Abstract
Introduction
Methanol poisoning is an uncommon but potent central nervous system toxin. We describe here the CT and MR findings in nine patients following an outbreak of methanol poisoning.
Methods
Five patients with a typical clinical presentation and elevated anion and osmolar gaps underwent conventional brain MRI with a 1.5-T Gyroscan Interna scanner. In addition nonenhanced CT was performed in another three patients with more severe toxicity.
Results
Bilateral hemorrhagic or nonhemorrhagic necrosis of the putamina, diffuse white matter necrosis, and subarachnoid hemorrhage were among the radiological findings. Various patterns of enhancement of basal ganglial lesions were found including no enhancement, strong enhancement and rim enhancement.
Conclusion
A good knowledge of the radiological findings in methanol poisoning seems to be necessary for radiologists. The present study is unique in that it enables us to include in a single report most of the radiological findings that have been reported previously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methanol is a component of varnishes, paint removers, perfumes, antifreeze, copy machine fluid and gasoline mixtures, and may be ingested accidentally or intentionally. It is a CNS depressant that is potentially toxic after ingestion, inhalation or transdermal exposure [1, 2], and it also crosses the placenta [3]. Without treatment, ingestion of 30 ml pure methanol usually results in death and as little as 4 ml can result in blindness [1, 2, 4]. Blood methanol levels above 200 mg/l are considered toxic and levels above 1500 mg/l are potentially fatal [1, 2]
The peculiarity of methanol poisoning is the latency period between its ingestion and the appearance of manifestations. The latency may be related to the concomitant ingestion of ethanol which affects the metabolism of methanol [5]. Although the latency in symptom onset is variable, symptom progression may be rapid [6]. Early symptoms of methanol poisoning, except for visual disturbances, are nonspecific and include nausea, vomiting and abdominal pain. Late manifestations are due to acidosis secondary to the accumulation of formic acid and lactic acid [7]. The terminal event is often respiratory arrest and the fatal period is from 6 to 36 hours.
Current treatment of methanol poisoning consists of gastric lavage, ethanol therapy, alcohol dehydrogenase enzyme blockade by means of fomepizole, dialysis, alkalinization, and the use of cofactors such as folate [8, 9]. In spite of improved treatment, mortality in methanol poisoning remains high, mainly because of an often difficult and therefore delayed diagnosis.
Various imaging techniques have enabled a better understanding of the clinical manifestations of methanol toxicity. Modern neuroimaging techniques also lead to much earlier diagnosis of CNS damage by focusing attention on the lesions that are radiologically detectable. A good knowledge of the target areas in methanol poisoning can lead the radiologist to suspect methanol poisoning in a relevant clinical setting, and guide early treatment.
As far as we are aware, the imaging findings of methanol poisoning have been described in only a few small case series in the literature, the largest one including five patients [10]. We describe here the radiological manifestations of methanol poisoning in nine patients following an outbreak of methanol poisoning.
Materials and methods
The outbreak occurred in Shiraz, the southern city of Iran. There are severe restrictions on the production and consumption of alcoholic beverages in Iran for religious reasons, and the liquor causing the present outbreak of methanol poisoning was produced illegally in the country, and was sold in bottles looking much like original bottles. Most of the patients were admitted to two main university hospitals: Namazee Hospital and Faghighi Hospital. The diagnosis of methanol poisoning was based on the typical clinical presentation, elevated anion and osmolar gaps, the presence of methanol which was measured as part of an alcohol screen, and the absence of other toxins in a toxicology screen. All patients were treated with intravenous alcohol, hemodialysis, folate therapy and alkalinization.
Nonenhanced CT of the brain was the initial imaging study in all patients, and was repeated in some patients during the hospital course according to neurological manifestations. Conventional brain MRI with a 1.5-T Gyroscan Interna scanner was also performed in all patients except those who were severely ill who were admitted to the intensive care unit for close observation and monitoring. MRI was also performed in a patient who was referred after 1 month and claimed to have drunk liquor from the same common source that had caused the outbreak.
This study was approved by the bioethical committee of Shiraz University of Medical Sciences.
