Abstract
Introduction:
Magnetic resonance imaging (MRI) may help identify acute stroke patients with a higher potential benefit from thrombolytic therapy. The aim of our study was to assess the correlation between initial cerebral infarct (CI) volume (quantified on diffusion-weighted MRI) and the resulting clinical outcome in acute stroke patients with middle cerebral artery (MCA) (M1–2 segment) occlusion detected on MRI angiography treated by intravenous/intraarterial thrombolysis.
Methods:
Initial infarct volume (VDWI-I ) was retrospectively compared with neurological deficit evaluated using the NIH stroke scale on admission and 24 h later, and with the 90-day clinical outcome assessed using the modified Rankin scale in a series of 25 consecutive CI patients. The relationship between infarct volume and neurological deficit severity was assessed and, following the establishment of the maximum VDWI-I still associated with a good clinical outcome, the patients were divided into two groups (VDWI-I ≤70 ml and >70 ml).
Results:
VDWI-I ranged from 0.7 to 321 ml. The 24-h clinical outcome improved significantly (P=0.0001) in 87% of patients with a VDWI-I ≤70 ml (group 1) and deteriorated significantly (P=0.0018) in all patients with a VDWI-I >70 ml (group 2). The 90-day mortality was 0% in group 1 and 71.5% in group 2. The 90-day clinical outcome was significantly better in group 1 than in group 2 (P=0.026).
Conclusion:
Clinical outcome could be predicted from initial infarct volume quantified by MRI-DWI in acute CI patients with MCA occlusion treated by intravenous/intraarterial thrombolysis. Patients with a VDWI-I ≤70 ml had a significantly better outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Even though computerized tomography (CT) is still considered to be the standard brain imaging modality in acute ischemic stroke [1], recent studies have suggested that magnetic resonance imaging (MRI) may help to identify patients with the most potential to benefit from thrombolytic therapy [2–8]. MRI using diffusion-weighted images (DWI) improves the ability to diagnose acute cerebral infarction (CI) in terms of both quantification of actual CI volume and accurate CI localization [9–18]. MRI can also detect significant cerebral arterial occlusion or severe stenosis [19, 20]. MRI allows the evaluation of cerebral collateral flow and also imaging of reversible ischemic changes [21–24]. Nevertheless, to date, few studies have evaluated the impact of these pretreatment MRI parameters on clinical outcome [6–8, 15, 25–27].
The aim of our study was to assess the influence of initial CI volume measured on MRI-DWI sequences on clinical outcome in acute stroke patients with middle cerebral artery (MCA) (M1–2 segment) occlusion detected on MR angiography, who subsequently underwent intravenous/intraarterial thrombolysis (IVT/IAT). Initial CI volume (VDWI-I) was retrospectively compared with neurological deficit evaluated using the NIH stroke scale (NIHSS) on admission and 24 h later, and with the 90-day clinical outcome using the modified Rankin scale (mRS) in a series of 25 consecutive patients.
The MRI protocol used was based on actual data in the literature and the published results of several recent studies [1, 3–5, 7, 14, 15, 28–33].
Subjects and methods
A group of 25 consecutive acute ischemic stroke patients with MCA occlusion (M1-2 segment) detected on MR angiography, who underwent IVT or IAT according to recent guidelines [34, 35] between January and September 2005, were retrospectively analyzed. The demographic and clinical characteristics of the patients are shown in Table 1.
On admission, blood pressure was measured, electrocardiogram was recorded, and blood samples were taken. Clinical status was evaluated using the NIHSS by a certified neurologist. An MRI examination followed immediately. After admission, patients were treated according to published guidelines [34, 35].
MRI was performed on a Magnetom Symphony 1.5-T Maestro Class (Siemens, Erlangen, Germany) with quantum gradients (syngo2004A) and a standard head coil (CP head array coil).
The MRI protocol included the following sequences: (1) localizer, (2) T2-turbo spin echo (TSE), (3) fluid-attenuated inversion recovery (FLAIR), (4) DWI, (5) 3D time of flight magnetic resonance angiography (TOF MRA). The total acquisition time (AT) was 11 min 28 s. Sequences 2–4 were applied to acquire data from the same set of slices (standard number of slices 19, slice thickness 5 mm, distance factor 30%). The standard slice orientation was oblique axial, approximately parallel to skull base in order to minimize susceptibility artifacts in echo-planar imaging (EPI) sequences.
