Abstract
The purpose of this study was to determine the technical capacity and diagnostic accuracy of 3D time-of-flight magnetic resonance angiography (MRA) in suspected cerebral vasculitis in a retrospective analysis of MRA and digital subtraction angiography (DSA) in 14 young patients with clinical and/or radiological suspicion of cerebral vasculitis. A total of nine arteries were evaluated in each patient. Consensus review of DSA by three observers was the reference standard. The sensitivity for detecting a stenosis varied from 62 to 79% for MRA and from 76 to 94% for DSA, depending on the observer. The specificity for detecting a stenosis varied from 83 to 87% for MRA and from 83 to 97% for DSA. Using the criterion “more than two stenoses in at least two separate vascular distributions” to consider the examination as being true positive, the false-positive rates for MRA and DSA were comparable. MRA plays a role as the first angiographical examination in the diagnostic work-up of suspected cerebral vasculitis. When more than two stenoses in at least two separate vascular distributions are depicted on MRA, DSA is not expected to add a significant diagnostic contribution in a patient with suspected cerebral vasculitis. DSA remains necessary when MRA is normal or when less than three stenoses are seen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central nervous system vasculitides are a heterogeneous group of disorders that are characterized by inflammation of the blood vessel walls [1]. Cerebral vasculitis can be infectious or non-infectious. Some of the non-infectious vasculitides are due to immune complex deposition (e.g., systemic lupus erythematosus), while others are cell mediated (e.g., granulomatous angiitis, Wegener granulomatosis). There is also a poorly defined group of disorders (e.g., Behçet disease, drug-induced arteritis) that may present with cerebral vasculitis. In addition, intracranial atheromatosis and vasospasm can mimick the appearance of vasculitis on angiography.
The diagnosis can be extremely difficult and needs often to be based on careful evaluation of the clinical signs, radiological correlation and exclusion of other causes [2]. Brain MR imaging may remain normal in the setting of cerebral vasculitis, although abnormalities are detected in most patients with angiographical evidence of arterial stenosis. Brain biopsies yield high false-negative rates [3]. We studied 14 consecutive patients who underwent digital subtraction angiography (DSA) and magnetic resonance angiography (MRA) as part of the diagnostic work-up for suspected cerebral vasculitis.
DSA is the imaging modality of choice in assessing patients with suspected cerebral vasculitis, but the risk of a complication is present. Few data have been published on the efficacy of MRA in evaluating intracranial stenosis, and there has been no comparison between both angiographical techniques in patients with suspected cerebral vasculitis [4–7]. In this study we want to compare DSA and MRA in their ability to detect stenoses of intracranial arteries in young patients with suspected cerebral vasculitis in order to define the role of MRA.
Material and methods
We included 14 patients who had undergone cerebral DSA and 3D time-of-flight MRA to exclude vasculitis. The decision to perform an angiogram was based on the clinical suspicion of cerebral vasculitis and/or the brain MR finding in a young patient. There were four male and ten female patients ranging in age from 22 to 53 years with an average age of 38.2 years. All patients presented with acute neurological symptoms. Lumbar puncture showed abnormalities in four patients and inflammatory blood changes were observed in two other patients. Three patients had cardiovascular risk factors. Brain MR was normal in 3/14 patients. Both angiographical examinations were retrospectively reviewed separately and at random by one general radiologist (BV, reader 1) and two neuroradiologists (PD and GW, readers 2 and 3). After the assessment of the examinations in 14 patients, a consensus review of the DSA by the three observers was considered the reference standard for the statistical analysis.