Results
The demographic and clinical characteristics of the patient and the radiological findings are presented in Table 1. Nine patients (eight male and one female) with a mean age of 28.5 years (range 19–42 years) with documented methanol poisoning were investigated in this study. The mean methanol level in these patients was 24.3 mg/dl (range 13–49.5 mg/dl). Of the eight patients who were admitted to the hospital, seven (87%) presented with visual disturbances and six (75%) with gastrointestinal symptoms, and two (25%) were comatose. One patient presented with respiratory arrest. A 29-year-old woman also was presented 1 month after the outbreak with parkinsonian symptoms. There was no significant relationship between severity of symptoms at presentation, clinical outcome or extent of brain injury and methanol levels (P > 0.05).
Regarding outcome, only one patient recovered completely and was discharged from hospital without sequelae. Five patients recovered partially and were discharged with sequelae and the remaining two patients did not respond to the therapeutic measures and died.
MRI was performed in five patients during their hospital course and in another patient who presented 1 month after the outbreak; however MRI could not be performed in the other three patients who were too ill.
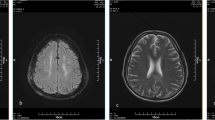
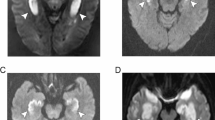
Regarding imaging findings, four patients had hemorrhagic or nonhemorrhagic necrosis of the basal ganglia (Figs. 1, 2, 3 and 4). One patient showed subarachnoid hemorrhage accompanied by global cerebral hypodensity (Fig. 5) and another patient revealed bilateral basal ganglia especially putaminal necrosis accompanied by diffuse white matter hypodensity and bilateral occipital necrosis more prominent on the left side (Fig. 6). The remaining three patients had no abnormal findings on brain CT or MRI.
MRI shows bilateral lentiform nucleus necrosis as shown by hyperintense putaminal lesions, less prominent globus pallidus involvement on the T2-weighted image (a), with reciprocal hypointensities on the T1-weighted image (b). Hypointense putaminal lesions with a hyperintense rim are evident on the FLAIR image (c). Slight peripheral enhancement is seen on the contrast-enhanced image (d)
Discussion
Radiological manifestations of methanol poisoning have been frequently described previously. Bilateral necrosis of the basal ganglia is accepted as the most characteristic radiological feature of methanol poisoning [9, 11–15]. Both hemorrhagic and nonhemorrhagic damage of the putamen can occur [9, 11]. Both CT and MRI give similar results showing bilateral areas of putaminal necrosis; however, MRI gives better anatomical detail and may reveal small hemorrhagic lesions [14]. If the patient survives the acute phase of the illness, resorption of the infarcted hemorrhagic putamen tissue occurs with formation of cystic cavities within the nucleus [16]. Other brain lesions occasionally described include edema, necrosis of subcortical white and gray matter, cerebellar cortical lesions, bilateral intracerebral hemorrhage, bilateral tegmental necrosis and diffuse cerebral edema [9, 11, 17–20]. Table 2 illustrates the main radiological features of methanol poisoning reported in previous series. As expected bilateral basal ganglia lesions were also the predominant pattern in our patients.
The mechanism responsible for selective putaminal necrosis is unknown. It has been postulated that the necrosis results from decreased blood flow through the basal veins of Rosenthal secondary to hypotension [30]. However, many cases described in the literature did not experience hypotension during their hospital course, thus making decreased venous flow as the cause of putaminal necrosis less likely [31]. Another possibility is that the necrosis occurs as a direct toxic effect of formic acid with higher concentrations of formic acid accumulating in the putamen than in other areas of the brain [31] or due to varying sensitivity of striatal neurons to toxic metabolites of methanol [32]. Finally the association of bilateral putaminal necrosis with Leigh disease which is characterized by congenital lactic acidosis may suggest that the putamen may indeed be more sensitive to an acidic environment than other areas of the brain [33, 34].
Putaminal changes on conventional MRI and CT scans are not specific to methanol intoxication and have been described in Wilson’s disease, Kearns-Sayre syndrome, Leigh disease, and various other neurodegenerative disorders [34–37]. In addition there are pathological similarities between methanol poisoning and carbon monoxide inhalation [38, 39], hypoxic–ischemic injury [40, 41] hemolytic–uremic syndrome [42], hydrogen sulfide poisoning [43], and (in the rare patient who survives) acute cyanide intoxication [44]. A common industrial solvent, 1,1,1-trichloroethane (methyl chloroform), which is found in typewriter correction fluid and is also used as a recreational drug, has also been shown to produce lesions in the basal ganglia similar to those observed in patients with methanol poisoning [45].
