairly large diameter of these vessels, a moderate degree of wall thickening may not be obvious on conventional angiography.
Medium vessel vasculitis
Medium vessel vasculitis affects the brain arteries distal to the MCA bifurcation and the communicating arteries. These vessels are much longer than the large brain arteries and wall thickening has a proportionally greater effect on the residual lumen. Due to their tortuous course and small diameter, these vessels are usually very difficult to image with MRA or MRI at 1.5 T.
Hence, a vasculitic process affecting these but sparing large brain vessels cannot be identified with MRI. Vasculitis of medium-sized brain arteries remains an indication for conventional cerebral angiography, preferably before significant quantities of steroids have been given (Fig. 3).
A wide spectrum of immunological and infectious diseases have been reported as possible causes of medium vessel arteritis. It appears to be more prevalent in older patients and is not or is only rarely associated with a varicella-zoster infection. Medium brain vessel involvement has been reported with panarteritis nodosa [24], lupus erythematosus [25, 26], Behçet’s disease [26], Crohn’s disease [27] and the Sneddon’s syndrome [28].
Small vessel vasculitis
Small vessel vasculitis affects arteries beyond the spatial resolution of conventional cerebral angiography. Brain parenchymal changes may be extensive but vascular imaging is usually normal. Leukocytoclastic vasculitis may occur in the course of systemic disorders such as Sjögren’s syndrome (own observation) but also without identified rheumatological disorder [29]. If the etiology cannot be established by other means, brain or leptomeningeal biopsy may be indicated, preferably before the onset of treatment. This type of vasculitis is thought to be rare [29].
Spinal cord vasculitis
Spinal cord signal changes and dysfunction are seen in the course of various vasculitic and rheumatological disorders. It is often difficult to differentiate an ischemic from an inflammatory etiology, caused by direct immunological damage to the neural tissue [30]. Spinal cord involvement has been reported in PACNS [31] Because the differential diagnosis of spinal cord lesions is even more difficult than in cerebral abnormalities, a brain MRI should be performed in patients with spinal cord pathology.
Biopsy
Although histological proof of vasculitis is still regarded as the gold standard, it is often negative or inconclusive [32, 33, 3]. Given the significant risk of brain or leptomeningeal tissue sampling, all efforts are made to identify the location with the highest diagnostic yield. Usually, contrast-enhanced images are acquired to search for leptomeningeal or parenchymal abnormalities thought to be inflammatory. However, there is no evidence that this does increase diagnostic accuracy compared to random sampling in a safe location such as the right frontal lobe. A biopsy of the large brain arteries is not possible. Histological confirmation has to be obtained from small arteries or branches of the external carotid artery.
Treatment
Although treatment of cerebral vasculitis is mainly by antiinflammatory or immunosuppressive medication [34], angioplasty of large artery stenoses [20] may be an emerging option which needs to be further investigated. Obviously, this option is only available if large vessels are affected.
Treatment control is an important role for imaging, especially for the noninvasive methods. Apart from repeat MRA to asses the degree of stenoses, the degree of vessel wall contrast enhancement may prove to be an indicator of disease activity. More work is required to address this problem.
Conclusion
MRI and MRA are valuable tools for the diagnosis of cerebral vasculitis, especially if supplemented by multiplanar, high-resolution contrast-enhanced T1-weighted images through the affected arteries.
In vasculitic disease of the large brain arteries, the demonstration of contrast enhancement in the wall of stenotic vessels may be the most sensitive test for an inflammatory disease, even exceeding that of biopsy. In vasculitis of medium sized brain vessels, MRA at 1.5 T is not sufficient to reliably diagnose vascular stenoses as vessel wall contrast enhancement is mostly invisible. However, MRI including diffusion-weighted sequences shows ischemic brain lesions in nearly all symptomatic patients. Intraarterial DSA is still required to prove vasculitic changes of medium sized arteries and should be performed in young patients with ischemic brain disease not explained otherwise [8]. At present, there is no reliable imaging test to diagnose small vessel vasculitis such as leukocytoclastic vasculitis without brain biopsy.
Hence, the present data support a staged imaging concept for patients suspected of having cerebral vasculitis. Initial imaging should be performed by MRI and MRA with high-resolution T1-weighted images after contrast material administration through areas of vascular stenoses detected on TOF-MRA. If MRI is not diagnostic, cerebral angiography should be performed to search for medium brain artery inflammatory changes [8]. Brain biopsy is still useful in patients with small vessel vasculitis [35] and occasionally for the further classification of vasculitic changes [36].
