Abstract
Objective
To evaluate anion and osmolal gaps as diagnostic tools in methanol poisoning.
Design and setting
Clinical observational study.
Patients and methods
In a recent methanol outbreak, the initial triage and treatment decisions in 28 patients were based mainly upon the values of the osmolal and anion gaps on admission. Methanol and formate levels were later compared to these gaps by linear regression analysis.
Results
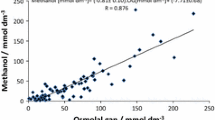
The correlation between the osmolal gaps and serum methanol concentrations on admission was linear (y = 1.03x+12.71, R2 = 0.94). The anion gaps correlated well with the serum formate concentrations (y = 1.12x+13.82, R2 = 0.86). Both gaps were elevated in 24 of the 28 subjects upon admission. Three patients had an osmolal gap within the reference area (because of low serum methanol), but elevated anion gap because of formate accumulation. One patient with probable concomitant ethanol ingestion had a high osmolal gap and a normal anion gap.
Conclusion
Osmolal and anion gaps are useful in the diagnosis and triage of methanol-exposed subjects. Confounders are low serum methanol and concomitant ethanol ingestion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methanol poisoning is characterized by increasing metabolic acidosis because of accumulation of the toxic metabolite formic acid, visual disturbances, and respiratory and cardiovascular failure [1, 2]. Despite effective treatment with alkali, antidotes (ethanol or fomepizole) and dialysis, patients suffer from complications such as visual impairment and a Parkinson-like syndrome because of delayed diagnosis [1, 2]. An important reason for this is that few hospitals analyze methanol on a 24-h basis.
Formic acid from methanol metabolism results in metabolic acidosis with high anion gap (AG) [3]. Ingestion of alcohols, such as methanol, significantly increases S-osmolality and the osmolal gap (OG) because of their high molar concentrations (Fig. 1). The use of these gaps as a diagnostic tool has not been evaluated in a larger methanol outbreak. Their usefulness has also been questioned [4]. We evaluated the use of the OG and the AG as a diagnostic tool in 28 patients from the latest methanol outbreak in Norway.
Patients and methods
Patients
During the outbreak, 46 patients were admitted to hospital and confirmed as poisoned by methanol, of whom six died. All consumed illegal spirits consisting of 20% methanol and 80% ethanol. Most patients were symptomatic upon admission, with dyspnea and visual disturbances being most common. Treatment was hypertonic (0.5 mmol/ml) sodium bicarbonate (27 of 28), antidote (ethanol or fomepizole), and hemodialysis (23 of 28). Three patients died and five were discharged with permanent visual and/or cerebral sequelae.
Methods
Methanol in serum was measured by gas chromatography using a headspace injector (Fisons GC 8000; Carlos Erba Instruments, Rodano, Italy) (sensitivity 1.3 mmol/l and day-to-day coefficient of variation 5%). Calibrators and controls were made by dilution of 100% methanol (Merck, Damstadt, Germany). Formate was measured enzymatically on a Cobas Mira analyzer (Roche Diagnostics, Basle, Switzerland) using formate dehydrogenase (Roche) and nicotinamid adenine dinucleotid (NAD) (Sigma, St. Louis, USA) (sensitivity 0.1 mmol/l, reference range ≤0.4 mmol/l, day-to-day coefficient of variation 5%). Statistical analyses were performed by the use of linear regression method.
Anion and osmolal gaps
The anion gap (AG) in serum was determined from the equation:
The reference range is 13±8 mmol/L (mean ± 2SD) [5].
The osmolal gap (OG) in serum is the difference between the measured osmolality (MO) and calculated osmolality, determined from the following equation [1]:
Osmolality was measured by freezing point depression method on a Fiske one-ten osmometer (Bergman Diagnostika, Oslo, Norway). The reference range for the OG is 5±14 mOsm/kgH2O (mean±2SD) [5].
The osmolal contribution from ethanol (four patients) was subtracted from the measured osmolality. Ethylene glycol was not detected. As such, these alcohols did not influence the OG presented here. The osmolal contribution of a 100 mg/dl concentration of methanol is 34 mOsm/kgH2O, ethanol 24 mOsm/kgH2O, isopropanol 18 mOsm/kgH2O, and ethylene glycol 17 mOsm/kgH2O.