In patients with putaminal necrosis who survive the initial insult, extrapyramidal symptoms and signs including rigidity, tremors, mask-like facies, and monotonous speech may develop. Imaging has the potential to play a role in the diagnosis of acute methanol poisoning weeks to months later in a patient presenting with dystonia and features of parkinsonism [46, 47]. Extrapyramidal symptoms are usually permanent, but improvement may occur following treatment with levodopa [48–50] although not in all patients [16]. We also encountered parkinsonian symptoms in one of our patients, but evaluation of this aspect in the other patients was impossible because they were lost to follow-up.
There is no general agreement between the extent of radiological abnormalities and clinical outcome in methanol poisoning. Patankar et al. noted that the severity and extent of necrosis of the lentiform nuclei do not necessarily correlate with the clinical outcome [19]; however, Aquilonius et al. stated that a clear relationship exists between the degree of necrotic change within the putamen and clinical outcome [20]. The presence of injury to both the subcortical white matter (with relative sparing of the centrum semiovale) and the putamen has also been claimed to be indicative of severe methanol toxicity [9]. In our patients there was a direct relationship between the degree of radiological abnormalities and clinical outcome, and the two patients with most severe radiological abnormalities succumbed to methanol poisoning.
Various studies have shown that there is no statistically significant association between methanol level and prognosis [51]. This lack of association is logical since severely poisoned patients may have a low methanol level because most of the ingested methanol is metabolized to formic acid [52]. Also repeated intake of the liquor over time could explain high methanol concentrations because ethanol is also ingested preventing methanol metabolism. Instead of methanol level, severe metabolic acidosis, coma or seizure at presentation and increased pCO2 have been shown to be associated with poor prognosis [53–55]. As expected we also found no significant relationship between methanol level and prognosis.
Methanol intoxication produces classic neuropathological changes. Optic neuropathy related to loss of myelin in the optic nerves is perhaps the best known neuropathological change after methanol poisoning [11, 56]. Post-mortem studies of poisoned patients who survive for a period of days or weeks have shown a distinctive pattern of brain injury characterized by bilateral putaminal necrosis and white matter hemorrhagic necrosis, especially affecting subcortical regions [16, 30]. These lesions spare the most peripheral white matter, the subcortical association fibers [11, 26]. Unfortunately, permission for autopsy could not be obtained in either of our two deceased patients.
Hemorrhage has been reported in up to 14% of patients with methanol poisoning [12, 46] and has been suggested to indicate a poor prognosis [17, 20]. In some studies hemorrhage was confined to the putamina [9, 12], while in others hemorrhagic necrosis occurred both in the putamina and subcortical white matter [22, 30]. In this series some type of hemorrhage (hemorrhagic putaminal necrosis in two patients and subarachnoid hemorrhage in one) occurred in one-third of our patients. To our knowledge following an extensive literature search, there is only one previous report of subarachnoid hemorrhage in methanol poisoning [29].
Although cerebral hemorrhage related to methanol poisoning is usually bilateral and not expansive [9, 21, 57], as in our patients, extensive hemorrhage with extension in to the ventricular system [46, 58] and massive hemorrhagic transformation of cerebral ischemic lesions [59] have also been reported.
Some believe that heparinization during dialysis may play some role in the development of brain hemorrhage [27, 46]. However, hemorrhage also is seen prior to and irrespective of dialysis [17, 19] or after hemodialysis without systemic heparin [59]. On the other hand, dialyzed patients are those most severely poisoned and therefore most likely to suffer from such hemorrhagic complications. Hemorrhagic basal ganglial necrosis was present in two of our patients who were dialyzed, but in patient 8 (Table 1), subarachnoid hemorrhage was evident on the CT scan before hemodialysis. It seems that although the direct role of hemodialysis has not been proved, avoidance of heparin in patients with methanol intoxication seems logical.
There are only a few studies in which diffusion-weighted imaging has been used in methanol poisoning. Deniz et al. [23] reported bilateral putaminal hyperintensity on diffusion-weighted images with decreased apparent diffusion coefficient (ADC) values, while Server et al. [22] reported high signal intensity and low ADC values in the subcortical white matter, basal ganglia, and right hippocampus. Takao et al. also reported bilateral putaminal lesions with restricted diffusion with development of new lesions in the subcortical white matter on the third day after admission [60]. These findings of restricted diffusion have been interpreted as cytotoxic edema which is less likely to be reversible and indicates nonviable tissue [22]. A possible explanation for diffusion abnormalities is that the accumulation of formic acid leads to metabolic acidosis and inhibition of cytochrome oxidase which causes anoxia [61] and the subsequent failure of the Na+/K+ adenosine triphosphatase pump which transports Na+ and K+ ions across the membrane. Failure of this pump leads to loss of ionic gradients and a flux of water from the extracellular to the intracellular space [62, 63] and reduction in ADC values [23]. Diffusion MR imaging has also been used during the early stages of acute carbon monoxide poisoning to show the early reversible white matter lesions [64]. Unfortunately, diffusion-weighted imaging was not performed in our patients due to some technical problems.