All patients were imaged at 1.0 or 1.5 T (Siemens, Erlangen, Germany). A circularly polarized head coil was used. A single 3D axial volume acquisition was obtained with a repetition time (TR) of 40 and 35 ms, respectively, at 1.0 and 1.5 T. Echo-time (TE) was 10 and 7.2 ms, respectively. Additional parameters included a 200×512 matrix with a 200 mm FOV. Twenty-four partitions were obtained with a slab thickness of 36 mm and a slice thickness of 1.5 mm. These parameters yielded a pixel size of 0.75×0.39 mm2. The flip angle at the center of the volume was 20°. Maximum intensity projections were calculated every 15–18° in the axial and in the sagittal plane. DSA, which was performed on all patients using a Siemens Angiostar system with a 1,024×1,024 non-interpolated matrix, was considered the reference standard after consensus review by the three observers after they had evaluated MRA and DSA separately. Anteroposterior and lateral views (six images per view) of the right and left internal carotid artery and the left vertebral artery were obtained and were available for review. Both angiographical examinations were performed within a time span of 1–9 days.
The following nine arteries were assessed in each of the 14 patients: the left and right anterior (ACA), middle (MCA) and posterior (PCA) cerebral artery, cavernous portion of the left and right internal carotid artery (CAR) and the basilar artery (BAS). All together, 126 intracranial arteries were evaluated. These vessels were evaluated with a five-point scale: 1, normal; 2, probably normal; 3, indeterminate; 4, probable stenosis; 5, definitive stenosis.
To assess the accuracy of MRA for detecting a stenosis at each artery, estimates of sensitivity and specificity were computed. The specificity of MRA was computed as the proportion of cases assigned a score 1 or 2 over all arteries that were normal on DSA. Sensitivity was defined as the proportion of DSA-positive cases assigned a score of 3, 4 or 5 on MRA.
To assess the overall accuracy of MRA for detecting intracranial stenosis, the maximum likelihood estimate of the area under receiver operating characteristic (ROC) curve was computed for each reader by pooling the results over all vessels. Asymptotic 95% confidence intervals were constructed.
Inter-observer agreement was assessed with a weighted kappa-statistic. The following weights were applied: 1.0 if both readers agreed about the presence of stenosis (scores of 4 or 5), the absence of stenosis (scores of 1 or 2) or described the case as indeterminate; 0.0 if one reader indicated the presence of stenosis and the other indicated absence; 0.5 if only one of the readers described the case as indeterminate. Kappa values are interpreted as <0.40, poor agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.0, almost perfect agreement. The hypothesis that kappa does not differ from zero was tested; a significance level of 0.01 was applied.
Results
Fourteen consecutive patients with the clinical suspicion of vasculitis who were referred to the department for DSA were retrospectively studied. The clinical and radiological findings are summarized in Table 1. Eleven patients presented with focal neurological signs, one patient with relapsing headache, one patient with seizures and one patient with cognitive deterioration.
No final diagnosis could be established in three patients. The diagnosis “vasculitis” was put forward in two patients, while three patients were diagnosed as “vasculopathy.” Intracranial atheromatosis was the final diagnosis in three patients. One patient was diagnosed as crash migraine, one as possible Sneddon syndrome (and/or Borrelia vasculitis) and one as pseudomigraine with lymphocytic CSF pleocytosis. The clinical status improved in ten patients, remained unchanged in two patients and deteriorated in two patients
Table 2 summarizes the distribution of intracranial stenoses on DSA (14 patients examined) obtained after consensus by the three observers after their blind analysis of both examinations. The sensitivity and specificity for all arteries on MRA and DSA for the three observers are given in Table 3. The sensitivity of MRA varied from 62 to 79% depending on the observer, while the sensitivity of DSA varied from 76 to 94%. The specificity of MRA varied from 83 to 87% depending on the observer, while the specificity of DSA varied from 83 to 97%.
False-positive and false-negative rates per patient for DSA and for MRA are listed in Table 4. Percentages are given with the absolute numbers in brackets. The number of false-positive MRA decreased when “more than two stenoses” was used as criterion for a true positive MRA. The number of false-negative MRA remained high.