The sequence parameters were as follows: T2-TSE TR/TE/ETL 4,000/99/9 ms, FOV 230×173 mm, matrix 256×256, AT 1 min 34 s; FLAIR 8,050/112/ETL 21/2 concatenation, FOV 230 mm, FOV phase 76.6%, matrix 256×151, AT 2 min 26 s. These sequences were used to assess hemorrhage and detect local demyelination changes including sites of ischemic demyelination.
The EPI-DWI sequence parameters were as follows: 3,200/94/EPI factor 128/3 averages, FOV 230×230 mm, matrix 128×128 with interpolation, TA 1 min 20 s. MRI data were acquired with three diffusion weightings: b=0, DWI b=500, and DWI b=1,000. The fourth type of image was an automatically created apparent diffusion coefficient (ADC) map. DWI traces show average local diffusivity in the brain tissue examined when b is 500 and 1,000. This sequence was applied to assess hemorrhage (b=0: T2*-EPI, susceptibility-sensitive sequence) and detect sites of reduced diffusion (DWI, b=500 and 1,000). The 3D-TOF MRA sequence parameters were as follows: 43/7.15, 3 slabs, 32 partitions/slab, slice thickness 1 mm, FOV 200×150 mm, matrix 512×192, AT 5:59 min. The images obtained—maximum intensity projection (MIP) and sublayers—would illustrate closure of the main arterial trunk of the circle of Willis or its branches.
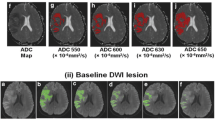
Infarct volumes were measured on DWI trace images (b=1,000) and calculated as total hyperintense area in single slices multiplied by effective slice thickness [(actual slice thickness + distance factor)/interslice gap]. Follow-up MRI was performed in all patients after 24 h. Clinical outcome was evaluated 3 months after CI using the mRS. The relationship between the initial infarct volume and neurological deficit severity was assessed, defining a cut-off point for maximum VDWI-I still associated with a good clinical outcome. Several cut-off points of VDWI-I were subsequently tested with the aim of maximizing both sensitivity and specificity for a good clinical outcome (mRS 0–2).
Pearson’s correlation analysis, an independent sample test, and the non-parametric Mann-Whitney test were used to assess the significance of differences.
Results
The analysis included 25 patients with acute stroke. The mean delay between symptom onset and MRI was 149 min. The demographic and clinical baseline data are shown in Table 1. VDWI-I ranged from 1.3 ml to 321 ml, quantified from hyperintense lesions on DWI sequences.
The relationship between VDWI-I and neurological deficit on admission is demonstrated in Fig. 1, and clinical evolution after 24 h and 90 days is shown in Figs. 2 and 3, respectively. The correlation analysis demonstrated a strong positive correlation between VDWI-I and 24-h clinical outcome (r=0.74) and a moderate positive correlation between VDWI-I and 90-day clinical outcome (r=0.67; Figs. 4 and 5).
The cut-off point of 70 ml was found to be the maximum VDWI-I still associated with a good clinical outcome and corresponding to the maximum sensitivity and specificity (80%) achieved. Figure 6 demonstrates this resulting cut-off point, on the basis of which the patients were divided into two groups (VDWI-I ≤70 ml and >70 ml; Table 2).
At 24 h (Fig. 7), patients with VDWI-I >70 ml had a significantly higher NIHSS score (P=0.0018).
Intracranial hemorrhage was present at 24 h in one patient with VDWI-I ≤70 ml and in three patients with VDWI-I >70 ml (Table 2). There was a statistically significant difference in mRS score between patients with VDWI-I ≤70 ml and those with VDWI-I >70 ml (P=0.003, Fisher’s exact test). The median mRS scores in the two groups are shown in Table 2. The independent samples test showed significantly higher mRS (3–5) in those with VDWI-I >70 ml than in those with VDWI-I ≤70 ml (P=0.026; Fig. 8). The significance of this difference was confirmed by the non-parametric Mann-Whitney test (P=0.003).
Mortality at 90 days was 71.5% in those with VDWI-I >70 ml and 0% in those with VDWI-I ≤70 ml. All these patients died during the first 7 days after thrombolysis; brain edema was the cause of death in all cases. Intracranial hemorrhage also occurred in three of these patients.