Contrast enhancement of brain lesions can be observed after methanol poisoning. Anderson et al. reported a 47-year-old man with significant methanol intoxication who had enhancing lesions in the caudate nuclei, putamina, hypothalamus, and subcortical white matter on MRI [11]. In another patient tiny foci of enhancement were found in the frontal region [22]. Meningeal and gyral enhancement of the cerebral cortex has also been reported on contrast-enhanced CT scans [28] as has peripheral contrast enhancement of subcortical white matter lesions [21]. Metabolic dysfunction and consequent endothelial cell injury may cause disruption of the blood–brain barrier which may be represented by contrast enhancement [11]. In our patients almost all the patterns, including nonenhancement, strong enhancement and rim enhancement of basal ganglial lesions, occurred.
Conclusion
With the increasing use of modern neuroimaging techniques in the evaluation of unconscious patients, a knowledge of the radiological findings in methanol poisoning seems to be necessary for radiologists. The radiological manifestations include a wide variety of abnormalities involving many areas of the brain. The present study is unique in that it enables us to include in a single report most of the radiological findings that have been reported previously.
References
Birnbaumer DM, Bessen HA (1998) Other alcohols. In: Rosen P, Barkin R (eds) Emergency medicine: concept and clinical practice. Mosby, St Louis, pp 1292–1300
Kruse JA (1992) Methanol poisoning. Intensive Care Med 18:391–397
Belson M, Morgan BW (2004) Methanol toxicity in a newborn. J Toxicol Clin Toxicol 42:673–677
Gonda A, Gault A, Churchill D, Hollomby D (1978) Haemodialysis for methanol intoxication. Anger J Med 64:749–758
Jacobsen D, Jansen H, Wiik-Larsen E, Bredesen JE, Halvorsen S (1982) Studies on methanol poisoning. Acta Med Scand 212:5–10
Ravichandran R, Dudani RA, Almeida AF, Chawla KP, Acharya VN (1984) Methyl alcohol poisoning. (Experience of an outbreak in Bombay). J Postgrad Med 30:69–74
McMartin KE, Ambre JJ, Tephly TR (1980) Methanol poisoning in human subjects; role of formic acid accumulation in metabolic acidosis. Am J Med 68:414–418
Brent J, McMartin K, Phillips S, Araron C, Kulig K (2001) Fomepizole for the treatment of methanol poisoning. N Engl J Med 344:424–429
Kuteifan K, Oesterle H, Tajahmadi T, Guttbub AM, Laplatte G (1998) Necrosis and hemorrhage of the putamen in methanol poisoning shown on MRI. Neuroradiology 40:158–160
Onder F, Ilker S, Kansu T, Tatar T, Kural G (1998–1999) Acute blindness and putaminal necrosis in methanol intoxication. Int Ophthalmol 22:81–84
Anderson CA, Rubinstein D, Filley CM, Stears JC (1997) MR enhancing brain lesions in methanol intoxication. J Comput Assist Tomogr 21:834–836
Glazer M, Dross P (1993) Necrosis of putamen caused by methanol intoxication: MR findings. AJR Am J Roentgenol 160:1105–1106
Pelletier J, Habib MH, Khalil R, Salamon G, Bartoli D, Jean P (1992) Putaminal necrosis after methanol intoxication. J Neurol Neurosurg Psychiatry 55:234–235
Bartoli JM, Laurent C, Moulin G, Vignoli P, Graziani N, Jean P, Kasbarian M (1990) Intoxication au methanol. A propos de deux observations explorees par tomodensitometrie et imagerie par resonance magnetique. Ann Radiol (Paris) 33(4–5):257–259
Koopmans RA, Li DK, Paty DW (1988) Basal ganglia lesions in poisoning: MR appearance. J Comput Assist Tomogr 12:168–169
McLean DR, Jacobs H, Mielke BW (1980) Methanol poisoning: a clinical and pathological study. Ann Neurol 8:161–167
Gaul HP, Wallace CJ, Auer RN, Fong TC (1995) MR findings in methanol intoxication. AJNR Am J Neuroradiol 16:1783–1786