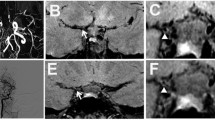
MRA showed comparable findings in six of them (Fig. 1). The examination was false-negative in two patients (Fig. 2). MRA showed abnormalities in one patient in whom DSA was normal (Fig. 3). Both examinations were normal in 5/14 patients (Table 1).
The estimated area under the ROC curve for detecting a stenosis on DSA was 0.95±0.03 for observer 1, 0.90±0.04 for observer 2 and 0.91±0.03 for observer 3. The 95% confidence intervals for the accuracy of DSA were 0.89–0.98, 0.83–0.95 and 0.85–0.96, respectively.
The estimated area under the ROC curve for detecting a stenosis on MRA was 0.79±0.05 for observer 1, 0.83±0.05 for observer 2 and 0.82±0.05 for observer 3. The 95% confidence intervals for the accuracy of MRA were 0.71–0.86, 0.75–0.89 and 0.74–0.88, respectively.
The difference between the areas under the ROC curve for DSA and MRA was 0.159 (P=0.002) for observer 1, 0.074 (P=0.072) for observer 2 and 0.097 (P=0.058) for observer 3 (Fig. 4).
A comparison of the areas under the ROC curves between the different observers showed no significant statistical differences. The difference between the ROC curves for MRA in observer 1 and 3 was 0.028±0.044 (P=0.520), 0.037±0.051 (P=0.465) for observer 1 and 2, and 0.009±0.045 (P=0.836) for observer 2 and 3. The difference between the ROC curves for DSA in observer 1 and 3 was 0.034±0.041 (P=0.416), 0.048±0.041 (P=0.249) for observer 1 and 2, and 0.014±0.047 (P=0.767) for observer 2 and 3.
The kappa statistics revealed a fair agreement (0.35) between DSA and MRA for observer 1, a substantial agreement (0.68) for observer 2 and a moderate agreement (0.46) for observer 3.
The kappa-statistics for inter-rater agreement in the assessment of MRA showed substantial agreement (0.60–0.68, prevalence-adjusted bias-adjusted kappa 0.67–0.73). The kappa-statistics for inter-rater agreement in the assessment of DSA showed moderate agreement (0.44–0.51, prevalence-adjusted bias-adjusted kappa 0.51–0.57).
Discussion
Central nervous system vasculitis is a rare disorder and is often difficult to diagnose. MR imaging is considered very sensitive in most series, but the specificity remained poor [8]. Opinions concerning the value of a normal brain MR vary [1, 9]. While several authors have reported a very high sensitivity for MR imaging, others have stated that a normal brain MR imaging does not exclude the presence of abnormalities angiographically [1, 9]. Although angiographical lesions without corresponding parenchymal abnormality have been reported in a particular vascular territory on DSA of patients with vasculitis and several brain MR lesions, it is accepted that the disease is extremely unlikely in the presence of a normal brain MR examination [9, 10]. The angiographical and the MR imaging findings provide different information about the extent of the disease [1, 11]. An angiographical examination in a patient with suspected cerebral vasculitis therefore always is required, and usually DSA is requested. The classical pattern was defined as long segmental or multiple focal areas of narrowing in at least two separate vascular distributions. Up to 30% false-negative results have been reported for DSA [12]. The biopsy yields the highest specificity (80%), but remains often false-negative (53%) and is associated with a serious morbidity (0.5–2%) [3].
DSA is the imaging reference standard in the diagnostic work-up of patients with suspected vasculitis [3, 12]. However, the sensitivity of 60–80% and a specificity of 30%, together with a limited risk of procedure-related complications, have to be taken into account. The positive predictive value is 22–37%.