Discussion
No direct relationship was found between the initial infarct volume measured on DWI and the neurological deficit on admission (Fig. 1). Two patients with the same severity of deficit and the same type of arterial occlusion (MCA, M1–2 segment) could have VDWI-I differing by as much as tens of milliliters (Fig. 9). This difference was mainly caused by the actual individual state of cerebral collateral flow.
The actual infarct volume is considered to be an important factor in the decision to perform thrombolysis. Initial infarct volume is considered to be an independent predictor of subsequent spontaneous CI hemorrhagic transformation and also for intracerebral hemorrhage growth after thrombolysis [36]. Based on the results of this study, one may also predict eventual CI progression and the resulting clinical outcome from the initial infarct volume.
Davalos et al. retrospectively found infarct volume progression in patients with VDWI-I ≤25 ml and initial NIHSS score >8, on average about 68 ml in a group of 166 acute CI patients, examined within 12 h of stroke onset. This progression occurred during the first 6 h in one of three of these patients—mainly in those who did not receive thrombolysis [8].
In the group of patients with VDWI-I ≤70 ml and initial NIHSS >8, infarct volume progression was found in nine patients with a mean volume of progression of 27.5 ml.
Derex et al. observed a significantly worse clinical outcome in association with a larger pretreatment DWI infarct volume in 49 stroke patients treated by IVT. They consider the initial DWI lesion volume and the recanalization to be independent factors in determining the final infarct size [27].
Oppenheim et al. consider quantification of the initial CI volume to be an accurate method for assessing the risk of malignant infarct progression in patients with MCA occlusion. They identified a threshold volume of 145 ml in their group of 28 patients. No patient with a CI volume below this value had a malignant MCA infarction in spite of a high NIHSS score on admission (16.5±4) [15]. In our study, 71.5% of the patients with MCA occlusion and with VDWI-I >70 ml died within 7 days from CI onset (Fig. 4).
Although perfusion-weighted imaging (PWI) is performed as a standard part of the MRI protocol in many stroke centers and has been used in previously reported studies, we did not use it in our MRI stroke protocol and therefore also did not subsequently interpret the ischemic penumbra according to the PWI/DWI mismatch for several reasons. We believe that PWI/DWI mismatch is an inaccurate approximation of the real ischemic penumbra [37–39]. Furthermore, quantification of PWI lesion volume generally uses only qualitative indices and quantitative calculations are very time-consuming and use controversial mathematical models [40]. Definitive infarct volume is usually far smaller than the initial PWI area [37–39]. For example, Heiss et al. compared the size of the PWI/DWI mismatch area with the area of reduced cerebral blood flow and increased oxygen extraction (over 150%) using positron emission tomography (PET) in acute stroke patients. Despite a good correlation between PWI and PET in the detection of perfusion defect, mismatch volume did not correspond to the volume of the area with increased oxygen extraction on PET scans. Thus PWI/DWI mismatch may not be a reliable correlate of ischemic penumbra [41].
Several methodological limitations of the presented study should be mentioned. The first one is the relatively small number of patients examined. This was a result of the generally low number of patients fulfilling the strict time criteria for thrombolytic therapy, event with MCA occlusion. Secondly, quantification of infarct volume was performed manually, because no semiautomatic quantification software was available. Therefore the quantification could have been affected by subjective operator error. Finally, interpretation of the results may have been limited by nonhomogeneity of the group: the patients had received two types of recanalization therapy, but both with the same effect on the occluded artery.
Conclusion
Quantification of initial CI volume can be considered important for an accurate and safe evaluation of the indications for thrombolytic therapy in acute stroke patients.
The MRI-DWI quantification of initial CI volume was able to predict clinical outcome in patients with acute CI with MCA occlusion treated by IVT or IAT.