Chen JC, Schneiderman JF, Wortzman G (1991) Methanol poisoning: bilateral putaminal and cerebellar cortical lesions on CT and MR. J Comput Assist Tomogr 15:522–524
Patankar T, Bichile L, Karnad D, Prasad S, Rathod K (1999) Methanol poisoning: brain computed tomography scan in four patients. Australas Radiol 43:526–528
Aquilonius SM, Bergstrom K, Enoksson P, et al (1980) Cerebral computed tomography in methanol intoxication. J Comput Assist Tomogr 4:425–428
Blanco M, Casado R, Vazquez F, Pumar JM (2006) CT and MR imaging findings in methanol intoxication. AJNR Am J Neuroradiol 27:452–454
Server A, Hovda KE, Nakstad PH, Jacobsen D, Dullerud R, Haakonsen M (2003) A case report: conventional and diffusion-weighted MRI in the evaluation of methanol poisoning. Acta Radiol 44:691–695
Deniz S, Oppenheim C, Lehericy S, Sharshar T, Lalam TF, Dormont D, Marsault C (2000) Diffusion-weighted magnetic resonance imaging in a case of methanol intoxication. Neurotoxicology 21:405–408
Hantson P, Duprez T, Mahieu P (1997) Neurotoxicity to the basal ganglia shown by magnetic resonance imaging (MRI) following poisoning by methanol and other substances. J Toxicol Clin Toxicol 35:151–161
Hsu HH, Chen CY, Chen FH, Lee CC, Chou TY, Zimmerman RA (1997) Optic atrophy and cerebral infarcts caused by methanol intoxication. Neuroradiology 39:192–194
Rubinstein D, Escott E, Kelly J (1995) Methanol intoxication with putaminal and white matter necrosis: MR and CT findings. AJNR Am J Neuroradiol 16:1492–1494
Giudicissi Filho M, Holanda CV, Nader NA, Gomes SR, Bertolucci PH (1995) Bilateral putaminal hemorrhage related to methanol poisoning: a complication of hemodialysis? Case report. Arq Neuropsiquiatr 53:485–487
Hsieh FY, Leu TM, Chia LG (1992) Bilateral putaminal necrosis caused by methanol poisoning: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 49:283–288
del Carpio-O’Donovan R, Glay J (1992) Subarachnoid hemorrhage resulting from methanol intoxication: demonstration by computed tomography. Can Assoc Radiol J 43:299–301
Feaney MB, Anthony DC, Frosch MP (2001) Two cases with necrosis and hemorrhage in the putamen and white matter. Brain Pathol 11:121–122
Fontenot AP, Pelak VS (2002) Development of neurologic symptoms in a 26-year-old woman following recovery from methanol intoxication. Chest 122:1436–1439
LeWitt PA, Martin SD (1988) Dystonia and hypokinesis with putaminal necrosis after methanol intoxication. Clin Neuropharmacol 11:161–167
Kamei S, Takasu T, Mori N, Yoshihashi K, Shikata E (1996) Serial imaging of bilateral striatal necrosis associated with acidaemia in adults. Neuroradiology 38:437–440
Medina L, Chi TL, Devivo DC, Hilal SK (1990) MR findings in patients with subacute necrotizing encephalomyelopathy (Leigh syndrome): correlation and biochemical defect. AJNR Am J Neuroradiol 11:379–384
Lawler GA, Pennock JM, Steiner RE, Jenkins WJ, Sherlock S, Young IR (1983) Nuclear magnetic resonance imaging in Wilson disease. J Comput Assist Tomogr 7:1–8
Seidenwurm D, Novotny E, Marshall W, Enzmann D (1986) MR and CT in cytoplasmically inherited striatal degeneration. AJNR Am J Neuroradiol 7:629–632
Starosta-Rubinstein S, Young AB, Kluin K, et al (1987) Clinical assessment of 31 patients with Wilson’s disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol 44:365–370
Kim KS, Weinberg PE, Suh JH, Ho SU (1980) Acute carbon monoxide poisoning: computed tomography of the brain. AJNR Am J Neuroradiol 1:399–402
Zeiss J, Brinker R (1988) Case report. Role of contrast enhancement in cerebral CT of carbon monoxide poisoning. J Comput Assist Tomogr 12:341–343