Results
Many patients (18 of 28) were admitted late with pronounced metabolic acidosis (base deficit ≥20 mmol/l). The median pH on admission was 7.12 (range 6.57–7.50), mean pCO2 was 3.2 kPa (range 1.3–7.9), mean HCO3- 7.3 mmol/l (range 1.0–28.0), and mean base deficit 21 mmol/l (range 0–30). Mean S-methanol was 54.3 mmol/l (range 8.4–146.9), mean S-ethanol 7.6 mmol/l (range 2.2–10.9, n=4), mean OG 68 mOsm/kgH2O (range 14–159), and mean AG 35 mmol/l (range 16–50).
The regression line between S-methanol concentrations and OG on admission (y = 1.03x+12.71, R2 = 0.94) showed a very good correlation (Fig. 2). The sensitivity was, however, poor for the detection of S-methanol concentrations below 20 mmol/l (65 mg/dl). The mean reference value for the OG in this population was 13 mOsm/kgH2O (x = 0).
Hypertonic sodium bicarbonate infusion did not affect the usefulness of the OG as a diagnostic tool; regression line was then: y = 1.02x+4.33, R2 = 0.96 [n = 47, time from admission: 1.5–40 h, mean S-methanol 34.5 mmol/l (range 2.8–138.1 mmol/l), mean OG 40 (range 1–140 mOsm/kgH2O)].
Figure 3 presents the correlation between several concomitantly measured AGs and S-formate levels in eight patients. The correlation was good (y = 1.12x + 13.82, R2 = 0.86), indicating a mean reference value of an AG of 14 mmol/l in this population. The factor 1.12 indicates that the increase in the AG was slightly higher than the increase in the respective S-formate. This was most probably accounted for by an increasing accumulation of lactate in the most acidotic patients. In six patients (Table 1), S-lactate was measured (2.4–14.1 mmol/l); reference range 0.3–1.5 mmol/l. As seen from Table 1, there was a trend towards more lactate in the most acidotic patients.
Discussion
Formic acid is responsible for metabolic acidosis in the early stage of methanol poisoning [3]. Later, formate inhibition of cytochrome oxidase may explain the lactate production reported in some late admitted cases [1] and in our patients with the most severe metabolic acidosis (Table 1). In contrast to a small series of patients where formate accumulation alone accounted for the increased AG [3], the present series of patients were more seriously poisoned as judged from clinical features and their more pronounced metabolic acidosis. The present patients were admitted late because of their own symptoms, while in the other series the patients were admitted early because of recommendations through other media, most before clinical symptoms had developed [3, 6]. This fact, combined with more alcohol abuse among the present patients, may explain the difference in anion composition, with more lactate in our patients [7]. Only one of our patients had a normal AG, probably because of early admission and/or concomitant ethanol ingestion.
Methanol metabolism is slow (about 2.5 mmol·l·h or 8 mg·dl·h) [8] and symptomatic acidosis therefore does not develop before 15–20 h after ingestion, and even later if the antidote ethanol is co-ingested [2]. Latency periods as long as 96 h have been reported in such patients [6]. In the present outbreak, a mixture of methanol in ethanol was ingested, thus increasing the latency period even longer than this. Diagnosis based on the history was therefore difficult to obtain.
Most drugs do not raise the OG significantly because of their low molar concentrations [2]. However, if ethanol is also ingested in methanol-poisoned patients, the osmolal contribution from ethanol must be subtracted (see methods). As methanol metabolism proceeds, the OG decreases and the AG increases (formic acid accumulates; Fig. 1). An elevated OG was found in 25 of our patients. The very good correlation between the S-methanol concentration and the OGs (Fig. 2) was still present after start of bicarbonate treatment. The diagnostic value of the OG was therefore not limited to the samples taken upon admission. The three patients without an elevated OG all had low S-methanol concentrations (≤ 15.6 mmol/l or 50 mg/dl) and no S-ethanol. They were all acidotic with high AGs, reflecting the accumulation of a significant amount of formate from methanol metabolism (Fig. 1).