References
Jansen O, Knauth M, Sartor K (1999) Advances in clinical neuroradiology. Akta Neurol 26:1–7
Rotta J (1997) Should thrombolytic therapy be the first-line treatment for acute ischemic stroke? N Engl J Med 337:1309–1310
Schellinger PD, Jansen O, Fiebach JB, Pohlers O, Ryssel H, Heiland S, Steiner T, Hacke W, Sartor K (2000) Feasibility and practicality of MR imaging of stroke in the management of hyperacute cerebral ischemia. AJNR Am J Neuroradiol 21:1184–1189
Fiebach JB, Jansen O, Schellinger PD, Knauth M, Hartmann M, Heiland S, Ryssel H, Pohlers O, Hacke W, Sartor K (2001) Comparison of CT with diffusion-weighted MRI in patients with hyperacute stroke. Neuroradiology 43:628–632
Schellinger PD, Fiebach JB, Jansen O, Ringleb PA, Mohr A, Steiner T, Heiland S, Schwab S, Pohlers O, Ryssel H, Orakcioglu B, Sartor K, Hacke W (2001) Stroke magnetic resonance imaging within 6 hours after onset of hyperacute cerebral ischemia. Ann Neurol 49:460–469
Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA, Davis SM (2002) Diffusion and perfusion-weighted MRI response on thrombolysis in stroke. Ann Neurol 51:28–37
Röther J, Schellinger PD, Gass A, Sieber M, Villringer A, Fiebach JB, Fiehler J, Jansen O, Kucinski T, Schoder V, Szabo K, Junge-Hülsing GJ, Hennerici M, Zeumer H, Sartor K, Weiller C, Hacke W (2002) Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 h. Stroke 33:2438–2445
Davalos A, Blanco M, Pedraza S, Leira B, Castellanos M, Pumar JM, Silva Y, Serena J, Castillo J (2004) The Clinical-DWI mismatch: a new diagnostic approach to the brain tissue risk of infarction. Neurology 62:2187–2192
Warach S, Chien D, Li W, Ronthal M, Edelman RR (1992) Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology 42:1717–1723
Baird AE, Warach S (1998) Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab 18:583–609
Lovblad KO, Laubach HJ, Baird AE, Curtin F, Schlaug G, Edelman RR, Warach S (1998) Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 19:1061–1066
Ay H, Buonanno FS, Rodorf G, Schaefer PW, Schwamm LH, Wu O, Gonzalez RG, Yamada K, Sorensen GA, Koroshetz WJ (1999) Normal diffusion-weighted MRI during stroke-like deficits. Neurology 52:1784–1792
Neumann-Haefelin T, Moseley ME, Albers GW (2000) New magnetic resonance imaging methods for cerebrovascular disease: emerging clinical applications. Ann Neurol 47:559–570
Oppenheim C, Logak M, Dormont D, Lehericy S, Manai R, Samson Y, Marsault C, Rancurel G (2000) Diagnosis of acute ischemic stroke with fluid attenuated inversion recovery and diffusion-weighted MR sequences. Neuroradiology 42:602–607
Oppenheim C, Samson Y, Manai R, Laam T, Vandamme X, Crozier S, Srour A, Cornu P, Dormont D, Rancurel G, Marsault C (2000) Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke 31:2175–2181
Jansen O, Brückmann H (2002) Ischemic brain diseases. In: Sartor K (ed) Diagnostic and interventional neuroradiology. Thieme, Stuttgart New York, p 148
Mullins ME, Lev MH, Schellingerhout D, Koroshetz WJ, Gonzales RG (2002) Influence of availability of clinical history on detection of early stroke using unenhanced CT and diffusion-weighted MR imaging. AJR Am J Roentgenol 179:223–228
Mullins ME, Schaefer PW, Sorensen AG, Halpern EF, Ay H, He J, Koroshetz WJ, Gonzalez RG (2002) CT and conventional and diffusion weighted MR Imaging in acute stroke: study in 961 patients at presentation to the emergency department. Radiology 224:353–360
Warach S, Li W, Ronthal M, Edelman RR (1992) Acute cerebral ischemia: evaluation with dynamic contrast-enhanced MR imaging and MR angiography. Radiology 182:41–47
Evans AJ, Richardson DB, Tien R, Mac Fall JR, Hedlund LW, Heinz ER, Boyko O, Sostman HD (1993) Poststenotic signal loss in MR angiography; effects of echo time, flow compensation and fractional echo. AJNR Am J Neuroradiol 14:721–729
Schellinger PD, Jansen O, Fiebach JB, Heiland S, Steiner T, Schwab S, Pohlers O, Ryssel H, Sartor K, Hacke W (2000) Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke 31:1318–1328
Grant PE, He HJ, Halpern E, Wu O, Schaefer PW, Schwamm LH, Budzik RF, Sorensen AG, Koroshetz WJ, Gonzalez RG (2001) Frequency and clinical context of decreased apparent diffusion coefficient reversal in the human brain. Radiology 221:43–50