References
Scolding NJ, Wilson H, Hohlfeld R, Polman C, Leite I, Gilhus N (2002) The recognition, diagnosis and management of cerebral vasculitis: a European survey. Eur J Neurol 9:343–347
Stone JH, Pomper MG, Roubenoff R, Miller TJ, Hellmann DB (1994) Sensitivities of noninvasive tests for central nervous system vasculitis: a comparison of lumbar puncture, computed tomography, and magnetic resonance imaging. J Rheumatol 21:1277–1282
Alrawi A, Trobe JD, Blaivas M, Musch DC (1999) Brain biopsy in primary angiitis of the central nervous system. Neurology 53:858–860
Aviv RI, Benseler SM, Silverman ED, Tyrrell PN, Deveber G, Tsang LM, Armstrong D (2006) MR imaging and angiography of primary CNS vasculitis of childhood. AJNR Am J Neuroradiol 27:192–199
Spitzer C, Mull M, Rohde V, Kosinski CM (2005) Non-traumatic cortical subarachnoid haemorrhage: diagnostic work-up and aetiological background. Neuroradiology 47:525–531
Moritani T, Hiwatashi A, Shrier DA, Wang HZ, Numaguchi Y, Westesson PL (2004) CNS vasculitis and vasculopathy: efficacy and usefulness of diffusion-weighted echoplanar MR imaging. Clin Imaging 28:261–270
Pomper MG, Miller TJ, Stone JH, Tidmore WC, Hellmann DB (1999) CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography. AJNR Am J Neuroradiol 20:75–85
Demaerel P, De Ruyter N, Maes F, Velghe B, Wilms G (2004) Magnetic resonance angiography in suspected cerebral vasculitis. Eur Radiol 14:1005–1012
Kadkhodayan Y, Alreshaid A, Moran CJ, Cross DT, 3rd, Powers WJ, Derdeyn CP (2004) Primary angiitis of the central nervous system at conventional angiography. Radiology 233:878–882
Greenan TJ, Grossman RI, Goldberg HI (1992) Cerebral vasculitis: MR imaging and angiographic correlation. Radiology 182:65–72
Nishikawa M, Sakamoto H, Katsuyama J, Hakuba A, Nishimura S (1998) Multiple appearing and vanishing aneurysms: primary angiitis of the central nervous system. Case report. J Neurosurg 88:133–137
Krings T, Willmes K, Becker R, Meister IG, Hans FJ, Reinges MH, Mull M, Thron A (2006) Silent microemboli related to diagnostic cerebral angiography: a matter of operator’s experience and patient’s disease. Neuroradiology 48:387–393
Jennette JC, Falk RJ (2007) Nosology of primary vasculitis. Curr Opin Rheumatol 19:10–16
Hunder GG, Arend WP, Bloch DA, Calabrese LH, Fauci AS, Fries JF, Leavitt RY, Lie JT, Lightfoot RW Jr, Masi AT, et al (1990) The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum 33:1065–1067
Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG, et al (1994) Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 37:187–192
Gilden DH, Mahalingam R, Cohrs RJ, Kleinschmidt-DeMasters BK, Forghani B (2002) The protean manifestations of varicella-zoster virus vasculopathy. J Neurovirol 8 [Suppl 2]:75–79
Calabrese LH, Furlan AJ, Gragg LA, Ropos TJ (1992) Primary angiitis of the central nervous system: diagnostic criteria and clinical approach. Cleve Clin J Med 59:293–306
Calabrese LH (2002) Diagnostic strategies in vasculitis affecting the central nervous system. Cleve Clin J Med 69 [Suppl 2]:SII105–SII108
Berkefeld J, Enzensberger W, Lanfermann H (2000) MRI in human immunodeficiency virus-associated cerebral vasculitis. Neuroradiology 42:526–528
Brisman JL, Hinduja A, McKinney JS, Gerhardstein B (2006) Successful emergent angioplasty of neurosarcoid vasculitis presenting with strokes. Surg Neurol 66:402-404
Jorens PG, Parizel PM, Demey HE, Smets K, Jadoul K, Verbeek MM, Wevers RA, Cras P (2005) Meningoencephalitis caused by Streptococcus pneumoniae: a diagnostic and therapeutic challenge. Diagnosis with diffusion-weighted MRI leading to treatment with corticosteroids. Neuroradiology 47:758–764
Gaa J, Weidauer S, Sitzer M, Lanfermann H, Zanella FE (2004) Cerebral vasculitis due to Treponema pallidum infection: MRI and MRA findings. Eur Radiol 14:746–747