Several conditions in medicine increase the AG or the OG [2], but except for methanol or ethylene glycol poisoning, few conditions increase both at the same time. The few exceptions previously reported include diabetic coma [9], acidosis in alcoholics [10], chronic renal failure [11] and patients with shock following major trauma [12]. However, these reports were published before the new reference range of OG in acutely admitted patients was proposed (−9 to 19 mOsm/kgH2O) [5]. Patients with chronic renal failure and alcoholic ketoacidosis will, according to this, have OGs within the reference range [10, 11]. In diabetic ketoacidosis, S-acetone may rise to 13 mmol/l [9], an osmolal contribution of 13/0.93 = 14 mOsm/kgH2O, which will hardly raise the OG above 30 mOsm/kgH2O. In these patients a high S-glucose will also point to the diagnosis.
In patients with metabolic acidosis of unknown origin, we propose a new decision level for the OG of 25 mOsm/kgH2O before considering therapeutic interventions with antidotes, provided that the osmolal contribution from ethanol is subtracted. This approach reduces sensitivity, but increases specificity. The high cost of fomepizole also requires a high degree of specificity concerning the diagnosis of methanol poisoning. The proposed algorithm can be used in order to simplify the understanding of diagnosis and triage of methanol poisoning (Fig. 4).
Algorithm for diagnosis and triage in suspected methanol poisoning based on the different combinations of gaps (osmolal contribution from ethanol subtracted). The principles of the algorithm may also be also valid in ethylene glycol poisoning. For details in treatment, see [1, 2]. (VD visual disturbances, HD hemodialysis)
Patients with suspected methanol intoxication and OG<25 (below the decision level), can be separated into two categories (Fig. 4): patients with a small intake of methanol, or late admitted patients who have already metabolized most of the methanol to formic acid. The first category are not severely poisoned and may not be candidates for fomepizole treatment (if necessary, ethanol can be used). In the second group, the diagnosis of methanol poisoning may be supported by history, symptoms (visual disturbances), findings (hyperventilation and pseudopapilitis) [2], and always an elevated AG. In our material, all seven patients with an OG<25 (Table 2) belonged to this latter category, whereas six had visual disturbances [2].
In conclusion, the use of the AG and OG in patients presenting with metabolic acidosis of unknown origin helps in diagnosing methanol (or ethylene glycol) poisoning at an early stage where effective treatment may still reduce morbidity and mortality. The proposed decision level for the OG (25 mOsm/kgH2O) improves diagnostic specificity.
References
Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA (2002) American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol 40:415–446
Jacobsen D, McMartin KE (1997) Antidotes for methanol and ethylene glycol poisoning. J Toxicol Clin Toxicol 35:127–143
Sejersted OM, Jacobsen D, Ovrebo S, Jansen H (1983) Formate concentrations in plasma from patients poisoned with methanol. Acta Med Scand 213:105–110
Hoffman RS, Smilkstein MJ, Howland MA, Goldfrank LR (1993) Osmol gaps revisited: normal values and limitations. J Toxicol Clin Toxicol 31:81–93
Aabakken L, Johansen KS, Rydningen EB, Bredesen JE, Ovrebo S, Jacobsen D (1994) Osmolal and anion gaps in patients admitted to an emergency medical department. Hum Exp Toxicol 13:131–134
Jacobsen D, Jansen H, Wiik-Larsen E, Bredesen JE, Halvorsen S (1982) Studies on methanol poisoning. Acta Med Scand 212:5–10
Hojer J (1996) Severe metabolic acidosis in the alcoholic: differential diagnosis and management. Hum Exp Toxicol 15:482–488
Jacobsen D, Webb R, Collins TD, McMartin KE (1988) Methanol and formate kinetics in late diagnosed methanol intoxication. Med Toxicol Adverse Drug Exp 3:418–423
Sulway MJ, Malins JM (1970) Acetone in diabetic ketoacidosis. Lancet 2:736–740
Cooperman MT, Davidoff F, Spark R, Pallotta J (1974) Clinical studies of alcoholic ketoacidosis. Diabetes 23:433–439
Sklar AH, Linas SL (1983) The osmolal gap in renal failure. Ann Intern Med 98:481–482
Boyd DR, Folk FA, Condon RE, Nyhus LM, Baker RJ (1970) Predictive value of serum osmolality in shock following major trauma. Surg Forum 21:32–33
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hovda, K.E., Hunderi, O.H., Rudberg, N. et al. Anion and osmolal gaps in the diagnosis of methanol poisoning: clinical study in 28 patients. Intensive Care Med 30, 1842–1846 (2004). https://doi.org/10.1007/s00134-004-2373-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2373-7