Crisostomo RA, Garcia MM, Tong DC (2003) Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack. Correlation with clinical characteristics. Stroke 34:932–937
Schaefer PW, Hassankhani A, Pulman C, Sorensen G, Schwamm L, Koroshetz W, Gonzales GR (2004) Characterization and evolution of diffusion MR imaging abnormalities in stroke patients undergoing intra-arterial thrombolysis. AJNR Am J Neuroradiol 25:951–957
Kidwell CS, Saver J, Mattiello J, Starkman S, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR (2000) Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol 47:462–469
Nighoghossian N, Hermier M, Adeleine P, Derex L, Durgor JF, Philippeau F, Ylmaz H, Honnorat J, Dardel P, Berthezene Y, Froment JC, Trouillas P (2003) Baseline magnetic resonance imaging parameters and stroke outcome in patients treated by intravenous tissue plasminogen activator. Stroke 34:458–463
Derex L, Nighoghossian N, Hermier M, Adeleine P, Berthezène, Philippeau F, Honnorat J, Froment J-C, Trouillas P (2004) Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci 225:3–9
Albers GW (1999) Expanding the window for thrombolytic therapy in acute stroke. The potential role of acute MRI for patient selection. Stroke 30:2230–2237
Barber PA, Davis SM, Darby DG, Desmond PM, Gerraty RP, Yang Q, Jolley D, Donnan GA, Tress BM (1999) Absent middle cerebral artery flow predict the presence and evolution of the ischemic penumbra. Neurology 52:1125–1132
Dugar N, Hoggard N, Wilkinson ID, Griffiths PD (2001) MR Imaging in acute stroke. Imaging Decis 5:2–19
Samson Y, Crozier S, Deltour S, Obadia M, Manai R, Oppenheim C, Marro B, Dormont D, Marsault C, Rancurel G (2002) L’IRM en urgence avant la thrombolyse (abstract). Rev Neurol Paris 158:1S20
Fiebach JB, Schellinger PD, Sartor K, Heiland S, Warach S, Hacke W (2003) Stroke MRI. Steinkopff Verlag, Darmstadt, pp 54–61
Schellinger PD, Fiebach JB, Hacke W (2003) Imaging-based decision making in thrombolytic therapy for ischemic stroke. Stroke 34:575–583
Hacke W, Kaste M, Bogousslavsky J, Brainin M, Chamorro A, Lees K, Leys D, Kwiecinski H, Toni P, Langhorne P, Diener C, Hennerici M, Ferro J, Sivenius J, Gunnar N, Bath P, Olsen TS, Gugging M, European Stroke Initiative Executive Committee and the EUSI Writing Committee (2003) European Stroke Initiative Recommendations for Stroke Management – update 2003. Cerebrovasc Dis 16:311–337
Highasida RT, Furlan AJ (2003) Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34:109–137
Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y, Linfante I, Schlaug G (2002) Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator. Stroke 33:2047–2052
Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, Donnan GA, Tress BM, Davis SM (1998) Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 51:418–426
Neumann-Haefelin T, Witsack HJ, Wenserski F, Siebler M, Seitz RJ, Modder U, Freund HJ (1999) Diffusion and perfusion-weighted MRI: the DWI/PWI mismatch region in acute stroke. Stroke 30:1591–1597
Grandin CB, Duprez TP, Smith AM, Mataigne F, Peeters A, Oppenheim C, Cosnard G (2001) Usefulness magnetic resonance-derived quantitative measurements of cerebral blood flow and volume in prediction of infarct growth in hyperacute stroke. Stroke 32:1147–1153
Sorensen AG, Copen WA, Ostergaard L, Buomanno FS, Gonzales RG, Rordorf G, Rosen BR, Schwamm LH, Weisskoff RM, Koroshetz WJ (1999) Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology 210:519–527
Heiss WD, Sobesky J, Hesselmann V (2004) Identifying thresholds for penumbra and irreversible tissue damage. Stroke 35:2671–2674
Acknowledgements
We thank Ms. Lucie Kopuletá and Ms. Michaela Kotzmundová, Department of Radiology, Division of MRI, University Hospital, Olomouc, Czech Republic, for their technical help in MRI data processing. This work was supported by the IGA Ministry of Health, Czech Republic (grant number NR/8579-3/2005).
Study protocol was in compliance with the Declaration of Helsinki (1964) and was approved by the Ethical Committee of the University Hospital, Olomouc, Czech Republic.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šaňák, D., Nosál′, V., Horák, D. et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology 48, 632–639 (2006). https://doi.org/10.1007/s00234-006-0105-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0105-0