Intracranial MRA has been restricted to some extent by limited resolution, saturation of flow and degree of background suppression. Tortuous blood vessels and/or decreased blood flow velocities may affect the images. This may lead to an overestimation of a stenosis and sometimes to the wrong diagnosis of a stenosis [4]. MRA in older patients may fail for these reasons. The artifacts are due to higher-order motion and intravoxel dephasing. In order to minimize artifacts due to higher-order motion and intravoxel dephasing, it is important to use the shortest achievable echo-time and voxel size. In this regard phase-contrast MRA is known to be less susceptible to turbulent flow and intravoxel dephasing. A comparison between the two different MRA techniques revealed a superiority of the time-of-flight method [14]. The advantages of MRA are tremendous and include absence of ionizing radiation, no contrast injection and non-invasiveness. The complication rate of DSA in the clinical setting of “suspected vasculitis” varies from 14 to 20%. In our series, one patient developed a stroke during the DSA.
Heiserman et al. have assessed the usefulness of MRA in the characterization of intracranial arterial stenoses and occlusions using a 3D TOF technique [4]. They found that 61% of the stenoses were correctly graded.
Recently MRA has been used in the assessment of patients with vasculitis, but comparison with DSA was available in only six patients [7]. The authors concluded that MRA was a valuable tool. Both angiographical techniques showed abnormalities in three patients, and both were normal in one patient. MRA was abnormal in one patient, while DSA was normal. Finally, DSA was abnormal in one patient who had normal MRA findings. From their observations the authors suggested to use MRA as the initial examination [6, 7].
We compared both techniques in 14 young patients with suspected cerebral vasculitis. We performed this study for the detection of intracranial stenoses in these patients and not for grading the stenosis. The area under the ROC curves showed that there was good agreement among the three observers both for the analysis of MRA and for the analysis of DSA. From the individual analysis of the three observers, it could be demonstrated that the assessment of MRA requires some experience, a finding that has recently been reported in the detection of intracranial aneurysms on MRA [15].
DSA yielded a limited number of false-positive and false-negative results, compared to the consensus analysis of the DSA by the three observers (Table 4). The false-positive and false-negative rate of MRA was higher when the detection of one stenosis was considered abnormal. However, with the criterion “more than two stenoses in at least two separate vascular distributions” to consider an examination to be compatible with vasculitis, the number of false-positive examinations decreased to a figure that was comparable with the DSA findings (Table 4). The number of false-negative examinations remained high. Eight patients showed two or more stenoses on DSA. MRA was found to be negative in two (observer 1) or three (observer 2 and 3) of these.
The additional use of CT angiography to improve the accuracy of the diagnosis of a stenosis has recently been studied [16]. The authors reported a reduction in the tendency to overestimate a stenosis at MRA, and they also improved the specificity for detecting a stenosis of 50% or more.
It is important to know that MRA has its own limitations. As the technique depends on flow velocity, the image quality is patient-dependent and can be variable for the same patient age. MRA does not offer information regarding hemodynamics and does not visualize the entire cerebral vasculature. Stenoses in more peripheral branches may have been missed. A false-negative rate of 20–30% is generally accepted because of the limited resolution of DSA [12]. The resolution of MRA is even lower than that of DSA, and therefore a higher false-negative can be expected.
Our study has several limitations. It would have been preferable to study a large cohort of patients with MRA and DSA. Sensitivity and specificity of MRA may vary with the number of patients with and without stenoses on intracranial arteries. The use of a five-point scale to assess the degree of stenosis also had an influence on the sensitivity and specificity of MRA. We considered the scores 3 (indeterminate), 4 and 5 as positive cases in the calculation of the sensitivity and specificity. When the score 3 was considered as negative, sensitivity slightly decreased, while specificity increased compared to the results in this paper. Our study also has a selection bias and therefore the statistical analysis may not reflect the analysis for an unselected population.
On the other hand, minor technical hardware improvements have led to a significantly better MRA image quality. We recommend MRA in the suspected clinical setting of cerebral vasculitis as the first non-invasive angiographical imaging modality. At our institution, we obtain maximum intensity projections every 4–5° over 360° in the axial and sagittal plane, and viewing is usually performed in a dynamic mode. Provided that MRA depicts more than two stenoses in at least two separate vascular distributions, DSA can be avoided (Fig. 5). In all other instances, and particularly when MRA is normal, DSA is still indicated. With the advent of 3.0 T units and/or eight-channel head coils, which increase the image resolution 2–4 times, it is to be expected that MRA will substitute DSA in the diagnostic work-up of suspected cerebral vasculitis in the near future.