Bianco F, Floris R (1987) Computed tomography abnormalities in hanging. Neuroradiology 29:297–298
Birbaner G, Aichner F, Felber J, et al (1991) MRI of cerebral hypoxia. Neuroradiology 33:53–55
Ho VB, Fitz CR, Chuang SH, Geyer CA (1993) Bilateral basal ganglia lesions: pediatric differential considerations. Radiographics 13:269–292
Matuso F, Cummins JW, Anderson RE (1979) Neurological sequelae of massive hydrogen sulfide inhalation. Arch Neurol 36:451–452
Rachinger J, Fellner FA, Stieglbauer K, Trenkler J (2002) MR changes after acute cyanide intoxication. AJNR Am J Neuroradiol 23:1398–1401
Del Amo M, Berenguer J, Pujol T, Mercader JM (1996) MR in trichloroethane poisoning. AJNR Am J Neuroradiol 17:1180–1182
Phang PT, Passerini L, Mielke B, et al (1988) Brain hemorrhage associated with methanol poisoning. Crit Care Med 16:137–140
Halavaara J, Valanne L, Setala K (2002) Neuroimaging supports the clinical diagnosis of methanol poisoning. Neuroradiology 44:924–928
Ley CO, Gali FG (1983) Parkinsonian syndrome after methanol intoxication. Eur Neurol 22:405–409
Bourrat C, Riboullard L, Flocard F, Chalumeau A, Guillaume C (1986) Voluntary methanol poisoning. Severe regressive encephalopathy with anomalies on X-ray computed tomography. Rev Neurol (Paris) 142:530–534
Davis LE, Adair JC (1999) Parkinsonism from methanol poisoning: benefit from treatment with anti-parkinson drugs. Mov Disord 14:520–522
Meyer RJ, Beard ME, Ardagh MW, Henderson S (2000) Methanol poisoning. N Z Med J 113:11–13
Hovda KE, Hunderi OH, Rudberg N, Froyshov S, Jacobsen D (2004) Anion and osmolal gaps in the diagnosis of methanol poisoning: clinical study in 28 patients. Intensive Care Med 30:1842–1846
Hovda KE, Hunderi H, Tafjord AB, Dunlop O, Rudberg N, Jacobsen D (2005) Methanol outbreak in Norway 2002–2004: epidemiology, clinical features and prognostic signs. J Intern Med 258:181–190
Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA (2002) American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol 40:415–446
Liu JJ, Daya MR, Carrasquillo O, Kales SN (1998) Prognostic factors in patients with methanol poisoning. J Toxicol Clin Toxicol 36:175–181
Martin-Amat G, McMartin K, Hayreh S, Hayreh M, Tephly T (1997) Methanol poisoning: ocular toxicity produced by formate. Toxicol Appl Pharmacol 45:201–208
Vazquez AV, Diaz Otero F, Marcos A, Varela De Seijas E (2001) Bilateral hemorrhagic necrosis of basal ganglia as a result of methanol poisoning. Neurologia 16:433
Erlandson P, Fritz H, Hagstam KE, Lilyenberg B, Tryding N, Voigt G (1968) Severe methanol intoxication. Acta Med Scand 177:393–408
Hernandez MA, Holanda MS, Tejerina EE, Gonzalez C, Lopez M, Hernandez JL (2004) Methanol poisoning and heparin: a dangerous couple? Am J Emerg Med 22:620–621
Takao H, Doi I, Watanabe T (2006) Serial diffusion-weighted magnetic resonance imaging in methanol intoxication. J Comput Assist Tomogr 30:742–744
Jacobsen D, McMartin KE (1997) Antidotes for methanol and ethylene glycol poisoning. J Toxicol Clin Toxicol 35:127–148
Roberts TPL, Rowley HA (2003) Diffusion weighted magnetic resonance imaging in stroke. Eur J Radiol 45:185–194
Schaefer PW, Grant PE, Gonzalez RG (2000) Diffusion-weighted MR imaging of the brain. Radiology 217:331–345
Sener RN (2003) Acute carbon monoxide poisoning: diffusion MR imaging findings. AJNR Am J Neuroradiol 24:1475–1477
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sefidbakht, S., Rasekhi, A.R., Kamali, K. et al. Methanol poisoning: acute MR and CT findings in nine patients. Neuroradiology 49, 427–435 (2007). https://doi.org/10.1007/s00234-007-0210-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-007-0210-8