Heinrich A, Khaw AV, Ahrens N, Kirsch M, Dressel A (2003) Cerebral vasculitis as the only manifestation of Borrelia burgdorferi infection in a 17-year-old patient with basal ganglia infarction. Eur Neurol 50:109–112
Valeyrie L, Bachot N, Roujeau JC, Authier J, Gherardi R, Hosseini H (2003) Neurological manifestations of polyarteritis nodosa associated with the antiphospholipid syndrome. Ann Med Interne (Paris) 154:479–482
Liem MD, Gzesh DJ, Flanders AE (1996) MRI and angiographic diagnosis of lupus cerebral vasculitis. Neuroradiology 38:134–136
Graham JW, Jan W (2003) MRI and the brain in systemic lupus erythematosus. Lupus 12:891–896
Schluter A, Krasnianski M, Krivokuca M, Spielmann RP, Neudecker S, Hirsch W (2004) Magnetic resonance angiography in a patient with Crohn’s disease associated cerebral vasculitis. Clin Neurol Neurosurg 106:110–113
Aquino Gondim Fde A, Leacock RO, Subrammanian TA, Cruz-Flores S (2003) Intracerebral hemorrhage associated with Sneddon’s syndrome: is ischemia-related angiogenesis the cause? Case report and review of the literature. Neuroradiology 45:368–372
Panchal NJ, Niku S, Imbesi SG (2005) Lymphocytic vasculitis mimicking aggressive multifocal cerebral neoplasm: MR imaging and MR spectroscopic appearance. AJNR Am J Neuroradiol 26:642–645
Hermisson M, Klein R, Schmidt F, Weller M, Kuker W (2002) Myelopathy in primary Sjogren’s syndrome: diagnostic and therapeutic aspects. Acta Neurol Scand 105:450–453
Campi A, Benndorf G, Filippi M, Reganati P, Martinelli V, Terreni MR (2001) Primary angiitis of the central nervous system: serial MRI of brain and spinal cord. Neuroradiology 43:599–607
Duna GF, Calabrese LH (1995) Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol 22:662–667
Chu CT, Gray L, Goldstein LB, Hulette CM (1998) Diagnosis of intracranial vasculitis: a multi-disciplinary approach. J Neuropathol Exp Neurol 57:30–38
Moore PM, Fauci AS (1981) Neurologic manifestations of systemic vasculitis. A retrospective and prospective study of the clinicopathologic features and responses to therapy in 25 patients. Am J Med 71:517–524
Benseler SM, deVeber G, Hawkins C, Schneider R, Tyrrell PN, Aviv RI, Armstrong D, Laxer RM, Silverman ED (2005) Angiography-negative primary central nervous system vasculitis in children: a newly recognized inflammatory central nervous system disease. Arthritis Rheum 52:2159–2167
Volcy M, Toro ME, Uribe CS, Toro G (2004) Primary angiitis of the central nervous system: report of five biopsy-confirmed cases from Colombia. J Neurol Sci 227:85–89
Conflict of interest statement
I declare that I have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Suggested diagnostic protocol for cerebral vasculitis
Suggested diagnostic protocol for cerebral vasculitis
Initial imaging: MRI
Mandatory
T2, T2* and DWI images of the whole brain. TOF-MRA of the large brain arteries and post-contrast T1-weighted images of the brain parenchyma. High-resolution post-contrast T1-weighted images (3 mm or less) of areas of abnormality on MRA. Vessel wall enhancement should be demonstrated in two planes. Axial sequences should be performed using fat suppression and flow compensation.
Optional
Perfusion-weighted imaging, CE-MRA.
Secondary imaging: cerebral angiography
Mandatory
Selective injections of both the internal carotid artery and at least one vertebral artery. Small field of view (25 cm or less), high spatial and temporal resolution (at least two frames per second) imaging of medium sized brain arteries in at least two planes.
Optional
A biplane angiography unit significantly reduces the amount of contrast material needed.
If imaging is inconclusive: brain or leptomeningeal biopsy.