3D time-of-flight MRA in two patients with suspected cerebral vasculitis, performed after the end of the study. DSA was not performed because at least three stenoses could be depicted (a and b, arrows). Note the better image quality of these examinations compared to those performed during the study. This is partly due to the use of an eight-channel head coil and partly due to the fact that the maximum intensity projections are now routinely obtained every 4–5°
References
Pomper MG, Miller TJ, Stone JH, Tidmore WC, Hellman DB (1999) CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography. Am J Neurorad 20:75–85
Chu CT, Gray L (1998) Diagnosis of intracranial vasculitis. J Neuropathol Exp Neurol 57:30–38
Duna GF, Calabrese LH (1995) Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol 22:662–667
Heiserman JE, Drayer BP, Keller PJ, Fram EK (1992) Intracranial vascular stenosis and occlusion: evaluation with three-dimensional time-of-flight MR angiography. Radiology 185:667–673
Dagirmanjian A, Ross JS, Obuchowski N et al (1995) High resolution, magnetization transfer saturation, variable flip angle, time-of-flight MRA in the detection of intracranial vascular stenoses. J Comp Assist Tomogr 19:700–706
Felber S, Auer A, Schmutzhard E (2000) Magnetresonanz-Angiographie bei entzündlichen Hirnerkrankungen. Radiologe 40:1077–1089
Schlüter A, Hirsch W, Jassoy A et al (2001) MR-angiographie in der Diagnostik von Vasculitiden und beignen Angiopathien des Zentralnervensystems. Fortschr Röntgenstr 173:522–527
Harris KG, Tran DD (1994) Diagnosing intracranial vasculitis. Am J Neuroradiol 15:317–330
Wasserman BA, Stone JH, Hellmann DB, Pomper MG (2001) Reliability of normal findings on MR imaging for excluding the diagnosis of vasculitis of the central nervous system. AJR 177:455–459
Greenan TJ, Grossman RI, Goldberg HI (1992) Cerebral vasculitis: MR imaging and angiographic correlation. Radiology 182:65–72
Calabrese LH (1995) Vasculitis of the central nervous system. Rheum Dis Clin North Am 21:1059–1076
Calabrese LH, Mallek JA (1987) Primary angiitis of the central nervous system: report of 8 new cases, review of the literature and proposal for diagnostic criteria. Medicine 67:20–39
Cloft HJ, Phillips CD, Dix JE, McNulty BC, Zagardo MT, Kallmes DF (1999) Correlation of angiography and MR imaging in cerebral vasculitis. Acta Radiol 40:83–87
Oelerich M, Lentschig MG, Zunker P, Reimer P, Rummeny EJ, Schuierer G (1998) Intracranial vascular stenosis and occlusion: comparison of 3D time-of-flight and 3D phase-contrast MR angiography. Neuroradiology 40:567–573
White PM, Wardlaw JM, Lindsay KW, Sloss S, Patel DKB, Teasdale EM (2003) The non-invasive detection of intracranial aneurysms: are neuroradiologists any better than other observers? Eur Radiol 13:389–396
Hirai T, Korogi Y, Ono K, Nagano M, Maruoka K, Uemura S, Takahashi M (2002) Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. Am J Neuroradiol 23:93–101
Acknowledgments
We are grateful to the W. Robberecht, MD, PhD, B. Dubois, MD, PhD and V. Thijs, MD, for referring the patients to the MR unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demaerel, P., De Ruyter, N., Maes, F. et al. Magnetic resonance angiography in suspected cerebral vasculitis. Eur Radiol 14, 1005–1012 (2004). https://doi.org/10.1007/s00330-004-2239-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2239-y